Cerium Niobate Hollow Sphere Engineered Graphitic Carbon Nitride for Synergistic Photothermal/Chemodynamic Cancer Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Required Chemicals
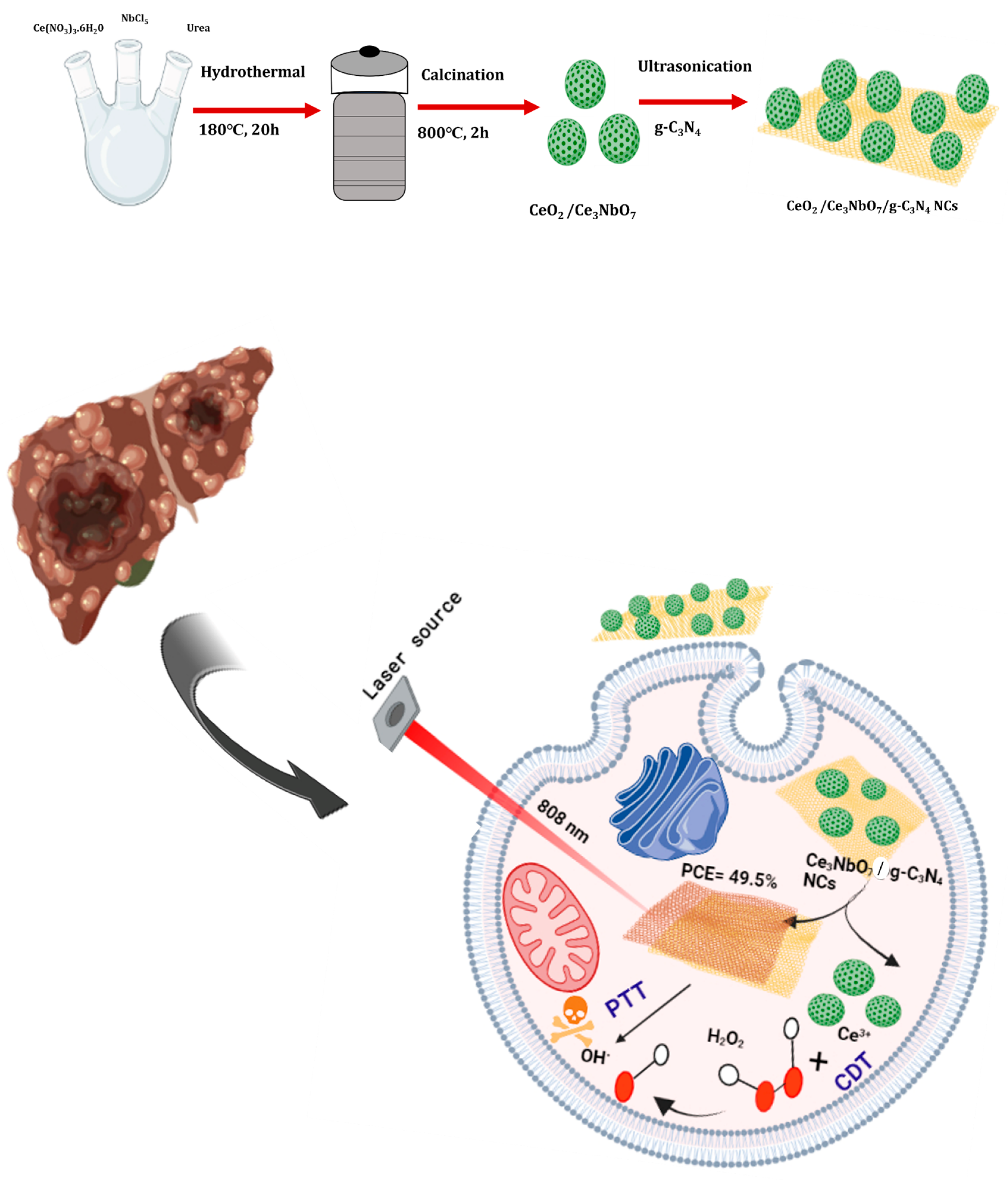
2.2. Preparation for CeO2/Ce3NbO7 Hollow Spheres
2.3. Preparation of g-C3N4
2.4. Modification of g-C3N4 on CeO2/Ce3NbO7 Hollow Spheres
2.5. Characterization of Synthesized g-C3N4-Coated NCs
2.6. Photothermal Response of the Synthesized CeO2/Ce3NbO7/g-C3N4 NCs
2.7. Cell Culture
2.8. OH• Generation of CeO2/Ce3NbO7/g-C3N4 NCs by MB
2.9. In Vitro Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
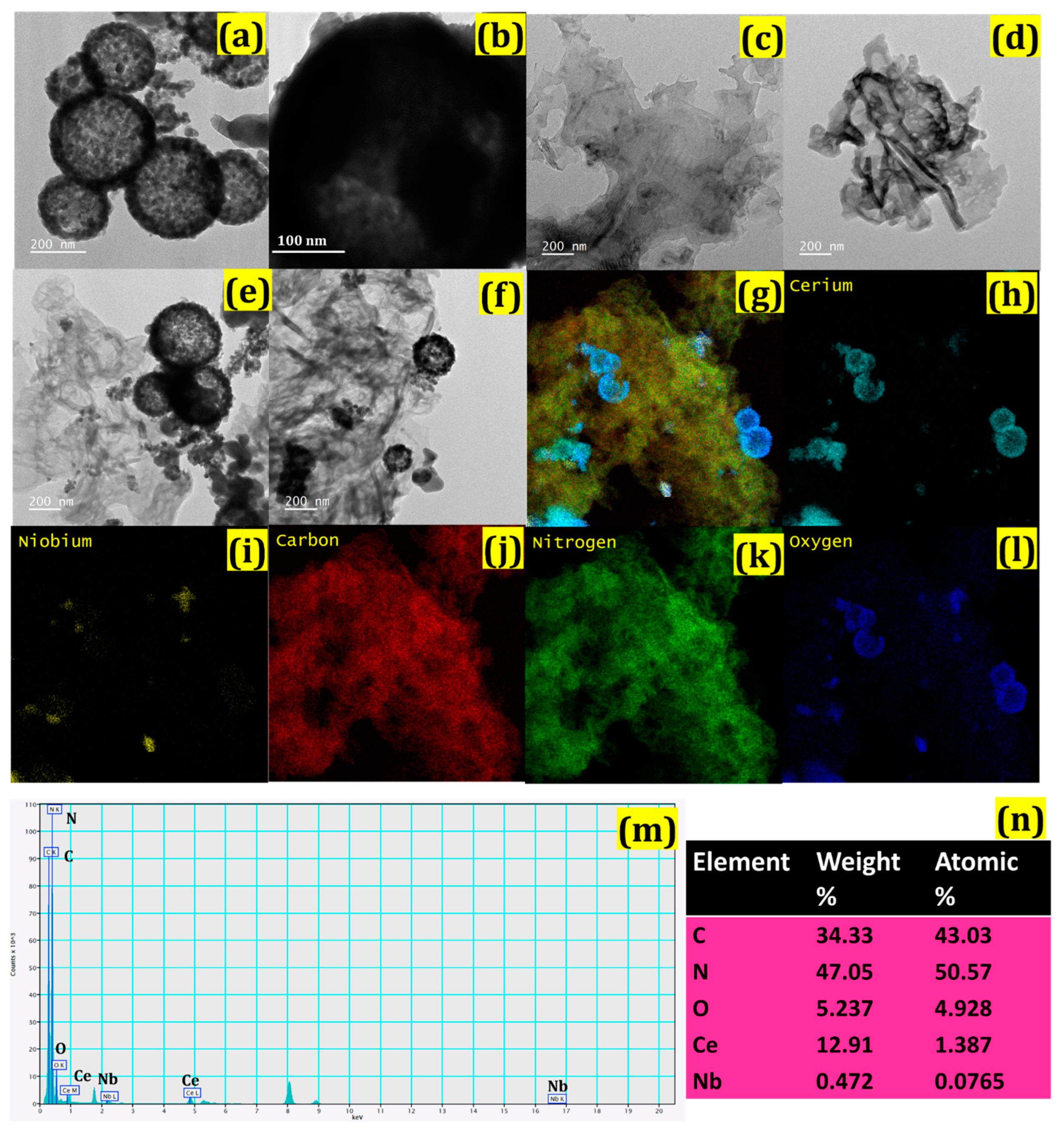
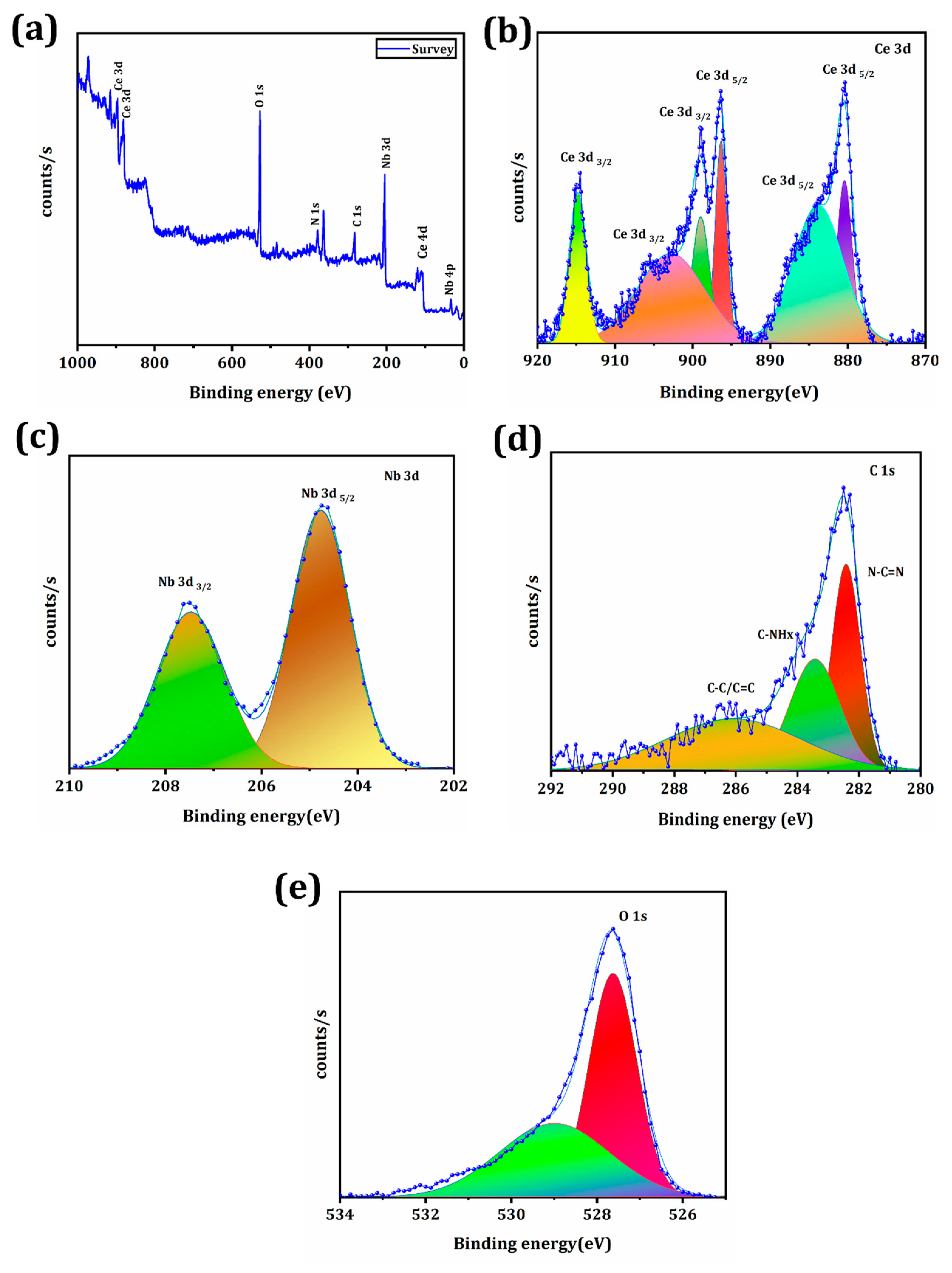
3.1. Synthesis and Characterization of CeO2/Ce3NbO7/g-C3N4 NCs
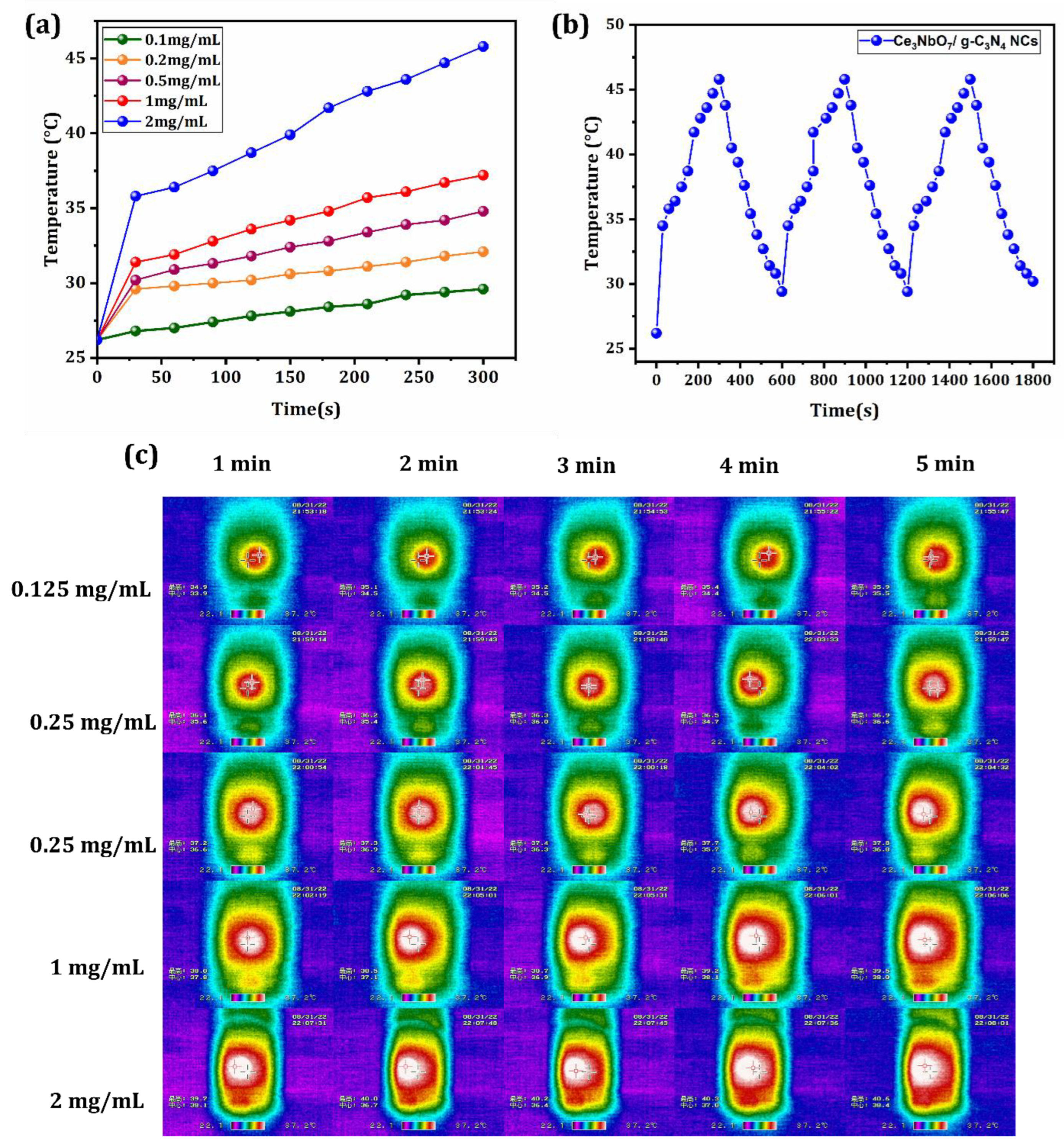
3.2. Photothermal Performance of CeO2/Ce3NbO7/g-C3N4 NCs
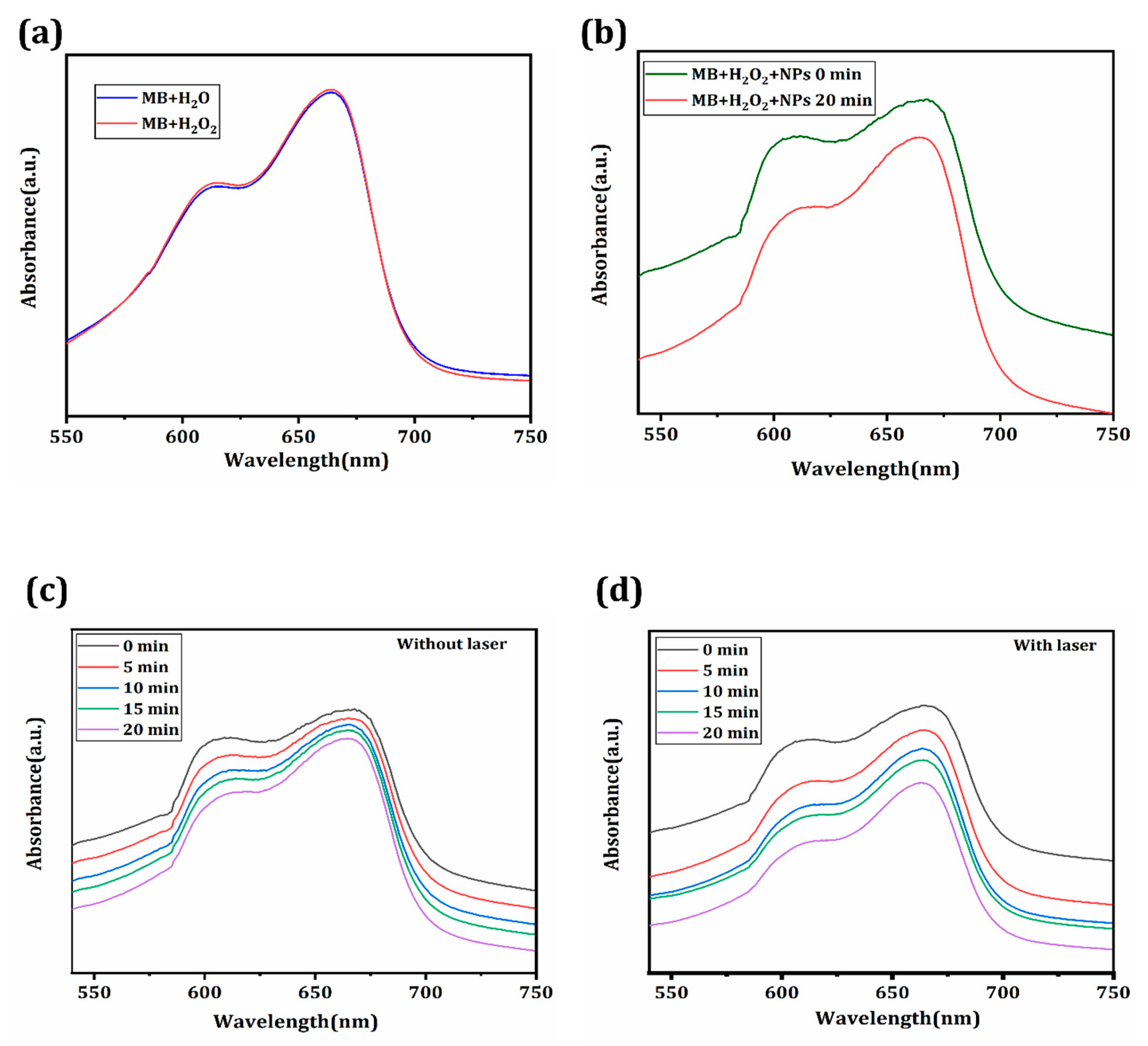
3.3. Fenton Catalytic Property of the Synthesized CeO2/Ce3NbO7/g-C3N4 NCs
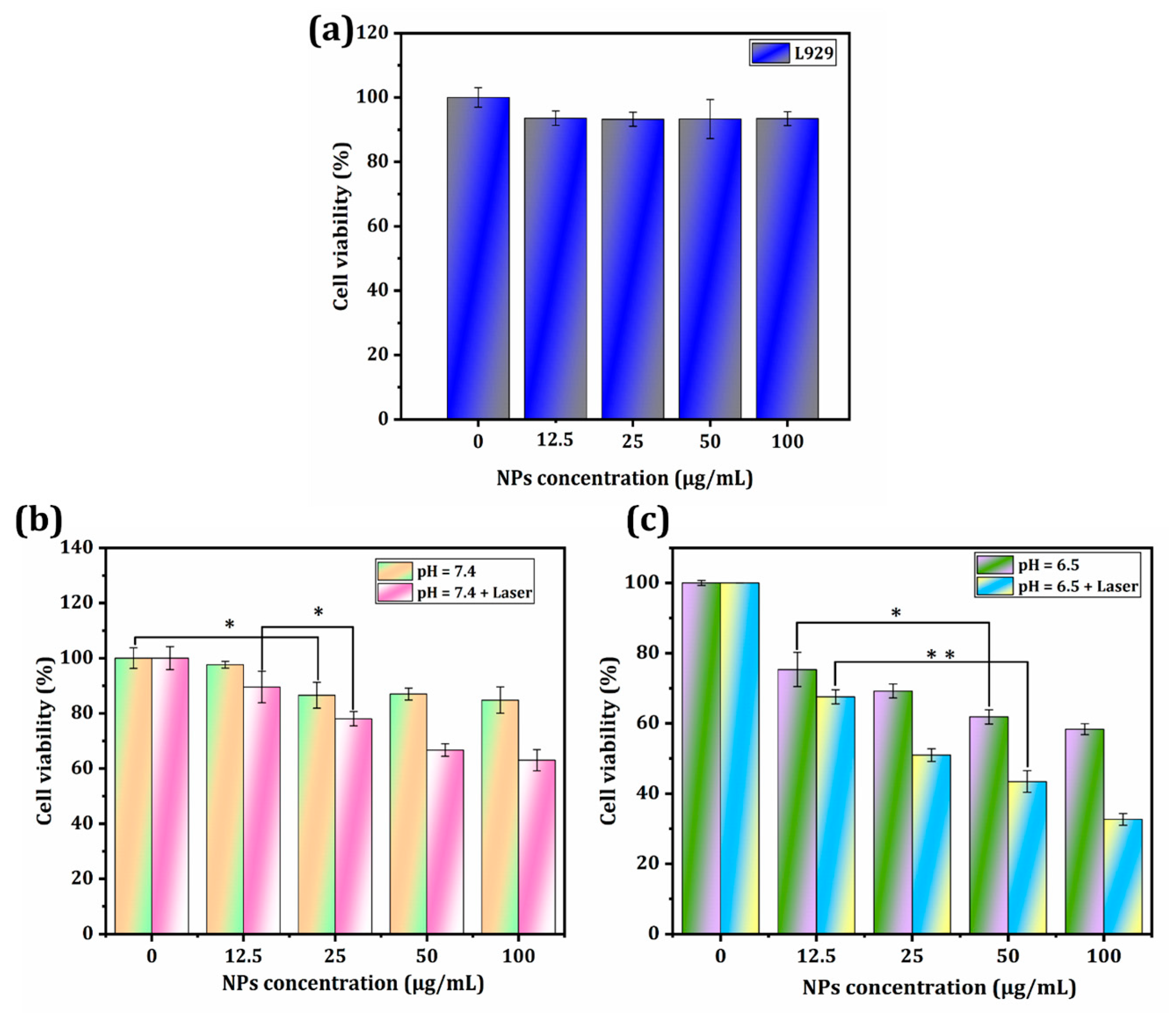
3.4. In Vitro PTT/CDT Performance of CeO2/Ce3NbO7/g-C3N4 NCs towards Liver Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Z.; Fang, C.; Ma, X.; Li, X.; Zeng, Y.-J.; Peng, X. One stone two birds: Zr-Fc metal–organic framework nanosheet for synergistic photothermal and chemodynamic cancer therapy. ACS Appl. Mater. Isnterfaces 2020, 12, 20321–20330. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Weiderpass, E. Lifestyle and cancer risk. J. Prev. Med. Public Health 2010, 43, 459–471. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hunter, D.J. Molecular epidemiology of cancer. CA A Cancer J. Clin. 2005, 55, 45–54. [Google Scholar] [CrossRef]
- Arico, M.; Schrappe, M.; Hunger, S.P.; Carroll, W.L.; Conter, V.; Galimberti, S.; Manabe, A.; Saha, V.; Baruchel, A.; Vettenranta, K. Clinical outcome of children with newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia treated between 1995 and 2005. J. Clin. Oncol. 2010, 28, 4755. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, J.; Kapur, J.; Malkin, D.; Babyn, P.S. Imaging of cancer predisposition syndromes in children. Radiographics 2011, 31, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A. International Trends in Liver Cancer Incidence RatesInternational Liver Cancer Incidence Trends. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2362–2368. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef]
- Pérez-López, A.; Martín-Sabroso, C.; Gómez-Lázaro, L.; Torres-Suárez, A.I.; Aparicio-Blanco, J. Embolization therapy with microspheres for the treatment of liver cancer: State-of-the-art of clinical translation. Acta Biomater. 2022, 149, 1–15. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Agency for Research on Cancer Cancer Today; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Starley, B.Q.; Calcagno, C.J.; Harrison, S.A. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar] [CrossRef]
- Feng, M.; Pan, Y.; Kong, R.; Shu, S. Therapy of primary liver cancer. Innovation 2020, 1, 100032. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, W.; Yu, N.; Li, X.; Feng, Y.; Geng, P.; Wen, M.; Li, M.; Zhang, H.; Chen, Z. Construction of CuS@ Fe-MOF nanoplatforms for MRI-guided synergistic photothermal-chemo therapy of tumors. Chem. Eng. J. 2020, 400, 125877. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for multimodal synergistic cancer therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Shi, J. Nanoenzyme-augmented cancer sonodynamic therapy by catalytic tumor oxygenation. ACS Nano 2018, 12, 3780–3795. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic therapy: Tumour microenvironment-mediated Fenton and Fenton-like reactions. Angew. Chem. 2019, 131, 958–968. [Google Scholar] [CrossRef]
- Jiang, F.; Ding, B.; Liang, S.; Zhao, Y.; Cheng, Z.; Xing, B.; Lin, J. Intelligent MoS2–CuO heterostructures with multiplexed imaging and remarkably enhanced antitumor efficacy via synergetic photothermal therapy/chemodynamic therapy/immunotherapy. Biomaterials 2021, 268, 120545. [Google Scholar] [CrossRef]
- Ng, C.W.; Li, J.; Pu, K. Recent progresses in phototherapy-synergized cancer immunotherapy. Adv. Funct. Mater. 2018, 28, 1804688. [Google Scholar] [CrossRef]
- Wang, C.; Xu, L.; Liang, C.; Xiang, J.; Peng, R.; Liu, Z. Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis. Adv. Mater. 2014, 26, 8154–8162. [Google Scholar] [CrossRef]
- Wu, C.; Wang, S.; Zhao, J.; Liu, Y.; Zheng, Y.; Luo, Y.; Ye, C.; Huang, M.; Chen, H. Biodegradable Fe (III)@ WS2-PVP nanocapsules for redox reaction and TME-enhanced nanocatalytic, photothermal, and chemotherapy. Adv. Funct. Mater. 2019, 29, 1901722. [Google Scholar] [CrossRef]
- Liu, Y.; Zhen, W.; Jin, L.; Zhang, S.; Sun, G.; Zhang, T.; Xu, X.; Song, S.; Wang, Y.; Liu, J. All-in-one theranostic nanoagent with enhanced reactive oxygen species generation and modulating tumor microenvironment ability for effective tumor eradication. ACS Nano 2018, 12, 4886–4893. [Google Scholar] [CrossRef]
- Wan, S.-S.; Cheng, Q.; Zeng, X.; Zhang, X.-Z. A Mn (III)-sealed metal–organic framework nanosystem for redox-unlocked tumor theranostics. ACS Nano 2019, 13, 6561–6571. [Google Scholar] [CrossRef]
- Ding, B.; Shao, S.; Jiang, F.; Dang, P.; Sun, C.; Huang, S.; Ma, P.a.; Jin, D.; Kheraif, A.A.A.; Lin, J. MnO2-disguised upconversion hybrid nanocomposite: An ideal architecture for tumor microenvironment-triggered UCL/MR bioimaging and enhanced chemodynamic therapy. Chem. Mater. 2019, 31, 2651–2660. [Google Scholar] [CrossRef]
- Gao, S.; Jin, Y.; Ge, K.; Li, Z.; Liu, H.; Dai, X.; Zhang, Y.; Chen, S.; Liang, X.; Zhang, J. Self-supply of O2 and H2O2 by a Nanocatalytic medicine to enhance combined chemo/Chemodynamic therapy. Adv. Sci. 2019, 6, 1902137. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, Q.; Guo, Q.; Wei, W.; Zhou, J. Simultaneously activating highly selective ratiometric MRI and synergistic therapy in response to intratumoral oxidability and acidity. Biomaterials 2018, 180, 104–116. [Google Scholar] [CrossRef]
- Duan, L.-Y.; Wang, Y.-J.; Liu, J.-W.; Wang, Y.-M.; Li, N.; Jiang, J.-H. Tumor-selective catalytic nanosystem for activatable theranostics. Chem. Commun. 2018, 54, 8214–8217. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lim, D.-H.; Lim, H.-J.; Kwon, T.; Choi, J.-s.; Jeong, S.; Choi, I.-H.; Cheon, J. Size dependent macrophage responses and toxicological effects of Ag nanoparticles. Chem. Commun. 2011, 47, 4382–4384. [Google Scholar] [CrossRef]
- Hu, R.; Fang, Y.; Huo, M.; Yao, H.; Wang, C.; Chen, Y.; Wu, R. Ultrasmall Cu2-xS nanodots as photothermal-enhanced Fenton nanocatalysts for synergistic tumor therapy at NIR-II biowindow. Biomaterials 2019, 206, 101–114. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, B.; Sun, Q.; Dong, S.; Kuang, Y.; Dong, Y.; He, F.; Gai, S.; Yang, P. Fusiform-like copper (II)-based metal–organic framework through relief hypoxia and GSH-depletion co-enhanced starvation and chemodynamic synergetic cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 17254–17267. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Liu, Y.; Dai, X.; Wang, Y.; Guo, Q.; Li, Q. Advanced applications of cerium oxide based nanozymes in cancer. RSC Adv. 2022, 12, 1486–1493. [Google Scholar] [CrossRef]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. 2009, 121, 2344–2348. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, K.; Ma, J.-l.; Gao, F. Cerium oxide nanoparticles in cancer. OncoTargets Ther. 2014, 7, 835–840. [Google Scholar] [CrossRef]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Bosio, G.N.; Mártire, D.O. Carbon nitride nanomaterials with application in photothermal and photodynamic therapies. Photodiagnosis Photodyn. Ther. 2022, 37, 102683. [Google Scholar] [CrossRef]
- Liu, X.; Tseng, C.-L.; Lin, L.-Y.; Lee, C.-A.; Li, J.; Feng, L.; Song, L.; Li, X.; He, J.-H.; Sakthivel, R. Template-free synthesis of mesoporous Ce3NbO7/CeO2 hollow nanospheres for label-free electrochemical immunosensing of leptin. Sens. Actuators B Chem. 2021, 341, 130005. [Google Scholar] [CrossRef]
- Sanjay, B.P.; Swamy, N.K.; Yashas, S.R.; Sandeep, S. Design of an electrochemical sensor using 2D sheet-like Cu@ g-C3N4 transducer matrix for electroanalysis of catechol. J. Electrochem. Soc. 2021, 168, 076511. [Google Scholar] [CrossRef]
- Guoguang, Z.; Qin, L.; Qianfeng, F. Structure and Dielectric Study of Ce3 NbO7+ δ. J. Rare Earths 2007, 25, 730–733. [Google Scholar] [CrossRef]
- Farahmandjou, M.; Zarinkamar, M.; Firoozabadi, T. Synthesis of Cerium Oxide (CeO2) nanoparticles using simple CO-precipitation method. Rev. Mex. De Física 2016, 62, 496–499. [Google Scholar]
- Feng, L.; He, F.; Yang, G.; Gai, S.; Dai, Y.; Li, C.; Yang, P. NIR-driven graphitic-phase carbon nitride nanosheets for efficient bioimaging and photodynamic therapy. J. Mater. Chem. B 2016, 4, 8000–8008. [Google Scholar] [CrossRef]
- Sakthivel, R.; Annalakshmi, M.; Chen, S.-M.; Kubendhiran, S.; Anbazhagan, R.; Tsai, H.-C. A novel sensitive and reliable electrochemical determination of palmatine based on CeO2/RGO/MWCNT ternary composite. J. Taiwan Inst. Chem. Eng. 2019, 96, 549–558. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Kong, X.; Lei, D.; Zhang, F.; Lei, X. A hierarchical Nb2O5@ NiFe-MMO rod array, fabricated and used as a structured photocatalyst. RSC Adv. 2019, 9, 6177–6183. [Google Scholar] [CrossRef]
- Baranowska, D.; Kędzierski, T.; Aleksandrzak, M.; Mijowska, E.; Zielińska, B. Influence of hydrogenation on morphology, chemical structure and photocatalytic efficiency of graphitic carbon nitride. Int. J. Mol. Sci. 2021, 22, 13096. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Li, Z.; Zou, Z. Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 2009, 25, 10397–10401. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, H.; Selke, M.; Wang, X. Selective cytotoxicity effect of cerium oxide nanoparticles under UV irradiation. J. Biomed. Nanotechnol. 2014, 10, 278–286. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthiah, K.S.; Thirumurugan, S.; Lin, Y.-C.; Sakthivel, R.; Dhawan, U.; Wang, A.-N.; Hsiao, M.; Chung, R.-J. Cerium Niobate Hollow Sphere Engineered Graphitic Carbon Nitride for Synergistic Photothermal/Chemodynamic Cancer Therapy. Crystals 2023, 13, 954. https://doi.org/10.3390/cryst13060954
Muthiah KS, Thirumurugan S, Lin Y-C, Sakthivel R, Dhawan U, Wang A-N, Hsiao M, Chung R-J. Cerium Niobate Hollow Sphere Engineered Graphitic Carbon Nitride for Synergistic Photothermal/Chemodynamic Cancer Therapy. Crystals. 2023; 13(6):954. https://doi.org/10.3390/cryst13060954
Chicago/Turabian StyleMuthiah, Kayalvizhi Samuvel, Senthilkumar Thirumurugan, Yu-Chien Lin, Rajalakshmi Sakthivel, Udesh Dhawan, An-Ni Wang, Michael Hsiao, and Ren-Jei Chung. 2023. "Cerium Niobate Hollow Sphere Engineered Graphitic Carbon Nitride for Synergistic Photothermal/Chemodynamic Cancer Therapy" Crystals 13, no. 6: 954. https://doi.org/10.3390/cryst13060954
APA StyleMuthiah, K. S., Thirumurugan, S., Lin, Y.-C., Sakthivel, R., Dhawan, U., Wang, A.-N., Hsiao, M., & Chung, R.-J. (2023). Cerium Niobate Hollow Sphere Engineered Graphitic Carbon Nitride for Synergistic Photothermal/Chemodynamic Cancer Therapy. Crystals, 13(6), 954. https://doi.org/10.3390/cryst13060954






