Preparation of Laterally Chloro-Substituted Schiff Base Ester Liquid Crystals: Mesomorphic and Optical Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis
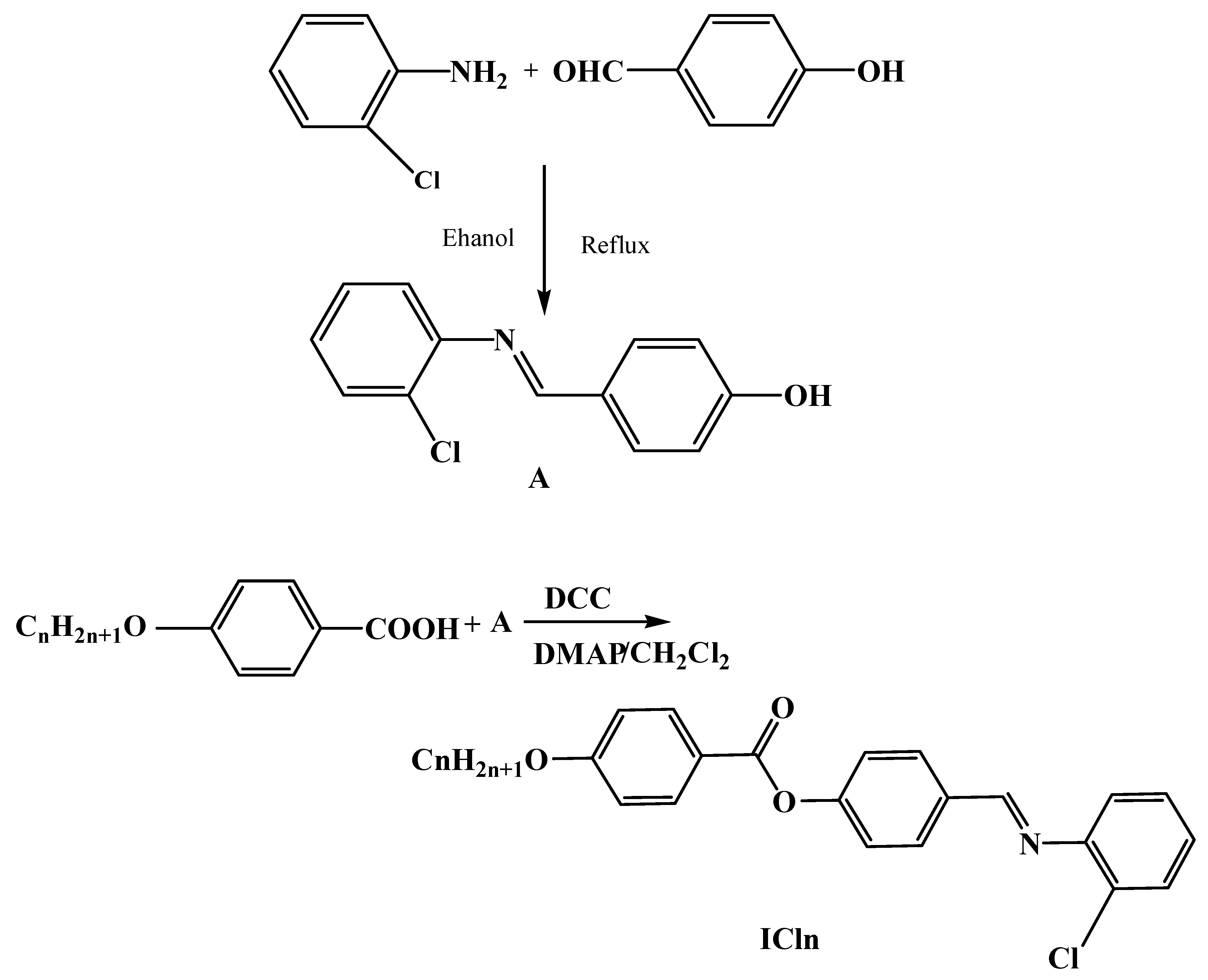
2.2.1. Synthesis of 4-((2′-Chlorophenylimino)methyl)phenol (A)
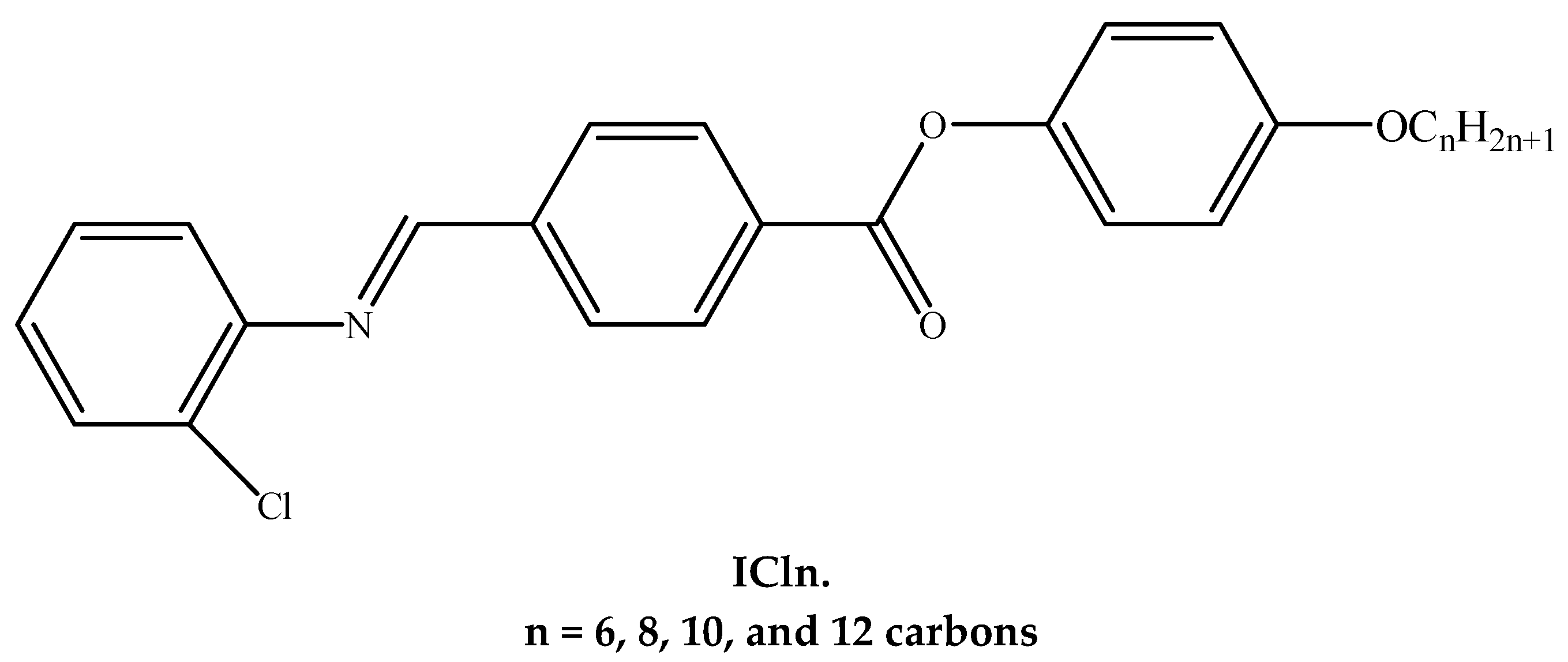
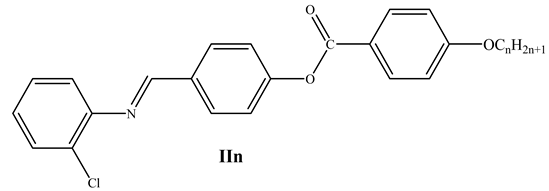
2.2.2. Synthesis of 4-((2′-Chlorophenylimino)methyl)phenyl-4″-alkoxy Benzoates, ICln
3. Results and Discussion
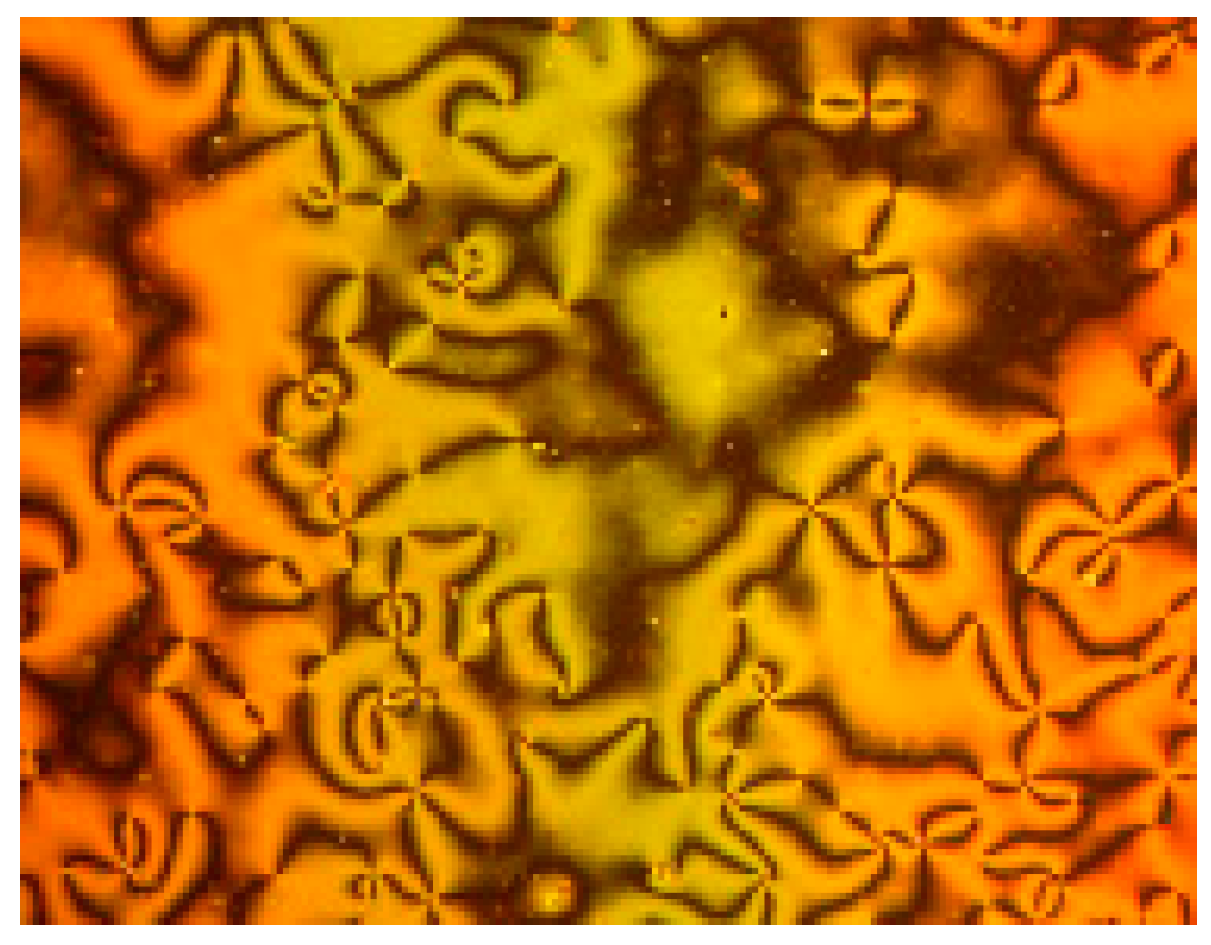
3.1. Liquid Crystalline Behavior
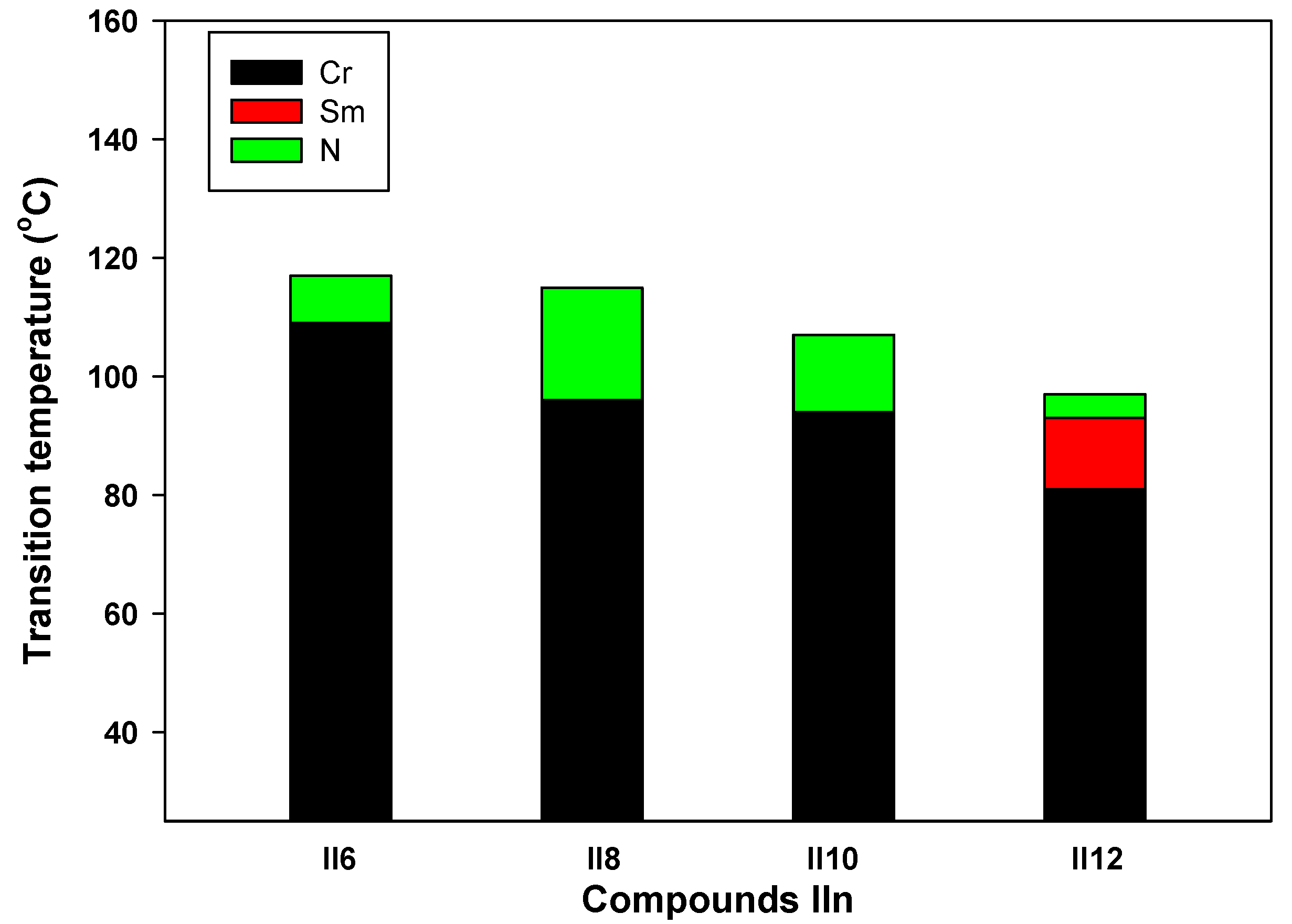
3.2. Comparison with Related Materials
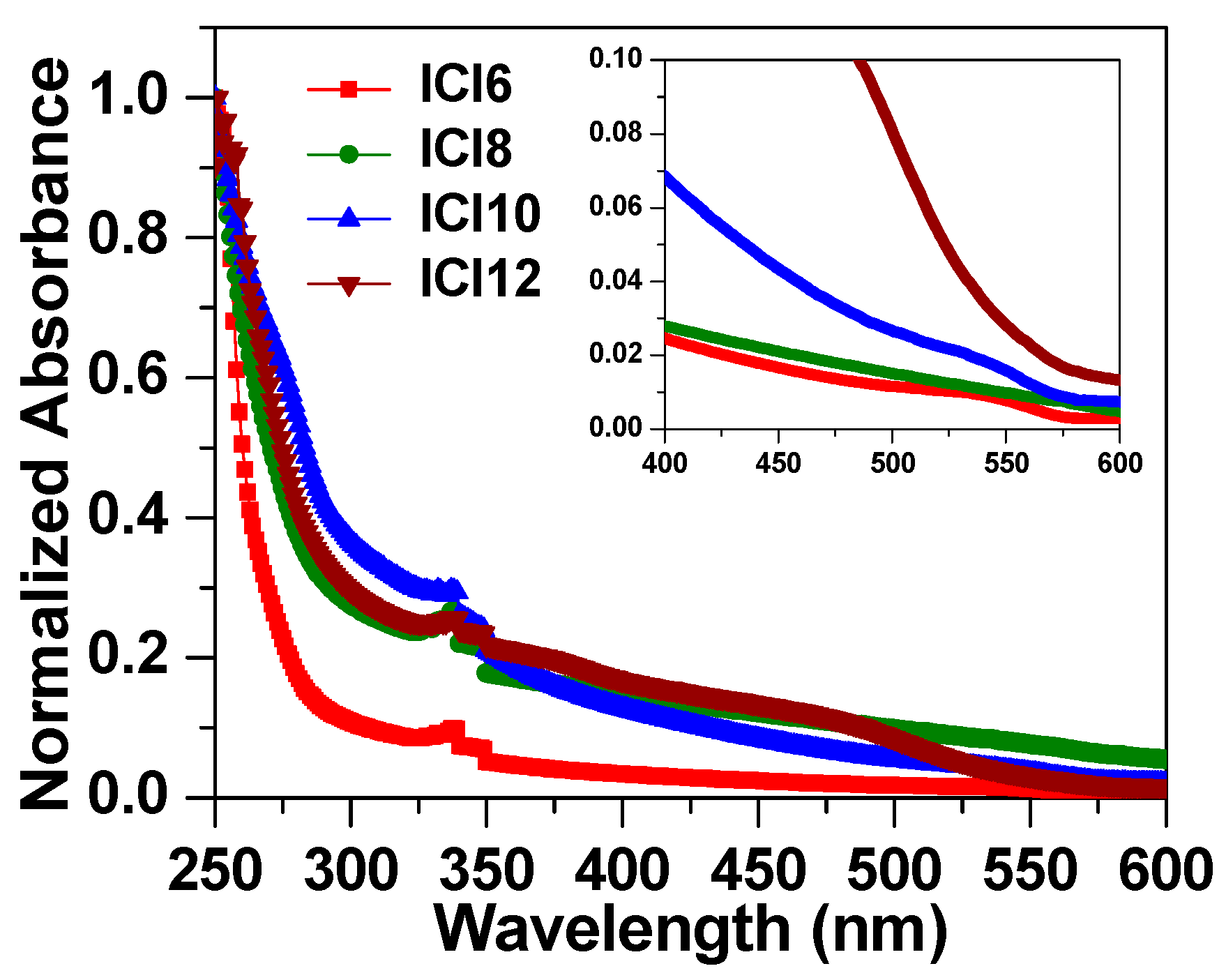
3.3. UV-Visible Absorption Spectra
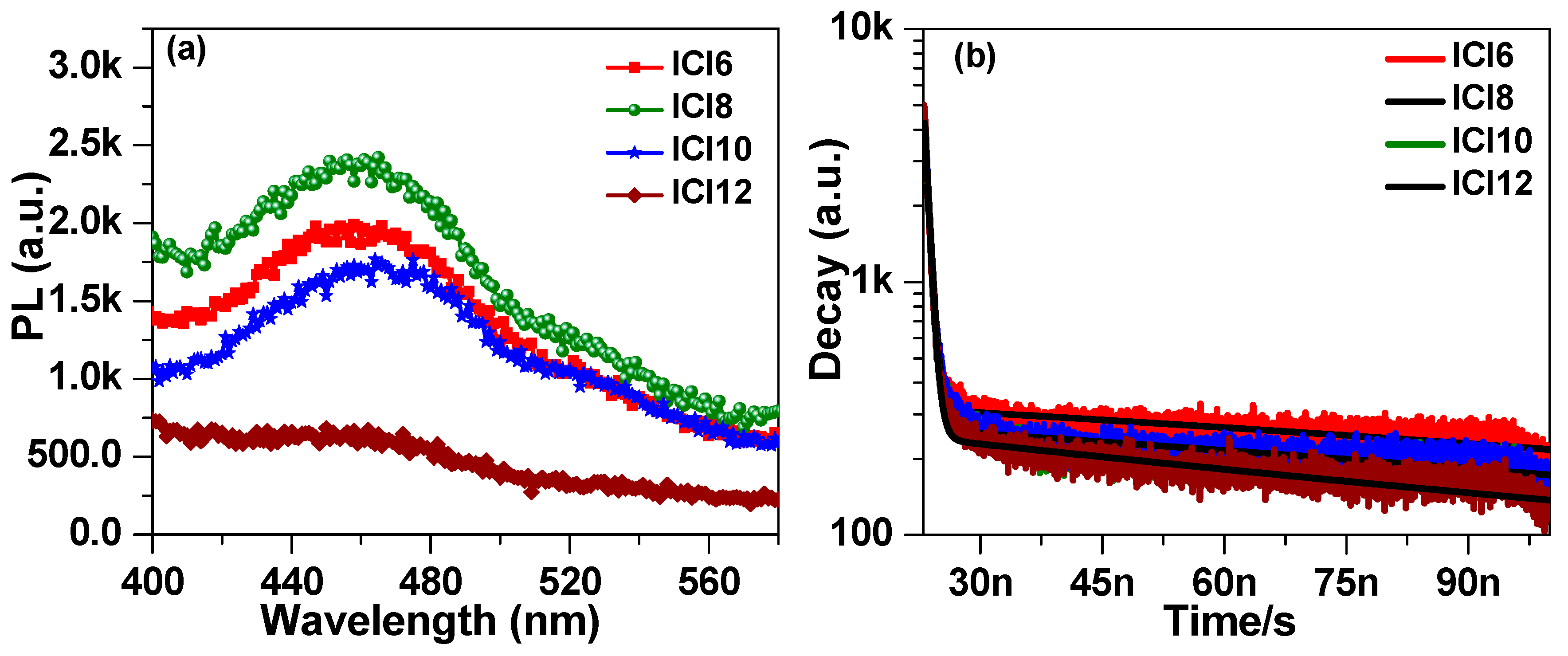
3.4. Photophysical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veerabhadraswamy, B.N.; Rao, D.S.S.; Yelamaggad, C.V. Ferroelectric Liquid Crystals: Synthesis and Thermal Behavior of Optically Active, Three-Ring Schiff Bases and Salicylaldimines. Chem.–Asian J. 2018, 13, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hsu, C.-C.; Chen, L.-W.; Cheng, Y.-L. The effect of position of (S)-2-octyloxy tail on the formation of frustrated blue phase and antiferroelectric phase in Schiff base liquid crystals. Soft Matter 2014, 10, 9343–9351. [Google Scholar] [CrossRef] [PubMed]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Gowda, A.; Jacob, L.; Joy, N.; Philip, R.; Pratibha, R.; Kumar, S. Thermal and nonlinear optical studies of newly synthesized EDOT based bent-core and hockey-stick like liquid crystals. New J. Chem. 2018, 42, 2047–2057. [Google Scholar] [CrossRef]
- Omar, A.Z.; Alazmi, M.L.; Alsubaie, M.S.; Hamed, E.A.; Ahmed, H.A.; El-Atawy, M.A. Synthesis of New Liquid-Crystalline Compounds Based on Terminal Benzyloxy Group: Characterization, DFT and Mesomorphic Properties. Molecules 2023, 28, 3804. [Google Scholar] [CrossRef]
- Alamro, F.S.; Gomha, S.M.; Shaban, M.; Altowyan, A.S.; Abolibda, T.Z.; Ahmed, H.A. Optical investigations and photoactive solar energy applications of new synthesized Schiff base liquid crystal derivatives. Sci. Rep. 2021, 11, 15046. [Google Scholar] [CrossRef]
- Gomha, S.M.; Ahmed, H.A.; Shaban, M.; Abolibda, T.Z.; Khushaim, M.S.; Alharbi, K.A. Synthesis, optical characterizations and solar energy applications of new Schiff base materials. Materials 2021, 14, 3718. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Gomha, S.M.; Abolibda, T.Z.; Shaban, M.; Ahmed, H.A. Synthesis and Mesomorphic and Electrical Investigations of New Furan Liquid Crystal Derivatives. Front. Chem. 2021, 9, 711862. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Khan, M.T.; Jevtovic, V.; Masood, N.; Jeilani, Y.A.; Ahmed, H.A.; Alfaidi, F.M. Liquid Crystalline Mixtures with Induced Polymorphic Smectic Phases Targeted for Energy Investigations. Crystals 2023, 13, 645. [Google Scholar] [CrossRef]
- Deng, X.; Wen, X.; Zheng, J.; Young, T.; Lau, C.F.J.; Kim, J.; Green, M.; Huang, S.; Ho-Baillie, A. Dynamic study of the light soaking effect on perovskite solar cells by in-situ photoluminescence microscopy. Nano Energy 2018, 46, 356–364. [Google Scholar] [CrossRef]
- Alamro, F.S.; Ahmed, H.A.; Gomha, S.M.; Shaban, M. Synthesis, Mesomorphic, and Solar Energy Characterizations of New Non-Symmetrical Schiff Base Systems. Front. Chem. 2021, 9, 686788. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, A.; Stoilova, A.; Dimov, D.; Yordanov, D.; Zhivkov, I.; Weiter, M. Synthesis and photochromic properties of some N-phthalimide azo-azomethine dyes. A DFT quantum mechanical calculations on imine-enamine tautomerism and trans-cis photoisomerization. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 210, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Ovdenko, V.; Kolendo, A. New bent-shaped azomethine monomers for optical applications. Mol. Cryst. Liq. Cryst. 2016, 640, 113–121. [Google Scholar] [CrossRef]
- Komissarova, O.A.; Lukyanov, B.S.; Lukyanova, M.B.; Ozhogin, I.V.; Mukhanov, E.L.; Korobov, M.S.; Minkin, V.I. New indoline spiropyrans containing azomethine fragment. Russ. Chem. Bull. 2017, 66, 2122–2125. [Google Scholar] [CrossRef]
- Stoilova, A.; Georgiev, A.; Nedelchev, L.; Nazarova, D.; Dimov, D. Structure-property relationship and photoinduced birefringence of the azo and azo-azomethine dyes thin films in PMMA matrix. Opt. Mater. 2019, 87, 16–23. [Google Scholar] [CrossRef]
- Georgiev, A.; Kostadinov, A.; Ivanov, D.; Dimov, D.; Stoyanov, S.; Nedelchev, L.; Yancheva, D. Synthesis, spectroscopic and TD-DFT quantum mechanical study of azo-azomethine dyes. A laser induced trans-cis-trans photoisomerization cycle. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 192, 263–274. [Google Scholar] [CrossRef]
- Reddy, D.S.; Reddy, G.S.; Beatriz, A.; Corey, E.J. Contrasting Diastereoselectivity between Cyclic Nitrones and Azomethine Ylides. Stereocontrolled Pathways to cis-anti-anti-cis-Oxazatetraquinanes from a Bicyclic Nitrone. Org. Lett. 2021, 23, 5445–5447. [Google Scholar] [CrossRef]
- Gray, G. Thermotropic Liquid Crystals; Wiley: New York, NY, USA, 1987. [Google Scholar]
- Ha, S.-T.; Ng, M.-Y.; Subramaniam, R.T.; Ito, M.M.; Saito, A.; Watanabe, M.; Lee, S.-L.; Bonde, N.L. Mesogenic azomethiane esters with different end groups: Synthesis and thermotropic properties. Int. J. Phys. Sci. 2010, 5, 1256–1262. [Google Scholar]
- Collings, P.; Goodby, J. Introduction to Liquid Crystals: Chemistry and Physics, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis: Abingdon, UK, 2019. [Google Scholar] [CrossRef]
- Yeap, G.; Osman, F.; Imrie, C. Non-symmetric dimers: Effects of varying the mesogenic linking unit and terminal substituent. Liq. Cryst. 2015, 42, 543–554. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Abaza, A.H.; Saad, G.R. Effect of exchange of terminal substituents on the mesophase behaviour of some azo/ester compounds. Liq. Cryst. 2014, 41, 1559–1568. [Google Scholar] [CrossRef]
- Aldahri, T.H.; Alaasar, M.; Ahmed, H.A. The influence of core fluorination on the phase behaviour of rod-like mesogens. Liquid Cryst. 2023, 1–10. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Omar, A.Z.; Alazmi, M.L.; Alsubaie, M.S.; Hamed, E.A.; Ahmed, H.A. Synthesis and characterization of new imine liquid crystals based on terminal perfluoroalkyl group. Heliyon 2023, 9, e14871. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.Z.; El-Atawy, M.A.; Alsubaie, M.S.; Alazmi, M.L.; Ahmed, H.A.; Hamed, E.A. Synthesis and Computational Investigations of New Thioether/Azomethine Liquid Crystal Derivatives. Crystals 2023, 13, 378. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Abd Eltawab, M.M.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Ahmed, H.; Saad, G. Mesophase behaviour of laterally di-fluoro-substituted four-ring compounds. Liq. Cryst. 2015, 42, 1765–1772. [Google Scholar] [CrossRef]
- Al-Zahrani, S.A.; Khan, M.T.; Jevtovic’, V.; Masood, N.; Jeilani, Y.A.; Ahmed, H.A. Design of Liquid Crystal Materials Based on Palmitate, Oleate, and Linoleate Derivatives for Optoelectronic Applications. Molecules 2023, 28, 1744. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Liquid crystalline behaviour of model compounds di-laterally substituted with different polar groups. Liq. Cryst. 2011, 38, 511–519. [Google Scholar] [CrossRef]
- Naoum, M.; Ahmed, H. Effect of dipole moment and conformation on the mesophase behavior of di-laterally substituted phenylazophenyl benzoate liquid crystals. Thermochim. Acta 2011, 521, 202–210. [Google Scholar] [CrossRef]
- Naoum, M.; Mohammady, S.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-) methyl phenyl-4′-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Vora, R.A.; Patel, D.N. Mesogenic Homologous Series Containing Chloro Group as Ortho and Para Substituent. Mol. Cryst. Liq. Cryst. 1983, 103, 127–135. [Google Scholar] [CrossRef]
- Goodby, J.; Collings, P.; Kato, T.; Tschierske, C. Handbook of Liquid Crystals, 2nd ed.; Wiley: Weinheim, Germany, 2014; Volume 8. [Google Scholar]
- Blinov, L. Structure and Properties of Liquid Crystals; Springer: London, UK, 2011. [Google Scholar]
- Patel, J.S.; Meyer, R.B. Flexoelectric electro-optics of a cholesteric liquid crystal. Phys. Rev. Lett. 1987, 58, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, B.; Lehmann, P.; Coles, H.J. A new series of chiral nematic bimesogens for the flexoelectro-optic effect. Liq. Cryst. 1999, 26, 1235–1249. [Google Scholar] [CrossRef]
- Gray, G.W. Molecular Structure and the Properties of Liquid Crystals; Academic Press: Cambridge, MA, USA, 1962. [Google Scholar]
- Gorkunov, M.V.; Osipov, M.A.; Kocot, A.; Vij, J.K. Molecular model of biaxial ordering in nematic liquid crystals composed of flat molecules with four mesogenic groups. Phys. Rev. E 2010, 81, 061702. [Google Scholar] [CrossRef] [PubMed]
- Klingbiel, R.T.; Genova, D.J.; Criswell, T.R.; Van Meter, J.P. Comparison of the dielectric behavior of several schiffbase and phenyl benzoate liquid crystals. J. Am. Chem. Soc. 1974, 96, 7651–7655. [Google Scholar] [CrossRef]
- Bogojawlenski, A.; Winogradov, N.Z. Über das Verhalten von Schmelz-und Klärungskurvenflüssiger Kristalle und ihrer Mischungen. Z. Phys. Chem. 1908, 64, 229–242. [Google Scholar] [CrossRef]
- Dewar, M.J.; Schroeder, J.P. P-alkoxy-and p-carbalkoxybenzoates of diphenols. A new series of liquid crystalline compounds. J. Org. Chem. 1965, 30, 2296–2300. [Google Scholar] [CrossRef]
- Khan, M.T.; Shkir, M.; Alhouti, B.; Almohammedi, A.; Ismail, Y.A.M. Modulation of optical, photophysical and electrical properties of poly(3-hexylthiophene) via Gd:CdS nanoparticles. Optik 2022, 260, 169092. [Google Scholar] [CrossRef]
- Khan, M.Y.; Almohammedi, A.; Shkir, M.; Aboud, S.W. Effect of Ag2S nanoparticles on optical, photophysical and electrical properties of P3HT thin films. Luminescence 2021, 36, 761–768. [Google Scholar] [CrossRef]
- Almohammedi, A.; Khan, M.T.; Benghanem, M.; Aboud, S.; Shakir, M.; Alfaify, S. Elucidating the impact of PbI2 on photophysical and electrical properties of poly(3-hexythiophene). Mater. Sci. Semicond. Process. 2020, 120, 105272. [Google Scholar] [CrossRef]
- Alhazime, A.A.; Mohamed, S.H.; Khan, M.T.; Awad, M.A. Effect of CuS buffer layer on the structural and optical properties of TeO2 nanowires: A comparative study. Phys. Scr. 2022, 97, 095807. [Google Scholar] [CrossRef]
- Khan, M.T.; Almohammedi, A.; Kazim, S.; Ahmed, S. Charge carrier dynamics in perovskite solar cells. In Perovskite Solar Cells: Materials, Processes, and Devices; Wiley-VCH: Hoboken, NJ, USA, 2021; pp. 389–429. ISBN 9783527347155. [Google Scholar] [CrossRef]
- Khan, F.; Khan, M.T.; Rehman, S.; Al-Sulaiman, F. Analysis of electrical parameters of p-i-n perovskites solar cells during passivation via N-doped graphene quantum dots. Surf. Interfaces 2022, 311, 02066. [Google Scholar] [CrossRef]
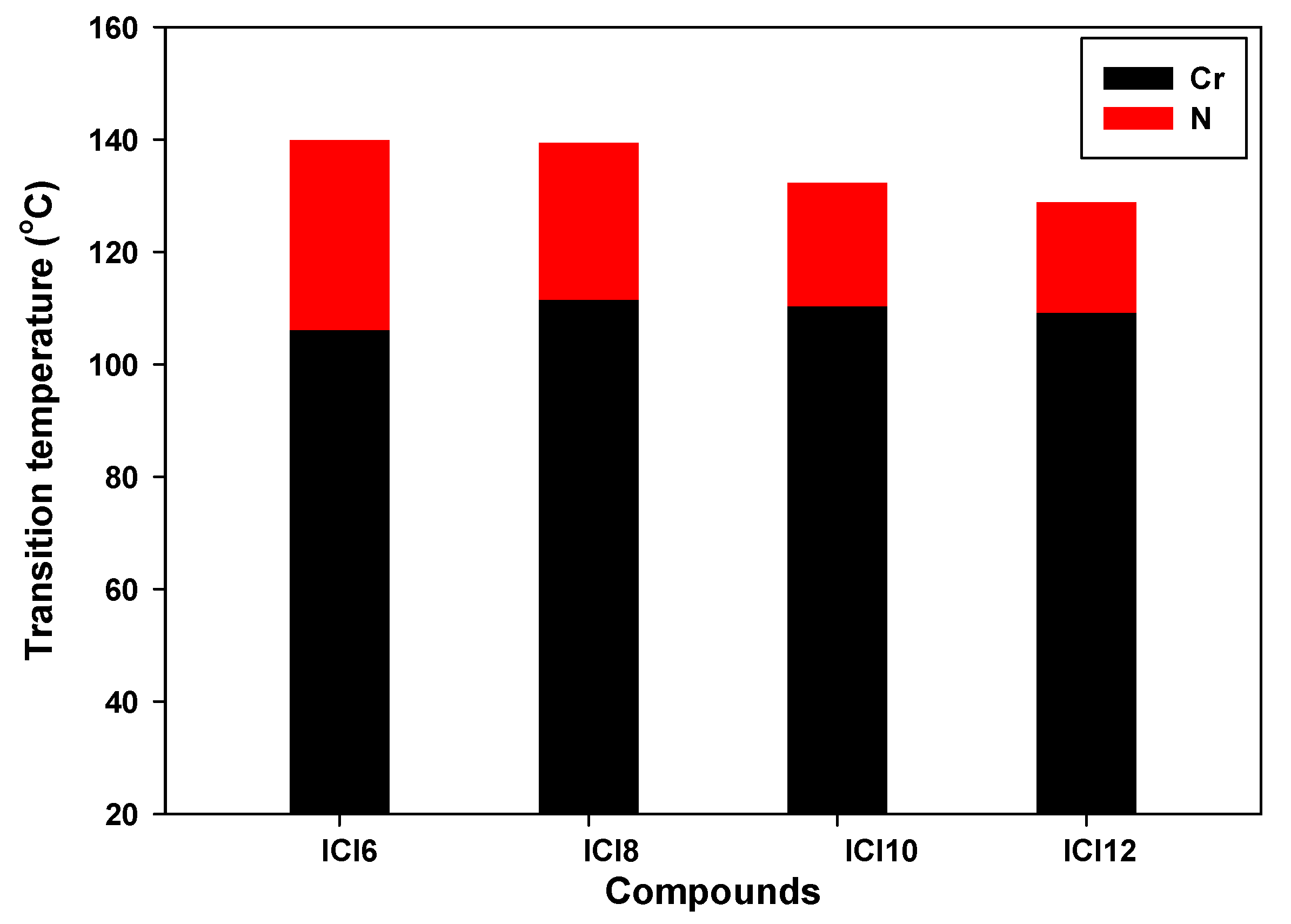
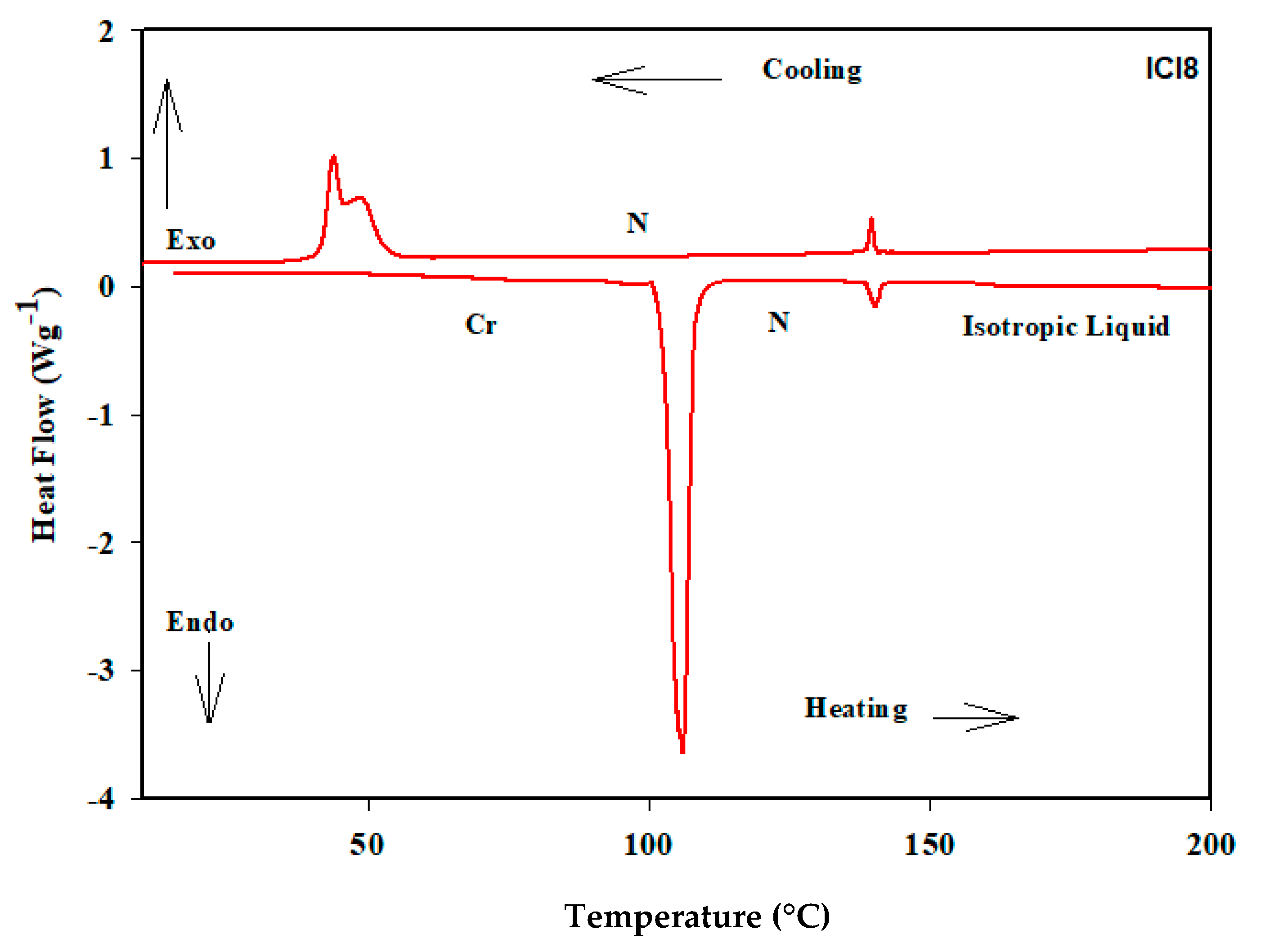


| Compound | TCr-N | ∆HCr-N | TN-I | ∆HN-I | ∆TN | ∆S/R |
|---|---|---|---|---|---|---|
| ICl6 | 106.1 | 38.4 | 139.9 | 1.49 | 33.8 | 0.43 |
| ICl8 | 111.5 | 41.6 | 139.4 | 1.73 | 27.9 | 0.50 |
| ICl10 | 110.4 | 46.9 | 132.3 | 1.18 | 21.9 | 0.35 |
| ICl12 | 109.2 | 47.1 | 128.8 | 1.32 | 19.6 | 0.39 |

| Comp. | Mesohase Transition Temperature and Enthalpy |
|---|---|
| I6 | Cr 110.8(26.15) N 156.8 (0.88) I |
| I8 | Cr 105.1(34.13) SmA 112.2 (0.81) N 150.4 (0.31) I |
| I10 | Cr 104.7(30.15) SmA 123.7 (0.78) N 144.6 (0.62) I |
| I12 | Cr 108.9(19.85) SmA 207.4 (3.27) I |
| Comp. | Phase Transition Temperature |
|---|---|
| II6 | Cr 109 N 117 I |
| II8 | Cr 96 N 115 I |
| II10 | Cr 94 N 109 I |
| II16 | Cr 81 Sm 93 N 97 I |
| Sample | Eg (eV) | A0 | A1 (%) | τ1 (ps) | A2 (%) | τ2 (ns) | τavg (ns) | χ2 |
|---|---|---|---|---|---|---|---|---|
| ICl6 | 2.168 | 149 | 17.1% | 557 | 82.9% | 91 | 4.48 | 2.17 |
| ICl8 | 2.022 | 117 | 21.09% | 635 | 78.91% | 66 | 2.84 | 2.26 |
| ICl10 | 2.012 | 98 | 14.17% | 618 | 85.83% | 99 | 3.59 | 2.25 |
| ICl12 | 1.868 | 47 | 10.88% | 571 | 89.12% | 100 | 5.02 | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtovic, V.; Ahmed, H.A.; Khan, M.T.; Al-Zahrani, S.A.; Masood, N.; Jeilani, Y.A. Preparation of Laterally Chloro-Substituted Schiff Base Ester Liquid Crystals: Mesomorphic and Optical Properties. Crystals 2023, 13, 835. https://doi.org/10.3390/cryst13050835
Jevtovic V, Ahmed HA, Khan MT, Al-Zahrani SA, Masood N, Jeilani YA. Preparation of Laterally Chloro-Substituted Schiff Base Ester Liquid Crystals: Mesomorphic and Optical Properties. Crystals. 2023; 13(5):835. https://doi.org/10.3390/cryst13050835
Chicago/Turabian StyleJevtovic, Violeta, Hoda A. Ahmed, Mohd Taukeer Khan, Salma A. Al-Zahrani, Najat Masood, and Yassin Aweis Jeilani. 2023. "Preparation of Laterally Chloro-Substituted Schiff Base Ester Liquid Crystals: Mesomorphic and Optical Properties" Crystals 13, no. 5: 835. https://doi.org/10.3390/cryst13050835
APA StyleJevtovic, V., Ahmed, H. A., Khan, M. T., Al-Zahrani, S. A., Masood, N., & Jeilani, Y. A. (2023). Preparation of Laterally Chloro-Substituted Schiff Base Ester Liquid Crystals: Mesomorphic and Optical Properties. Crystals, 13(5), 835. https://doi.org/10.3390/cryst13050835







