Multifunctional Polymeric Nanocomposites for Sensing Applications—Design, Features, and Technical Advancements
Abstract
:1. Introduction
2. Design and Features of Multifunctional Nanocomposites Applied for Sensing
2.1. Nanocarbon-Reinforced Nanocomposites
2.2. Inorganic Nanoparticle-Reinforced Nanocomposites
3. Technical Applications of Multifunctional Nanocomposites towards Various Sensors
3.1. Strain-Sensing
3.2. Gas-Sensing
3.3. Bio-Sensing or Chemical-Sensing
4. Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Z.; Li, R.; Meng, F.; Zhang, J.; Zuo, K.; Han, E. Approaches to enhancing gas sensing properties: A review. Sensors 2019, 19, 1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Hussein, N.A.; Wong, Y.H.; Burhanudin, Z.A.; Hawari, H.F. Ternary Hybrid Materials for Highly Sensitive Acetone Sensing at Room Temperature. Crystals 2023, 13, 845. [Google Scholar] [CrossRef]
- Yu, W.; Chen, D.; Li, J.; Zhang, Z. TiO2-SnS2 Nanoheterostructures for High-Performance Humidity Sensor. Crystals 2023, 13, 482. [Google Scholar] [CrossRef]
- Lai, Q.-T.; Sun, Q.-J.; Tang, Z.; Tang, X.-G.; Zhao, X.-H. Conjugated Polymer-Based Nanocomposites for Pressure Sensors. Molecules 2023, 28, 1627. [Google Scholar] [CrossRef]
- Kausar, A. Epitome of Fullerene in Conducting Polymeric Nanocomposite—Fundamentals and Beyond. Polym. Plast. Technol. Mater. 2023, 62, 618–631. [Google Scholar] [CrossRef]
- Khan, I.; Khan, I.; Saeed, K.; Ali, N.; Zada, N.; Khan, A.; Ali, F.; Bilal, M.; Akhter, M.S. Polymer nanocomposites: An overview. In Smart Polymer Nanocompos; Ali, N., Bilal, M., Khan, A., Nguyen, T.A., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 167–184. [Google Scholar]
- Pezzuoli, D.; Angeli, E.; Repetto, D.; Ferrera, F.; Guida, P.; Firpo, G.; Repetto, L. Nanofluidic-Based Accumulation of Antigens for Miniaturized Immunoassay. Sensors 2020, 20, 1615. [Google Scholar] [CrossRef] [Green Version]
- Prosa, M.; Bolognesi, M.; Fornasari, L.; Grasso, G.; Lopez-Sanchez, L.; Marabelli, F.; Toffanin, S. Nanostructured Organic/Hybrid Materials and Components in Miniaturized Optical and Chemical Sensors. Nanomaterials 2020, 10, 480. [Google Scholar] [CrossRef] [Green Version]
- Long, H.; Turner, S.; Yan, A.; Xu, H.; Jang, M.; Carraro, C.; Maboudian, R.; Zettl, A. Plasma assisted formation of 3D highly porous nanostructured metal oxide network on microheater platform for Low power gas sensing. Sens. Actuators B Chem. 2019, 301, 127067. [Google Scholar] [CrossRef]
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Developments in the field of conducting and non-conducting polymer based potentiometric membrane sensors for ions over the past decade. Sensors 2008, 8, 2331–2412. [Google Scholar] [CrossRef] [Green Version]
- Seyedin, S.; Razal, J.M.; Innis, P.C.; Wallace, G.G. A facile approach to spinning multifunctional conductive elastomer fibres with nanocarbon fillers. Smart Mater. Struct. 2016, 25, 035015. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, S.; Peng, S.; Sha, Z.; Wang, C.H. Synergism of binary carbon nanofibres and graphene nanoplates in improving sensitivity and stability of stretchable strain sensors. Compos. Sci. Technol. 2019, 172, 7–16. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Ganguly, S. Introduction to polymer composite-based sensors. In Polymeric Nanocomposite Materials for Sensor Applications; Parameswaranpillai, J., Ganguly, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–21. [Google Scholar]
- Albar, M.M.J.; Jamion, N.A.; Baharin, S.N.A.; Raoov, M.; Sambasevam, K.P. Preparation of novel commercial polyaniline composites for ammonia detection. In Book Preparation of Novel Commercial Polyaniline Composites for Ammonia Detection; Trans Tech Publications: Zurich, Switzerland, 2020; pp. 124–131. [Google Scholar]
- Sankar, S.; Naik, A.A.; Anilkumar, T.; Ramesan, M. Characterization, conductivity studies, dielectric properties, gas sensing performance of in situ polymerized polyindole/copper alumina nanocomposites. J. Appl. Polym. Sci. 2020, 137, 49145. [Google Scholar] [CrossRef]
- Su, S.; Wu, W.; Gao, J.; Lu, J.; Fan, C. Nanomaterials-based sensors for applications in environmental monitoring. J. Mater. Chem. 2012, 22, 18101–18110. [Google Scholar] [CrossRef]
- Rane, A.V.; Kanny, K.; Abitha, V.; Thomas, S. Methods for synthesis of nanoparticles and fabrication of nanocomposites. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 121–139. [Google Scholar]
- Rohilla, D.; Chaudhary, S.; Umar, A. An overview of advanced nanomaterials for sensor applications. Eng. Sci. 2021, 16, 47–70. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef]
- Pang, J.; Peng, S.; Hou, C.; Zhao, H.; Fan, Y.; Ye, C.; Zhang, N.; Wang, T.; Cao, Y.; Zhou, W. Applications of Graphene in Five Senses, Nervous System, Artificial Muscles. ACS Sensors 2023, 8, 482–514. [Google Scholar] [CrossRef]
- Xiao, Z.; Kong, L.B.; Ruan, S.; Li, X.; Yu, S.; Li, X.; Jiang, Y.; Yao, Z.; Ye, S.; Wang, C. Recent development in nanocarbon materials for gas sensor applications. Sens. Actuators B Chem. 2018, 274, 235–267. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting polymer-based nanostructures for gas sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Kushwaha, C.S.; Singh, P.; Shukla, S.K.; Chehimi, M.M. Advances in conducting polymer nanocomposite based chemical sensors: An overview. Mater. Sci. Eng. B 2022, 284, 115856. [Google Scholar] [CrossRef]
- D’Amico, A.; Di Natale, C. A contribution on some basic definitions of sensors properties. IEEE Sens. J. 2001, 1, 183–190. [Google Scholar] [CrossRef]
- Pilan, L.; Raicopol, M. Highly selective and stable glucose biosensors based on polyaniline/carbon nanotubes composites. UPB Sci. Bull. Ser. B 2014, 76, 155–166. [Google Scholar]
- Yang, D.; Wang, J.; Cao, Y.; Tong, X.; Hua, T.; Qin, R.; Shao, Y. Polyaniline-based biological and chemical sensors: Sensing mechanism, configuration design, perspective. ACS Appl. Electron. Mater. 2023, 5, 593–611. [Google Scholar] [CrossRef]
- Aycan, D.; Karaca, F.; Alemdar, N. Development of hyaluronic acid-based electroconductive hydrogel as a sensitive non-enzymatic glucose sensor. Mater. Today Commun. 2023, 35, 105745. [Google Scholar] [CrossRef]
- Wu, G.; Du, H.; Lee, D.; Cha, Y.L.; Kim, W.; Zhang, X.; Kim, D.-J. Polyaniline/Graphene-Functionalized Flexible Waste Mask Sensors for Ammonia and Volatile Sulfur Compound Monitoring. ACS Appl. Mater. Interfaces 2022, 14, 56056–56064. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sensors Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Ruecha, N.; Rodthongkum, N.; Cate, D.M.; Volckens, J.; Chailapakul, O.; Henry, C.S. Sensitive electrochemical sensor using a graphene–polyaniline nanocomposite for simultaneous detection of Zn (II), Cd (II), Pb (II). Anal. Chim. Acta 2015, 874, 40–48. [Google Scholar] [CrossRef]
- Kooti, M.; Keshtkar, S.; Askarieh, M.; Rashidi, A. Progress toward a novel methane gas sensor based on SnO2 nanorods-nanoporous graphene hybrid. Sens. Actuators B Chem. 2019, 281, 96–106. [Google Scholar] [CrossRef]
- Biswas, M.R.U.D.; Oh, W.-C. Comparative study on gas sensing by a Schottky diode electrode prepared with graphene–semiconductor–polymer nanocomposites. RSC Adv. 2019, 9, 11484–11492. [Google Scholar] [CrossRef]
- Wang, M.; Yang, L.; Hu, B.; Liu, Y.; Song, Y.; He, L.; Zhang, Z.; Fang, S. A novel electrochemical sensor based on Cu3P@ NH2-MIL-125 (Ti) nanocomposite for efficient electrocatalytic oxidation and sensitive detection of hydrazine. Appl. Surf. Sci. 2018, 445, 123–132. [Google Scholar] [CrossRef]
- Chuiprasert, J.; Srinives, S.; Boontanon, N.; Polprasert, C.; Ramungul, N.; Lertthanaphol, N.; Karawek, A.; Boontanon, S.K. Electrochemical Sensor Based on a Composite of Reduced Graphene Oxide and Molecularly Imprinted Copolymer of Polyaniline–Poly (o-phenylenediamine) for Ciprofloxacin Determination: Fabrication, Characterization, Performance Evaluation. ACS Omega 2023, 8, 2564–2574. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.; Wu, X.; Lu, C. Self-stabilized polyaniline@ graphene aqueous colloids for the construction of assembled conductive network in rubber matrix and its chemical sensing application. Compos. Sci. Technol. 2016, 125, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, T.W. Carbon nanotubes. Annu. Rev. Mater. Sci. 1994, 24, 235–264. [Google Scholar] [CrossRef]
- Eletskii, A.V. Carbon nanotubes. Phys. Uspekhi 1997, 40, 899. [Google Scholar] [CrossRef]
- Kumar, M.K.; Reddy, A.L.M.; Ramaprabhu, S. Exfoliated single-walled carbon nanotube-based hydrogen sensor. Sens. Actuators B Chem. 2008, 130, 653–660. [Google Scholar] [CrossRef]
- Song, X.-Y.; Chen, J.; Shi, Y.-P. Different configurations of carbon nanotubes reinforced solid-phase microextraction techniques and their applications in the environmental analysis. TrAC Trends Anal. Chem. 2017, 86, 263–275. [Google Scholar] [CrossRef]
- Cheng, G.; Xu, H.; Gao, N.; Zhang, M.; Gao, H.; Sun, B.; Gu, M.; Yu, L.; Lin, Y.; Liu, X. Carbon nanotubes field-effect transistor pressure sensor based on three-dimensional conformal force-sensitive gate modulation. Carbon 2023, 204, 456–464. [Google Scholar] [CrossRef]
- Paul, R.; Zhai, Q.; Roy, A.K.; Dai, L. Charge transfer of carbon nanomaterials for efficient metal-free electrocatalysis. Interdiscip. Mater. 2022, 1, 28–50. [Google Scholar] [CrossRef]
- Vadalkar, S.; Chodvadiya, D.; Som, N.N.; Vyas, K.N.; Jha, P.K.; Chakraborty, B. An Ab-initio Study of the C18 nanocluster for Hazardous Gas Sensor Application. ChemistrySelect 2022, 7, e202103874. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Xiao, S.; Yang, C.; Zhou, J.; Xiao, B. Single Ni atom doped WS2 monolayer as sensing substrate for dissolved gases in transformer oil: A first-principles study. Appl. Surf. Sci. 2022, 579, 152141. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Xu, Q.; Ying, Q.; Gu, B.; Yuan, W. Wireless strain sensing using carbon nanotube composite film. Compos. Part B Eng. 2023, 256, 110650. [Google Scholar] [CrossRef]
- Ahmed, S.; Sinha, S.K. Studies on nanomaterial-based p-type semiconductor gas sensors. Environ. Sci. Pollut. Res. 2023, 30, 24975–24986. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Ali, E.; Darwish, M.S. Recent Developments of Conductive Polymers/Carbon Nanotubes Nanocomposites for Sensor Applications. Polym. Plast. Technol. Mater. 2022, 61, 1456–1480. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Xin, Z.; Luo, Y.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Poly (lactic acid)/carbon nanotube composites with enhanced electrical conductivity via a two-step dispersion strategy. Compos. Commun. 2022, 30, 101087. [Google Scholar] [CrossRef]
- Pinjari, S.; Bera, T.; Kapur, G.; Kjeang, E. The mechanism and sorption kinetic analysis of hydrogen storage at room temperature using acid functionalized carbon nanotubes. Int. J. Hydrog. Energy 2023, 48, 1930–1942. [Google Scholar] [CrossRef]
- Morawska, L.; Thai, P.K.; Liu, X.; Asumadu-Sakyi, A.; Ayoko, G.; Bartonova, A.; Bedini, A.; Chai, F.; Christensen, B.; Dunbabin, M. Applications of low-cost sensing technologies for air quality monitoring and exposure assessment: How far have they gone? Environ. Int. 2018, 116, 286–299. [Google Scholar] [CrossRef]
- Panasenko, I.V.; Bulavskiy, M.O.; Iurchenkova, A.A.; Aguilar-Martinez, Y.; Fedorov, F.S.; Fedorovskaya, E.O.; Mikladal, B.; Kallio, T.; Nasibulin, A.G. Flexible supercapacitors based on free-standing polyaniline/single-walled carbon nanotube films. J. Power Sources 2022, 541, 231691. [Google Scholar] [CrossRef]
- Srivastava, S.; Sharma, S.; Agrawal, S.; Kumar, S.; Singh, M.; Vijay, Y. Study of chemiresistor type CNT doped polyaniline gas sensor. Synth. Met. 2010, 160, 529–534. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Chen, C.-H.; Hwang, B.-J.; Luo, J.-D.; Chiou, C.-C.; Tian, Y.-C.; Lin, C.-Y.; Cheng, C.-H.; Lai, C.-S. Spin-coated Au-nanohole arrays engineered by nanosphere lithography for a Staphylococcus aureus 16S rRNA electrochemical sensor. Biosens. Bioelectron. 2016, 77, 1086–1094. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, F.; Cao, P.; Liu, J.; Li, D.; Wu, J.; Wang, N.; Su, Y.; Zhao, Y. Winding-locked carbon nanotubes/polymer nanofibers helical yarn for ultrastretchable conductor and strain sensor. ACS Nano 2020, 14, 3442–3450. [Google Scholar] [CrossRef]
- Anantha-Iyengar, G.; Shanmugasundaram, K.; Nallal, M.; Lee, K.-P.; Whitcombe, M.J.; Lakshmi, D.; Sai-Anand, G. Functionalized conjugated polymers for sensing and molecular imprinting applications. Prog. Polym. Sci. 2019, 88, 1–129. [Google Scholar] [CrossRef]
- Luo, H.; Kaneti, Y.V.; Ai, Y.; Wu, Y.; Wei, F.; Fu, J.; Cheng, J.; Jing, C.; Yuliarto, B.; Eguchi, M. Nanoarchitectured porous conducting polymers: From controlled synthesis to advanced applications. Adv. Mater. 2021, 33, 2007318. [Google Scholar] [CrossRef]
- Rawal, I. Facial synthesis of hexagonal metal oxide nanoparticles for low temperature ammonia gas sensing applications. RSC Adv. 2015, 5, 4135–4142. [Google Scholar] [CrossRef]
- Navarrete, E.; Bittencourt, C.; Umek, P.; Llobet, E. AACVD and gas sensing properties of nickel oxide nanoparticle decorated tungsten oxide nanowires. J. Mater. Chem. C 2018, 6, 5181–5192. [Google Scholar] [CrossRef]
- Verma, A.; Gupta, R.; Verma, A.S.; Kumar, T. A review of Composite Conducting Polymer-based Sensors for detection of industrial waste gases. Sens. Actuators Rep. 2023, 11, 100143. [Google Scholar] [CrossRef]
- Reddy, Y.V.M.; Shin, J.H.; Palakollu, V.N.; Sravani, B.; Choi, C.-H.; Park, K.; Kim, S.-K.; Madhavi, G.; Park, J.P.; Shetti, N.P. Strategies, advances, challenges associated with the use of graphene-based nanocomposites for electrochemical biosensors. Adv. Colloid Interface Sci. 2022, 304, 102664. [Google Scholar] [CrossRef]
- Mutlaq, S.; Albiss, B.; Al-Nabulsi, A.A.; Jaradat, Z.W.; Olaimat, A.N.; Khalifeh, M.S.; Osaili, T.; Ayyash, M.M.; Holley, R.A. Conductometric Immunosensor for Escherichia coli O157: H7 Detection Based on Polyaniline/Zinc Oxide (PANI/ZnO) Nanocomposite. Polymers 2021, 13, 3288. [Google Scholar] [CrossRef]
- Nasirian, S. Enhanced carbon dioxide sensing performance of polyaniline/tin dioxide nanocomposite by ultraviolet light illumination. Appl. Surf. Sci. 2020, 502, 144302. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Q.; Xie, G.; Su, Y.; Zhao, K.; Du, H.; Jiang, Y. Gas sensors based on polyaniline/zinc oxide hybrid film for ammonia detection at room temperature. Chem. Phys. Lett. 2016, 665, 147–152. [Google Scholar] [CrossRef]
- Jose, S.; Das, S.; Vakamalla, T.R.; Sen, D. Electrochemical Glucose Sensing Using Molecularly Imprinted Polyaniline–Copper Oxide Coated Electrode. Surf. Eng. Appl. Electrochem. 2022, 58, 260–268. [Google Scholar] [CrossRef]
- Liang, J.; Wei, M.; Wang, Q.; Zhao, Z.; Liu, A.; Yu, Z.; Tian, Y. Sensitive electrochemical determination of hydrogen peroxide using copper nanoparticles in a polyaniline film on a glassy carbon electrode. Anal. Lett. 2018, 51, 512–522. [Google Scholar] [CrossRef]
- Karim, M.; Lee, C.; Lee, M. Synthesis of conducting polypyrrole by radiolysis polymerization method. Polym. Adv. Technol. 2007, 18, 916–920. [Google Scholar] [CrossRef]
- Singh, P.; Kushwaha, C.S.; Singh, V.K.; Dubey, G.; Shukla, S.K. Chemiresistive sensing of volatile ammonia over zinc oxide encapsulated polypyrrole based nanocomposite. Sens. Actuators B Chem. 2021, 342, 130042. [Google Scholar] [CrossRef]
- Bano, S.; Ahmad, N.; Sultana, S.; Sabir, S.; Khan, M.Z. Preparation and study of ternary polypyrrole-tin oxide-chitin nanocomposites and their potential applications in visible light photocatalysis and sensors. J. Environ. Chem. Eng. 2019, 7, 103012. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Xu, L.-H.; Wang, J.-M.; Wu, T.-M. Fabrication of polypyrrole/tin oxide/graphene nanoribbon ternary nanocomposite and its high-performance ammonia gas sensing at room temperature. Mater. Sci. Eng. B 2021, 272, 115317. [Google Scholar] [CrossRef]
- Sankar, S.; Parvathi, K.; Ramesan, M. Structural characterization, electrical properties and gas sensing applications of polypyrrole/Cu-Al2O3 hybrid nanocomposites. High Perform. Polym. 2020, 32, 0954008319899157. [Google Scholar] [CrossRef]
- Praveena, P.; Ann, M.S.; Dhanavel, S.; Kalpana, D.; Maiyalagan, T.; Narayanan, V.; Stephen, A. Camphor sulphonic acid doped novel polycarbazole-gC3N4 as an efficient electrode material for supercapacitor. J. Mater. Sci. Mater. Electron. 2019, 30, 8736–8750. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Hu, T.; Yu, X.; Wang, J.; Jia, C. Fabrication of Mn/O co-doped g-C3N4: Excellent charge separation and transfer for enhancing photocatalytic activity under visible light irradiation. Dyes Pigments 2020, 175, 108107. [Google Scholar] [CrossRef]
- Ramesan, M.; Santhi, V. In situ synthesis, characterization, conductivity studies of polypyrrole/silver doped zinc oxide nanocomposites and their application for ammonia gas sensing. J. Mater. Sci. Mater. Electron. 2017, 28, 18804–18814. [Google Scholar] [CrossRef]
- Ma, Y.; Hou, C.; Zhang, H.; Zhang, Q.; Liu, H.; Wu, S.; Guo, Z. Three-dimensional core-shell Fe3O4/Polyaniline coaxial heterogeneous nanonets: Preparation and high performance supercapacitor electrodes. Electrochim. Acta 2019, 315, 114–123. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, M.; Yin, X.; Shao, Q.; Lu, N.; Feng, Y.; Lu, Y.; Wujcik, E.K.; Mai, X.; Wang, C. Tuning polyaniline nanostructures via end group substitutions and their morphology dependent electrochemical performances. Polymer 2018, 156, 128–135. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M. Conductive carboxylated styrene butadiene rubber composites by incorporation of polypyrrole-wrapped halloysite nanotubes. Compos. Sci. Technol. 2017, 143, 56–66. [Google Scholar] [CrossRef]
- Chen, M.; Duan, S.; Zhang, L.; Wang, Z.; Li, C. Three-dimensional porous stretchable and conductive polymer composites based on graphene networks grown by chemical vapour deposition and PEDOT: PSS coating. Chem. Commun. 2015, 51, 3169–3172. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhang, Z.; Wang, X.; Tang, J.; Liu, H.; Shao, Q.; Ding, T.; Umar, A.; Guo, Z. Solvent-free graphene liquids: Promising candidates for lubricants without the base oil. J. Colloid Interface Sci. 2019, 542, 159–167. [Google Scholar] [CrossRef]
- Li, S.; Yang, P.; Liu, X.; Zhang, J.; Xie, W.; Wang, C.; Liu, C.; Guo, Z. Graphene oxide based dopamine mussel-like cross-linked polyethylene imine nanocomposite coating with enhanced hexavalent uranium adsorption. J. Mater. Chem. A 2019, 7, 16902–16911. [Google Scholar] [CrossRef]
- Guo, Y.; Ruan, K.; Yang, X.; Ma, T.; Kong, J.; Wu, N.; Zhang, J.; Gu, J.; Guo, Z. Constructing fully carbon-based fillers with a hierarchical structure to fabricate highly thermally conductive polyimide nanocomposites. J. Mater. Chem. C 2019, 7, 7035–7044. [Google Scholar] [CrossRef]
- Chen, J.-N.; Zhao, S.-P.; Hu, W.-H.; Zhang, J.; Dong, M.-Y.; Liu, H.; Huang, Z.; Liu, Y.-Q.; Wang, Z.; Guo, Z. Vinyl ester resin nanocomposites reinforced with carbon nanotubes modified basalt fibers. Sci. Adv. Mater. 2019, 11, 1340–1347. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Pan, Y.; Schubert, D.W.; Liu, C. Shear-induced rheological and electrical properties of molten poly (methyl methacrylate)/carbon black nanocomposites. Compos. Part B Eng. 2019, 164, 37–44. [Google Scholar] [CrossRef]
- Zhan, P.; Zhai, W.; Wang, N.; Wei, X.; Zheng, G.; Dai, K.; Liu, C.; Shen, C. Electrically conductive carbon black/electrospun polyamide 6/poly (vinyl alcohol) composite based strain sensor with ultrahigh sensitivity and favorable repeatability. Mater. Lett. 2019, 236, 60–63. [Google Scholar] [CrossRef]
- Gu, H.; Xu, X.; Dong, M.; Xie, P.; Shao, Q.; Fan, R.; Liu, C.; Wu, S.; Wei, R.; Guo, Z. Carbon nanospheres induced high negative permittivity in nanosilver-polydopamine metacomposites. Carbon 2019, 147, 550–558. [Google Scholar] [CrossRef]
- Sun, H.; Yang, Z.; Pu, Y.; Dou, W.; Wang, C.; Wang, W.; Hao, X.; Chen, S.; Shao, Q.; Dong, M. Zinc oxide/vanadium pentoxide heterostructures with enhanced day-night antibacterial activities. J. Colloid Interface Sci. 2019, 547, 40–49. [Google Scholar] [CrossRef]
- Huang, J.C. Carbon black filled conducting polymers and polymer blends. Adv. Polym. Technol. J. Polym. Process. Inst. 2002, 21, 299–313. [Google Scholar] [CrossRef]
- Ke, K.; Pötschke, P.; Jehnichen, D.; Fischer, D.; Voit, B. Achieving β-phase poly (vinylidene fluoride) from melt cooling: Effect of surface functionalized carbon nanotubes. Polymer 2014, 55, 611–619. [Google Scholar] [CrossRef]
- Coniglio, A. Cluster structure near the percolation threshold. J. Phys. A Math. Gen. 1982, 15, 3829. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.K. Correlations between percolation threshold, dispersion state, aspect ratio of carbon nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Wu, N.; Xu, D.; Wang, Z.; Wang, F.; Liu, J.; Liu, W.; Shao, Q.; Liu, H.; Gao, Q.; Guo, Z. Achieving superior electromagnetic wave absorbers through the novel metal-organic frameworks derived magnetic porous carbon nanorods. Carbon 2019, 145, 433–444. [Google Scholar] [CrossRef]
- Shi, X.; Wang, C.; Ma, Y.; Liu, H.; Wu, S.; Shao, Q.; He, Z.; Guo, L.; Ding, T.; Guo, Z. Template-free microwave-assisted synthesis of FeTi coordination complex yolk-shell microspheres for superior catalytic removal of arsenic and chemical degradation of methylene blue from polluted water. Powder Technol. 2019, 356, 726–734. [Google Scholar] [CrossRef]
- Ke, K.; Pötschke, P.; Wiegand, N.; Krause, B.; Voit, B. Tuning the network structure in poly (vinylidene fluoride)/carbon nanotube nanocomposites using carbon black: Toward improvements of conductivity and piezoresistive sensitivity. ACS Appl. Mater. Interfaces 2016, 8, 14190–14199. [Google Scholar] [CrossRef]
- Deng, H.; Lin, L.; Ji, M.; Zhang, S.; Yang, M.; Fu, Q. Progress on the morphological control of conductive network in conductive polymer composites and the use as electroactive multifunctional materials. Prog. Polym. Sci. 2014, 39, 627–655. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, Y. Tune the phase morphology to design conductive polymer composites: A review. Polym. Compos. 2018, 39, 2985–2996. [Google Scholar] [CrossRef]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly stretchable and sensitive strain sensor based on silver nanowire–elastomer nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Zhang, S.; Zhang, D.; Liu, X.; He, Y.; Mi, L.; Zhang, J.; Liu, C.; Shen, C. Superhydrophobic electrically conductive paper for ultrasensitive strain sensor with excellent anticorrosion and self-cleaning property. ACS Appl. Mater. Interfaces 2019, 11, 21904–21914. [Google Scholar] [CrossRef]
- Wang, J.; Lu, C.; Zhang, K. Textile-based strain sensor for human motion detection. Energy Environ. Mater. 2020, 3, 80–100. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-Q.; Zhu, W.-B.; Yu, X.-G.; Huang, P.; Fu, S.-Y.; Hu, N.; Liao, K. Multifunctional wearable device based on flexible and conductive carbon sponge/polydimethylsiloxane composite. ACS Appl. Mater. Interfaces 2016, 8, 33189–33196. [Google Scholar] [CrossRef]
- Yu, G.; Hu, J.; Tan, J.; Gao, Y.; Lu, Y.; Xuan, F. A wearable pressure sensor based on ultra-violet/ozone microstructured carbon nanotube/polydimethylsiloxane arrays for electronic skins. Nanotechnology 2018, 29, 115502. [Google Scholar] [CrossRef]
- Shanks, R.A. General purpose elastomers: Structure, chemistry, physics and performance. Adv. Elastom. I 2013, 11, 11–45. [Google Scholar]
- Ding, B.; Zhang, Y.; Wang, J.; Mei, S.; Chen, X.; Li, S.; Zhao, W.; Zhang, X.; Shi, G.; He, Y. Selective laser sintering 3D-Printed conductive thermoplastic polyether-block-amide elastomer/carbon nanotube composites for strain sensing system and electro-induced shape memory. Compos. Commun. 2022, 35, 101280. [Google Scholar] [CrossRef]
- Li, K.; Clarkson, C.M.; Wang, L.; Liu, Y.; Lamm, M.; Pang, Z.; Zhou, Y.; Qian, J.; Tajvidi, M.; Gardner, D.J. Alignment of cellulose nanofibers: Harnessing nanoscale properties to macroscale benefits. ACS Nano 2021, 15, 3646–3673. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, M.; Grunin, L.; Morris, R.H.; Imamutdinova, A. Nanocellulose-Based Thermoplastic Polyurethane Biocomposites with Shape Memory Effect. J. Compos. Sci. 2023, 7, 168. [Google Scholar] [CrossRef]
- Seto, C.; Chang, B.P.; Tzoganakis, C.; Mekonnen, T.H. Lignin derived nano-biocarbon and its deposition on polyurethane foam for wastewater dye adsorption. Int. J. Biol. Macromol. 2021, 185, 629–643. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Chang, W.C.; Yen, C.T. Novel flexible nerve conduits made of water-based biodegradable polyurethane for peripheral nerve regeneration. J. Biomed. Mater. Res. Part A 2017, 105, 1383–1392. [Google Scholar] [CrossRef]
- Ma, L.; Yang, W.; Wang, Y.; Chen, H.; Xing, Y.; Wang, J. Multi-dimensional strain sensor based on carbon nanotube film with aligned conductive networks. Compos. Sci. Technol. 2018, 165, 190–197. [Google Scholar] [CrossRef]
- Liu, L.; Niu, S.; Zhang, J.; Mu, Z.; Li, J.; Li, B.; Meng, X.; Zhang, C.; Wang, Y.; Hou, T. Bioinspired, omnidirectional, hypersensitive flexible strain sensors. Adv. Mater. 2022, 34, 2200823. [Google Scholar] [CrossRef]
- Wu, G.; Gu, Y.; Hou, X.; Li, R.; Ke, H.; Xiao, X. Hybrid nanocomposites of cellulose/carbon-nanotubes/polyurethane with rapidly water sensitive shape memory effect and strain sensing performance. Polymers 2019, 11, 1586. [Google Scholar] [CrossRef] [Green Version]
- Ehsani, M.; Rahimi, P.; Joseph, Y. Structure–Function Relationships of Nanocarbon/Polymer Composites for Chemiresistive Sensing: A Review. Sensors 2021, 21, 3291. [Google Scholar] [CrossRef]
- Cruz-Leal, M.; Ávila-Gutiérrez, M.; Coutino-Gonzalez, E. Surface stabilization and functionalities of nanostructures. In Nanochemistry; CRC Press: Boca Raton, FL, USA, 2023; pp. 16–29. [Google Scholar]
- Dharmalingam, G.; Sivasubramaniam, R.; Parthiban, S. Quantification of ethanol by metal-oxide-based resistive sensors: A review. J. Electron. Mater. 2020, 49, 3009–3024. [Google Scholar] [CrossRef]
- Ellmer, K.; Mientus, R.; Seeger, S. Metallic oxides (ITO, ZnO, SnO2, TiO2): Transparent conductive materials. In Materials, Synthesis, Characterization, Applications; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 31–80. [Google Scholar]
- Persaud, K. Cross-reactive sensors (or e-noses). In Volatile Biomarkers for Human Health: From Nature to Artificial Senses; Royal Society of Chemistry: London, UK, 2022; p. 364. [Google Scholar]
- Mane, A.; Navale, S.; Sen, S.; Aswal, D.; Gupta, S.; Patil, V. Nitrogen dioxide (NO2) sensing performance of p-polypyrrole/n-tungsten oxide hybrid nanocomposites at room temperature. Org. Electron. 2015, 16, 195–204. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kumar, V.; Bonyani, M.; Majhi, S.M.; Bang, J.H.; Kim, J.-Y.; Kim, H.W.; Kim, S.S.; Kim, K.-H. Conducting polymer nanofibers based sensors for organic and inorganic gaseous compounds. Asian J. Atmos. Environ. 2020, 14, 85–104. [Google Scholar] [CrossRef]
- Nellaiappan, S.; Shalini Devi, K.; Selvaraj, S.; Krishnan, U.M.; Yakhmi, J.V. Chemical, gas and optical sensors based on conducting polymers. In Adv. Hybrid Conduct. Polymer Technol; Shahabuddin, S., Pandey, A.K., Khalid, M., Jagadish, P., Eds.; Springer: Cham, Switzerland, 2021; pp. 159–200. [Google Scholar]
- Karmakar, N.; Jain, S.; Fernandes, R.; Shah, A.; Patil, U.; Shimpi, N.; Kothari, D. Enhanced Sensing Performance of an Ammonia Gas Sensor Based on Ag-Decorated ZnO Nanorods/Polyaniline Nanocomposite. ChemistrySelect 2023, 8, e202204284. [Google Scholar] [CrossRef]
- Miah, M.R.; Yang, M.; Khandaker, S.; Bashar, M.M.; Alsukaibi, A.K.D.; Hassan, H.M.; Znad, H.; Awual, M.R. Polypyrrole-based sensors for volatile organic compounds (VOCs) sensing and capturing: A comprehensive review. Sens. Actuators A Phys. 2022, 347, 113933. [Google Scholar] [CrossRef]
- Vijeth, H.; Ashokkumar, S.; Yesappa, L.; Vandana, M.; Devendrappa, H. Camphor sulfonic acid surfactant assisted polythiophene nanocomposite for efficient electrochemical hydrazine sensor. Mater. Res. Express 2020, 6, 125375. [Google Scholar]
- Kulkarni, S.; Patil, P.; Mujumdar, A.; Naik, J. Synthesis and evaluation of gas sensing properties of PANI, PANI/SnO2 and PANI/SnO2/rGO nanocomposites at room temperature. Inorg. Chem. Commun. 2018, 96, 90–96. [Google Scholar] [CrossRef]
- Su, P.-G.; Lu, P.-H. Electrical and Humidity-Sensing Properties of Impedance-Type Humidity Sensors that Were Made of Ag Microwires/PPy/SnO2 Ternary Composites. Chemosensors 2020, 8, 92. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Harussani, M.; Demon, S.; Halim, N.; Mohamad, I.; Bahruji, H.; Abdullah, N. Research Progress On Polythiophene And Its Application As Chemical Sensor. Zulfaqar J. Def. Sci. Eng. Technol. 2022, 5, 48–68. [Google Scholar]
- Farea, M.A.; Mohammed, H.Y.; Shirsat, S.M.; Ali, Z.M.; Tsai, M.-L.; Yahia, I.; Zahran, H.; Shirsat, M.D. Impact of reduced graphene oxide on the sensing performance of Poly (3, 4–ethylenedioxythiophene) towards highly sensitive and selective CO sensor: A comprehensive study. Synth. Met. 2022, 291, 117166. [Google Scholar] [CrossRef]
- Deshpande, N.; Gudage, Y.; Sharma, R.; Vyas, J.; Kim, J.; Lee, Y. Studies on tin oxide-intercalated polyaniline nanocomposite for ammonia gas sensing applications. Sens. Actuators B Chem. 2009, 138, 76–84. [Google Scholar] [CrossRef]
- Eising, M.; Cava, C.E.; Salvatierra, R.V.; Zarbin, A.J.G.; Roman, L.S. Doping effect on self-assembled films of polyaniline and carbon nanotube applied as ammonia gas sensor. Sens. Actuators B Chem. 2017, 245, 25–33. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J. Carbon nanofiber/polypyrrole nanocable as toxic gas sensor. Sens. Actuators B Chem. 2007, 122, 7–13. [Google Scholar] [CrossRef]
- Van Hieu, N.; Dung, N.Q.; Tam, P.D.; Trung, T.; Chien, N.D. Thin film polypyrrole/SWCNTs nanocomposites-based NH3 sensor operated at room temperature. Sens. Actuators B Chem. 2009, 140, 500–507. [Google Scholar] [CrossRef]
- Qiu, J.D.; Shi, L.; Liang, R.P.; Wang, G.C.; Xia, X.H. Controllable deposition of a platinum nanoparticle ensemble on a polyaniline/graphene hybrid as a novel electrode material for electrochemical sensing. Chem. A Eur. J. 2012, 18, 7950–7959. [Google Scholar] [CrossRef]
- Konwer, S.; Guha, A.K.; Dolui, S.K. Graphene oxide-filled conducting polyaniline composites as methanol-sensing materials. J. Mater. Sci. 2013, 48, 1729–1739. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Room temperature methane sensor based on graphene nanosheets/polyaniline nanocomposite thin film. IEEE Sens. J. 2012, 13, 777–782. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Qiu, R.; Shi, L.; Wu, W.; Zhou, S. Responsive fluorescent Bi2O3@ PVA hybrid nanogels for temperature-sensing, dual-modal imaging, drug delivery. Biomaterials 2012, 33, 3058–3069. [Google Scholar] [CrossRef]
- Joulazadeh, M.; Navarchian, A.H. Effects of Addition of ZnO and SnO2 Nanofillers on the Performance of Polyaniline-Based Ammonia Sensors; Scientific Information Database: Tehran, Iran, 2014. [Google Scholar]
- Rahman, M.M.; Khan, A.; Marwani, H.M.; Asiri, A.M. Hydrazine sensor based on silver nanoparticle-decorated polyaniline tungstophosphate nanocomposite for use in environmental remediation. Microchim. Acta 2016, 183, 1787–1796. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, W.; Strout, T.; Schempf, A.; Zhang, H.; Li, B.; Cui, J.; Lei, Y. Ammonia Gas Sensor Using Polypyrrole-Coated TiO2/ZnO Nanofibers. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2009, 21, 1432–1438. [Google Scholar] [CrossRef]
- Didier, C.M.; Orrico, J.F.; Cepeda Torres, O.S.; Castro, J.M.; Baksh, A.; Rajaraman, S. Microfabricated polymer-metal biosensors for multifarious data collection from electrogenic cellular models. Microsyst. Nanoeng. 2023, 9, 22. [Google Scholar] [CrossRef]
- Dakshayini, B.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in ampereometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.-Y.; Choi, J.-W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kaur, A.; Kaur, H. Review on nanomaterials/conducting polymer based nanocomposites for the development of biosensors and electrochemical sensors. Polym. Plast. Technol. Mater. 2021, 60, 504–521. [Google Scholar] [CrossRef]
- Segets, D.; Martinez Tomalino, L.; Gradl, J.; Peukert, W. Real-time monitoring of the nucleation and growth of ZnO nanoparticles using an optical hyper-Rayleigh scattering method. J. Phys. Chem. C 2009, 113, 11995–12001. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Q.; Feng, Q. Catalytic hairpin assembly-triggered DNA walker for electrochemical sensing of tumor exosomes sensitized with Ag@ C core-shell nanocomposites. Anal. Chim. Acta 2020, 1135, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, Y.; Wang, Y.; Zhao, Y.; Su, H. Detecting Pb2+ by a ‘turn-on’ fluorescence sensor based on DNA functionalized magnetic nanocomposites. Nanotechnology 2021, 33, 075603. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Shen, J.; Qi, W.; Wang, H. Design of organic/inorganic nanocomposites for ultrasensitive electrochemical detection of a cancer biomarker protein. Talanta 2020, 212, 120794. [Google Scholar] [CrossRef]
- Verma, M.S.; Rogowski, J.L.; Jones, L.; Gu, F.X. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol. Adv. 2015, 33, 666–680. [Google Scholar] [CrossRef]
- Hu, L.; Fu, X.; Kong, G.; Yin, Y.; Meng, H.-M.; Ke, G.; Zhang, X.-B. DNAzyme–gold nanoparticle-based probes for biosensing and bioimaging. J. Mater. Chem. B 2020, 8, 9449–9465. [Google Scholar] [CrossRef]
- Han, Y.; Qiu, Z.; Nawale, G.N.; Varghese, O.P.; Hilborn, J.; Tian, B.; Leifer, K. MicroRNA detection based on duplex-specific nuclease-assisted target recycling and gold nanoparticle/graphene oxide nanocomposite-mediated electrocatalytic amplification. Biosens. Bioelectron. 2019, 127, 188–193. [Google Scholar] [CrossRef]
- Lu, W.; Arumugam, S.R.; Senapati, D.; Singh, A.K.; Arbneshi, T.; Khan, S.A.; Yu, H.; Ray, P.C. Multifunctional oval-shaped gold-nanoparticle-based selective detection of breast cancer cells using simple colorimetric and highly sensitive two-photon scattering assay. ACS Nano 2010, 4, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Lacerda, S.H.D.P.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef] [PubMed]
- Dasary, S.S.; Singh, A.K.; Senapati, D.; Yu, H.; Ray, P.C. Gold nanoparticle based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene. J. Am. Chem. Soc. 2009, 131, 13806–13812. [Google Scholar] [CrossRef]
- Li, C.; Adamcik, J.; Mezzenga, R. Biodegradable nanocomposites of amyloid fibrils and graphene with shape-memory and enzyme-sensing properties. Nat. Nanotechnol. 2012, 7, 421–427. [Google Scholar] [CrossRef]
- Tanguy, N.R.; Wiltshire, B.; Arjmand, M.; Zarifi, M.H.; Yan, N. Highly sensitive and contactless ammonia detection based on nanocomposites of phosphate-functionalized reduced graphene oxide/polyaniline immobilized on microstrip resonators. ACS Appl. Mater. Interfaces 2020, 12, 9746–9754. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, Applications. Biosensors 2022, 13, 40. [Google Scholar] [CrossRef]
- Jose, S.; Thadathil, D.A.; Ghosh, M.; Varghese, A. Enzyme immobilized conducting polymer-based biosensor for the electrochemical determination of the eco-toxic pollutant p-nonylphenol. Electrochim. Acta 2023, 460, 142591. [Google Scholar] [CrossRef]
- Hamimed, S.; Mahjoubi, Y.; Abdeljelil, N.; Gamraoui, A.; Othmani, A.; Barhoum, A.; Chatti, A. Chemical sensors and biosensors for soil analysis: Principles, challenges, emerging applications. In Advanced Sensor Technology Biomedical, Environmental, and Construction Applications; Barhoum, A., Altintas, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 669–698. [Google Scholar]
- Wang, X.-D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2015–2019). Anal. Chem. 2019, 92, 397–430. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Ganguly, S. (Eds.) Polymeric Nanocomposite Materials for Sensor Applications; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Wang, X.; Li, Q.; Tao, X. Sensing mechanism of a carbon nanocomposite-printed fabric as a strain sensor. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106350. [Google Scholar] [CrossRef]
- Kyeong, D.; Kim, M.; Kwak, M. Thermally triggered multilevel diffractive optical elements tailored by shape-memory polymers for temperature history sensors. ACS Appl. Mater. Interfaces 2023, 15, 9813–9819. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Shi, H.; Chen, X.; Liu, G.; Meng, S.; Lu, J. Lightweight and geometrically complex ceramics derived from 4D printed shape memory precursor with reconfigurability and programmability for sensing and actuation applications. Chem. Eng. J. 2023, 455, 140655. [Google Scholar] [CrossRef]
- Moussa, M.; El-Kady, M.F.; Zhao, Z.; Majewski, P.; Ma, J. Recent progress and performance evaluation for polyaniline/graphene nanocomposites as supercapacitor electrodes. Nanotechnology 2016, 27, 442001. [Google Scholar] [CrossRef] [PubMed]
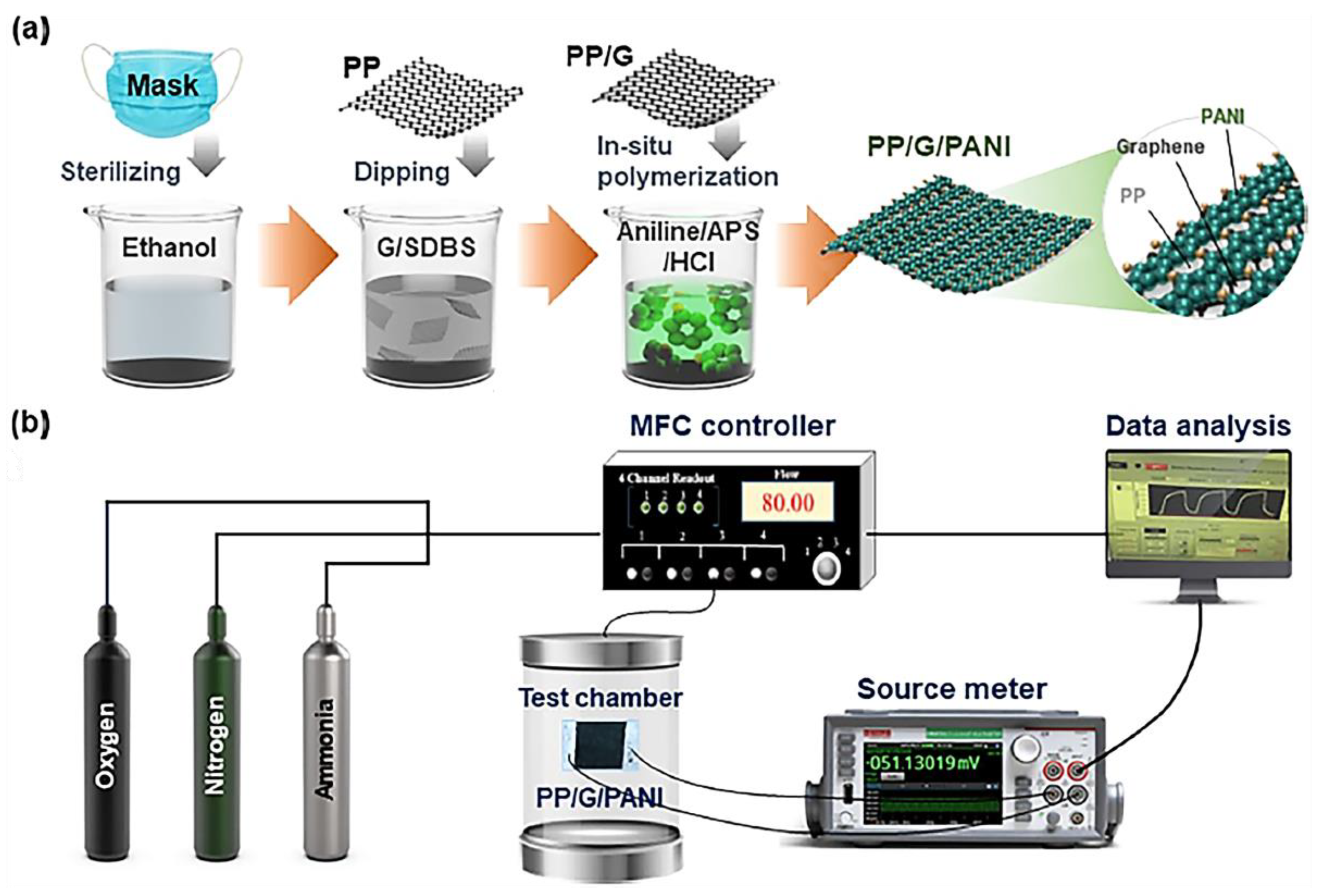
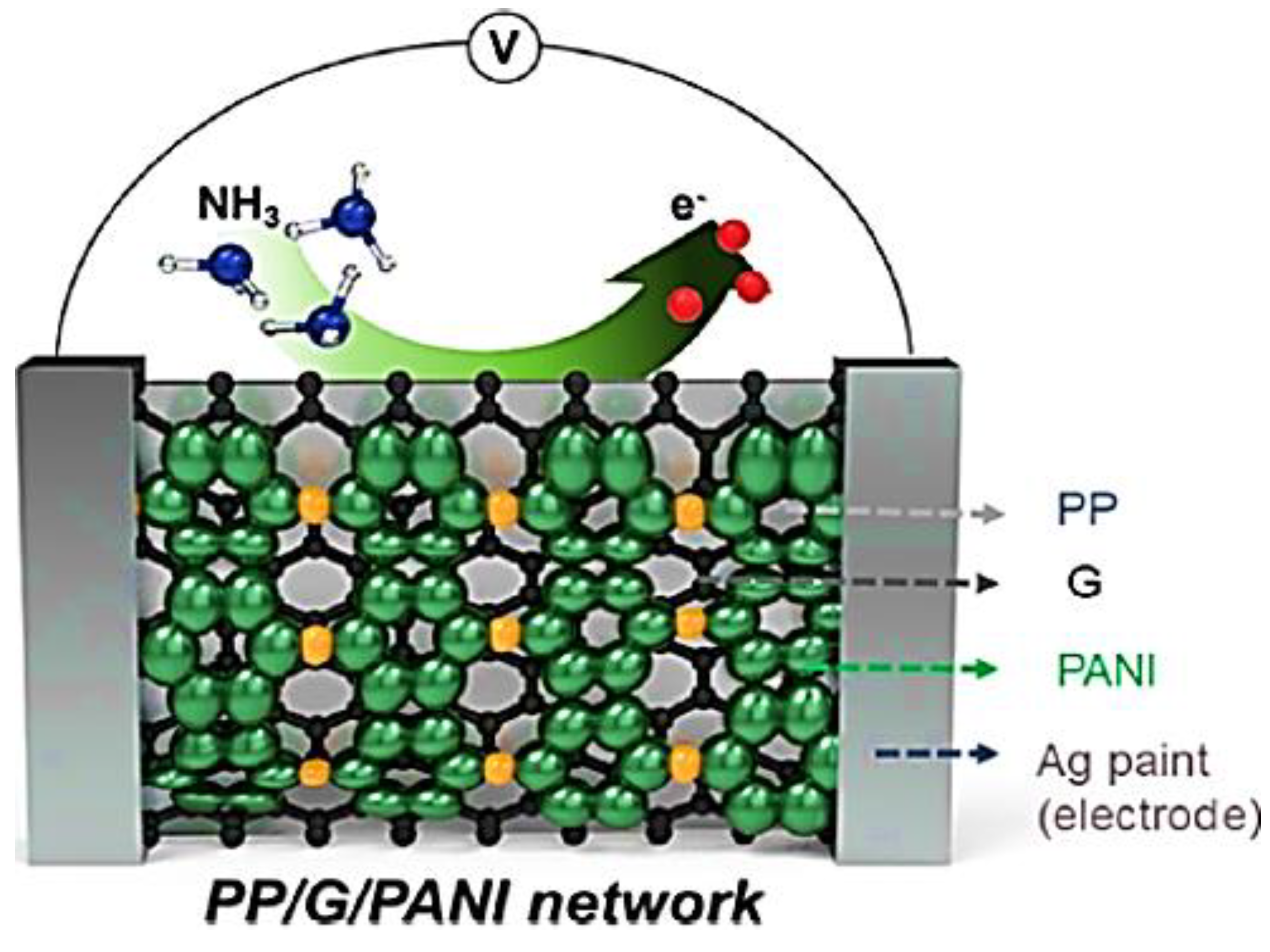
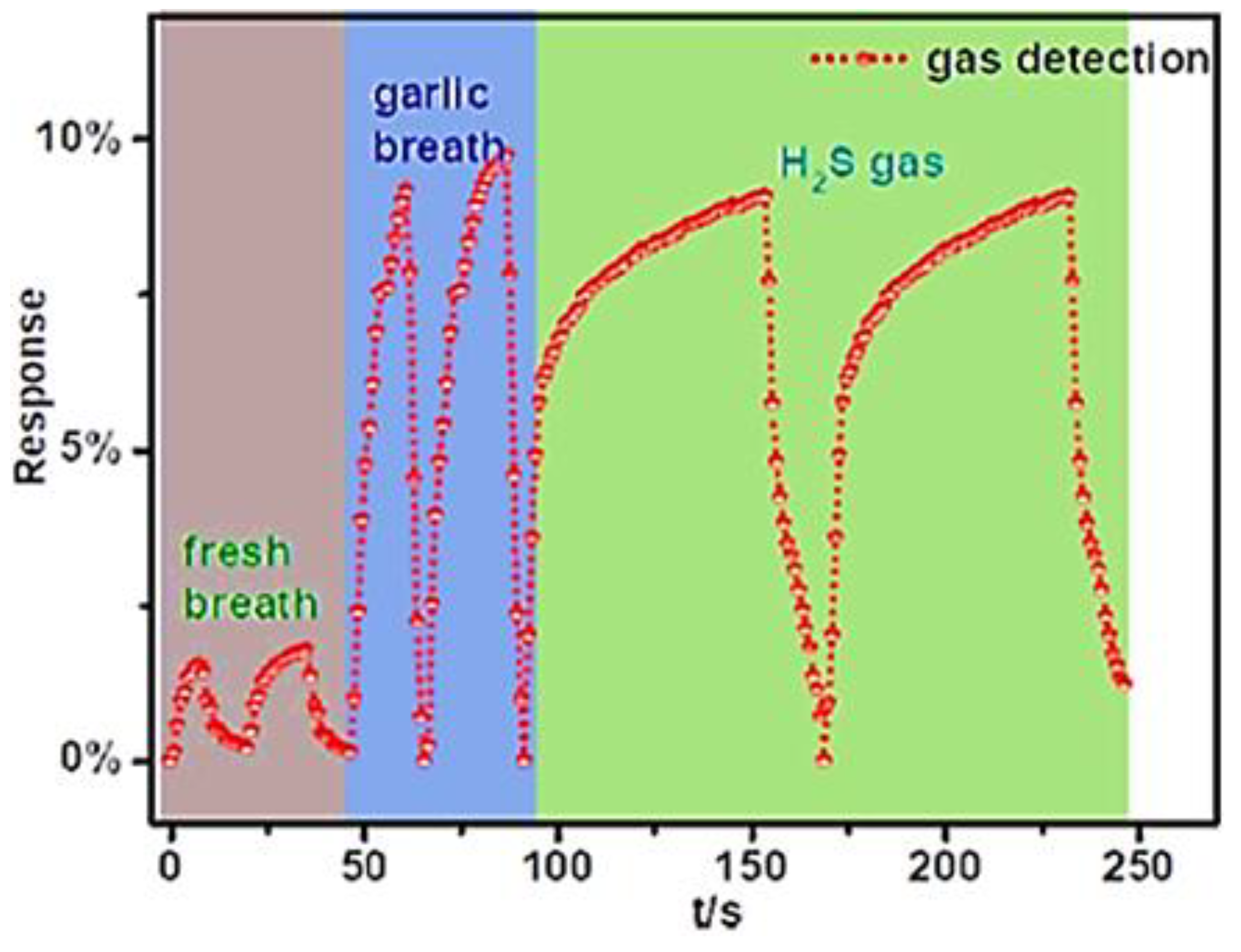
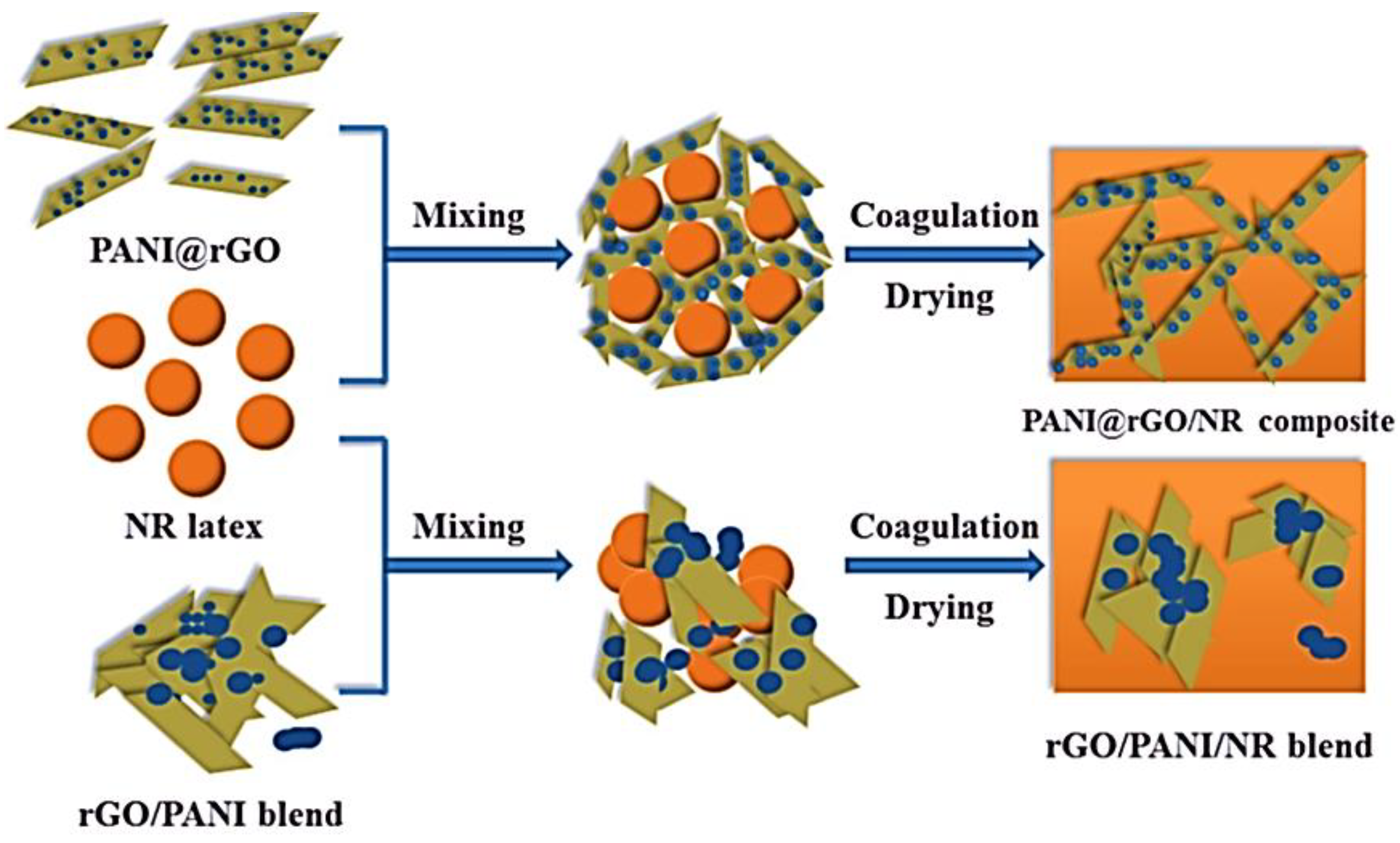
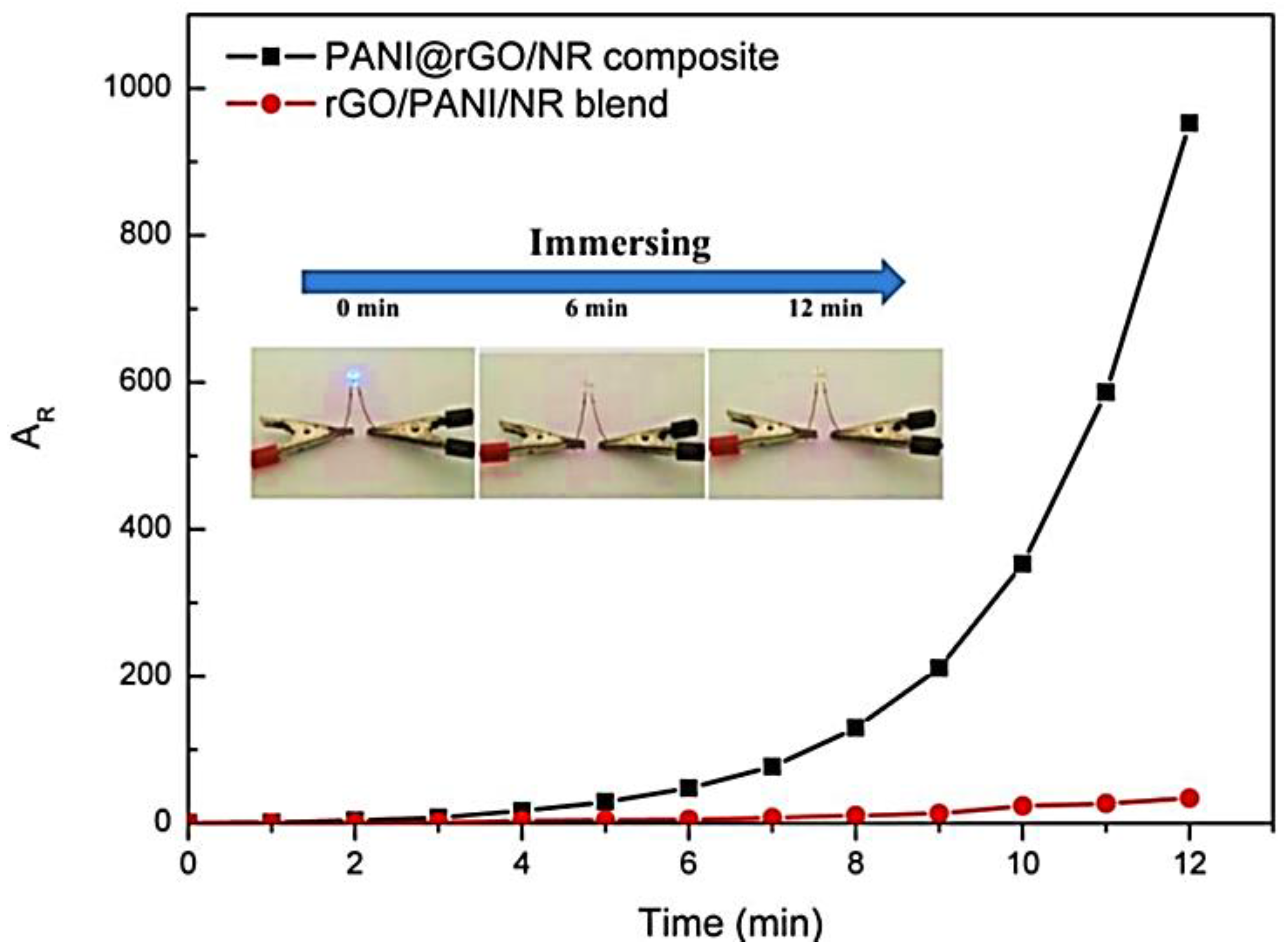
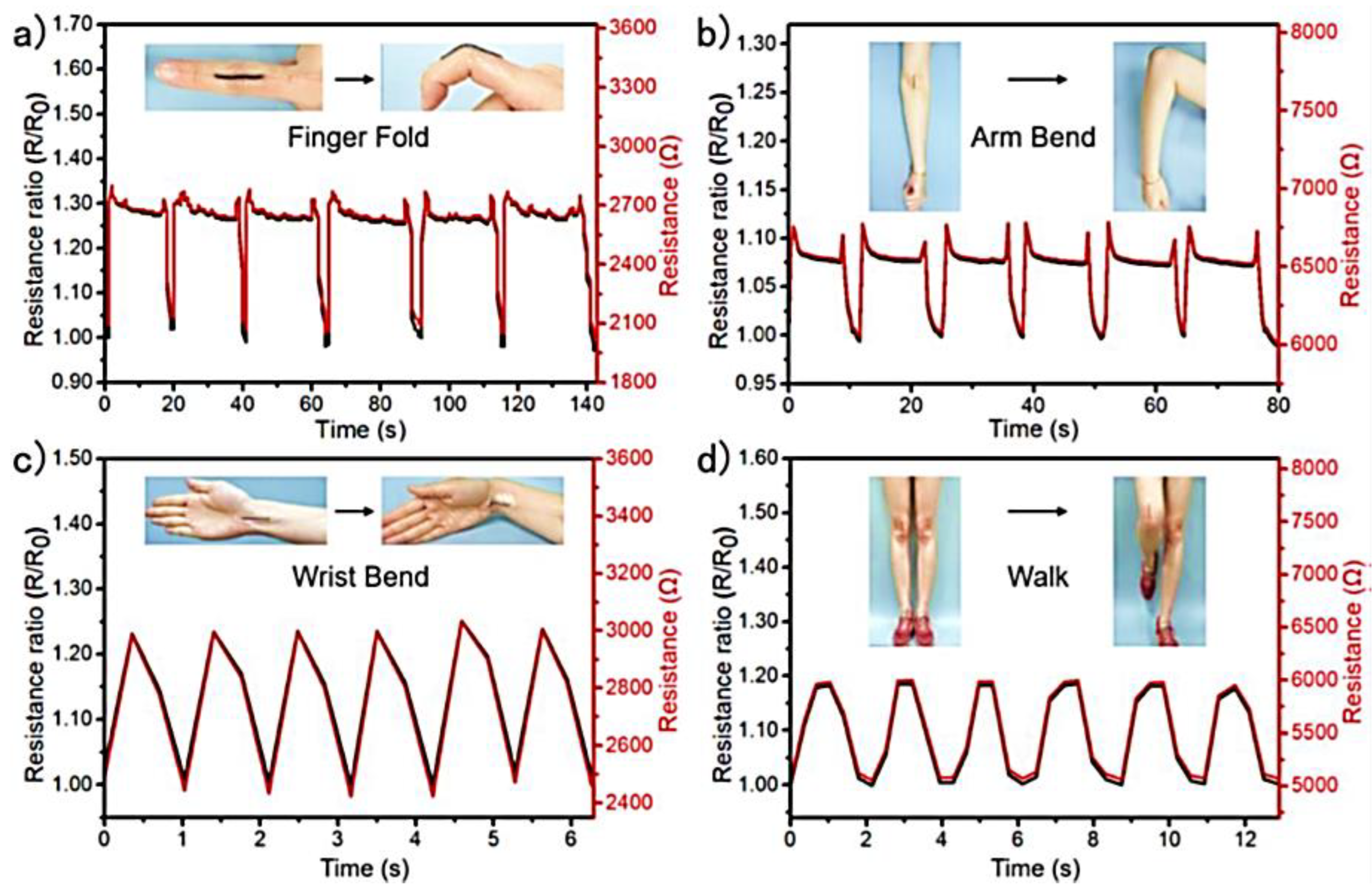
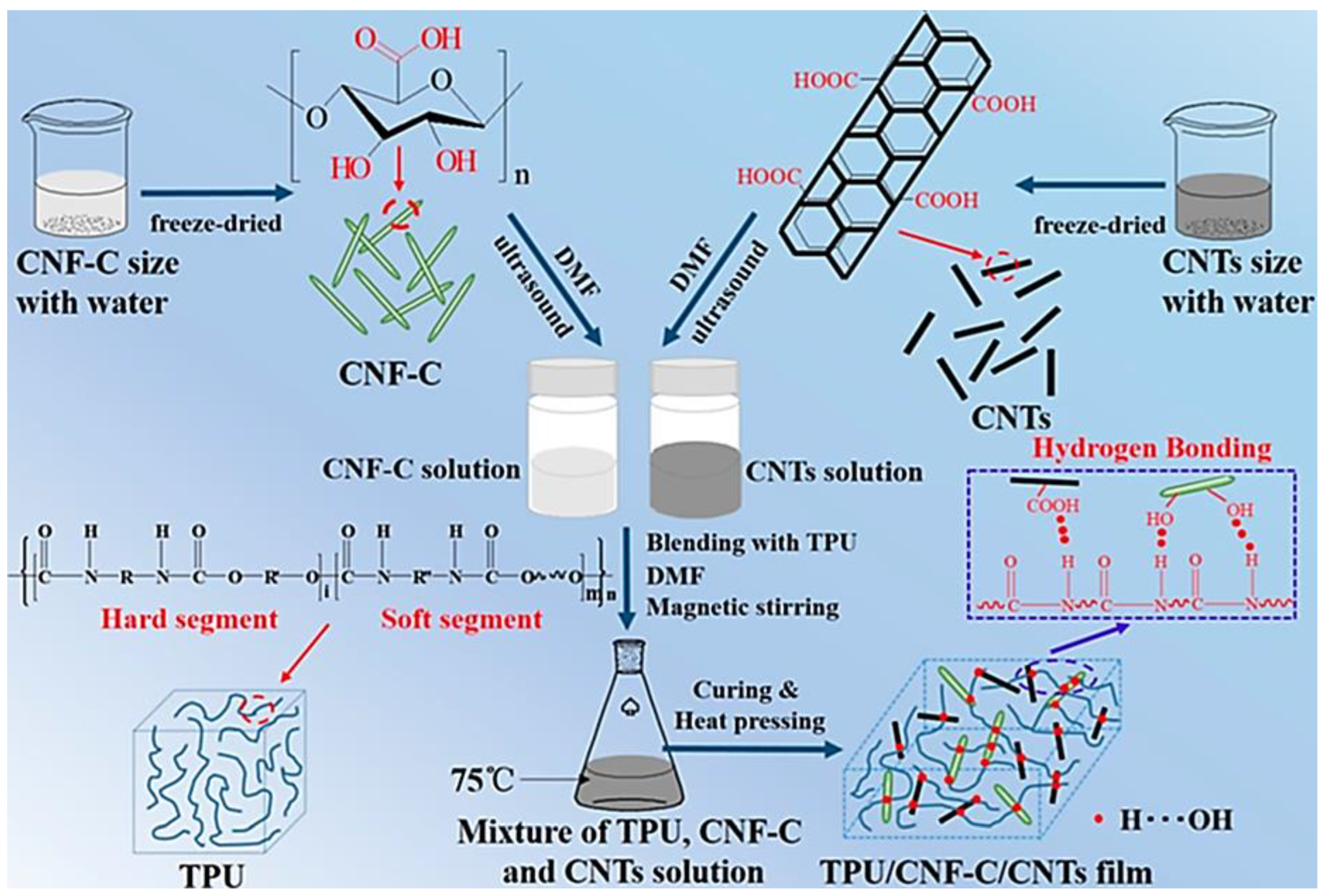

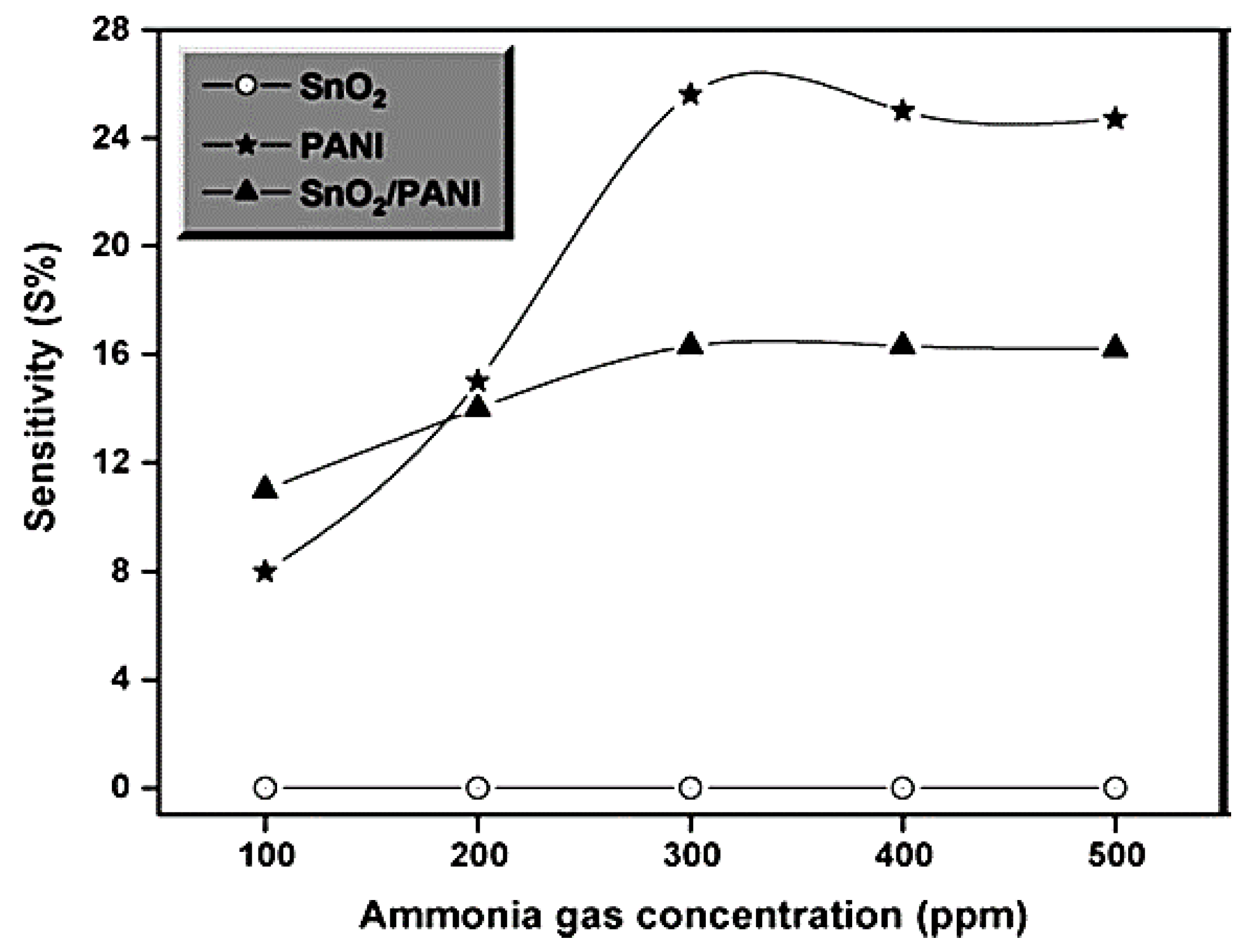

| Sample | Initial Resistance (Air) | Shift in Resistance | Response (Rg/R0) |
|---|---|---|---|
| (2% H2 in Air) | |||
| PANI film | 3.10 MΩ | 2.05 ± 0.02 MΩ | 1.66 |
| PANI/SWCNT | 69.2 kΩ | 57.2 ± 0.09 kΩ | 1.83 |
| PANI/MWCNT | 99.1 kΩ | 129 ± 0.1 kΩ | 2.30 |
| Conjugated Polymer | Nanofiller | Processing | Property/Application | Ref |
|---|---|---|---|---|
| Polyaniline | Carbon nanotube | Spin coating method | Hydrogen gas-sensing | [55] |
| Polyaniline | Carbon nanotube | Interfacial technique | Ammonia-sensing | [128] |
| Polypyrrole | Carbon nanotube | Spin coating | Ammonia-sensing | [129] |
| Polypyrrole | Carbon nanotube | In situ and | Ammonia sensor | [130] |
| spin coating methods | ||||
| Polyaniline | Graphene | Interfacial technique | H2O2-sensing | [131] |
| Polyaniline | Graphene oxide | In situ method | Methanol sensitivity; | [132] |
| electrical conductivity 241 Sm−1 | ||||
| Polyaniline | Graphene | Layer-by-layer technique | π–π conjugation; | [133] |
| high methane sensitivity | ||||
| Polythiophene | Reduced graphene oxide | In situ method | Humidity sensor | [134] |
| Polyaniline | ZnO-SnO2 | In situ chemical method | Ammonia-sensing | [135] |
| Polyaniline | Silver nanoparticle | In situ technique | Ammonia-sensing; | [136] |
| sensitivity ~12.5 μAmM−1 cm−2; response time 10 s | ||||
| Polypyrrole | ZnO-TiO2 | In situ method | Ammonia-sensing | [137] |
| Polypyrrole | SnO2-ZnO | In situ process | Ammonia-sensing | [73] |
| Polypyrrole | Ag-ZnO | In situ process | Ammonia-sensing | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A.; Ahmad, I.; Zhao, T.; Aldaghri, O.; Ibnaouf, K.H.; Eisa, M.H. Multifunctional Polymeric Nanocomposites for Sensing Applications—Design, Features, and Technical Advancements. Crystals 2023, 13, 1144. https://doi.org/10.3390/cryst13071144
Kausar A, Ahmad I, Zhao T, Aldaghri O, Ibnaouf KH, Eisa MH. Multifunctional Polymeric Nanocomposites for Sensing Applications—Design, Features, and Technical Advancements. Crystals. 2023; 13(7):1144. https://doi.org/10.3390/cryst13071144
Chicago/Turabian StyleKausar, Ayesha, Ishaq Ahmad, Tingkai Zhao, Osamah Aldaghri, Khalid H. Ibnaouf, and M. H. Eisa. 2023. "Multifunctional Polymeric Nanocomposites for Sensing Applications—Design, Features, and Technical Advancements" Crystals 13, no. 7: 1144. https://doi.org/10.3390/cryst13071144
APA StyleKausar, A., Ahmad, I., Zhao, T., Aldaghri, O., Ibnaouf, K. H., & Eisa, M. H. (2023). Multifunctional Polymeric Nanocomposites for Sensing Applications—Design, Features, and Technical Advancements. Crystals, 13(7), 1144. https://doi.org/10.3390/cryst13071144