Crystal Structure, Ionic Conductivity, Dielectric Properties and Electrical Conduction Mechanism of the Wyllieites Na1.5Mn3.5(AsO4)3 and Na1.5Mn3Fe0.5(AsO4)3
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Resolution and Structure Refinement
3.2. Structures Validation by CHARDI and BVS Analyzes
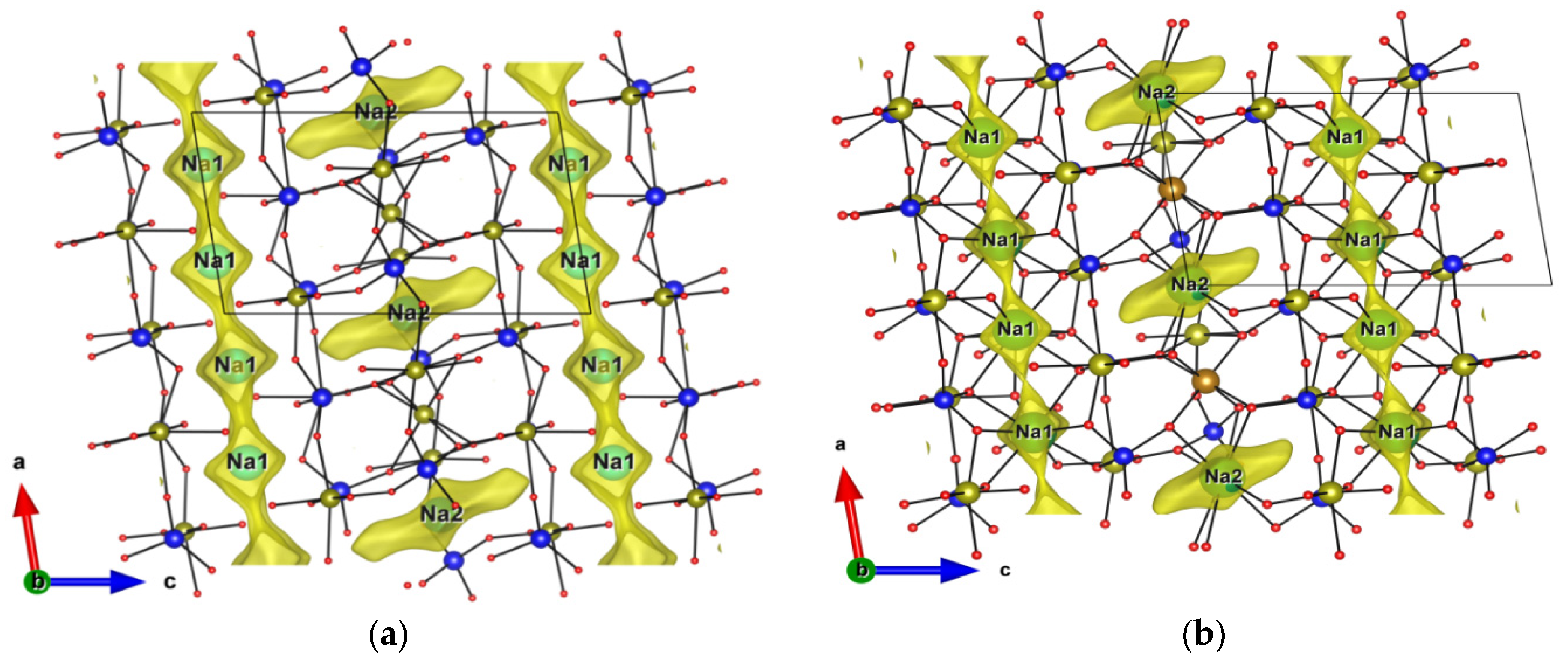
3.3. Structures Description and Discussion
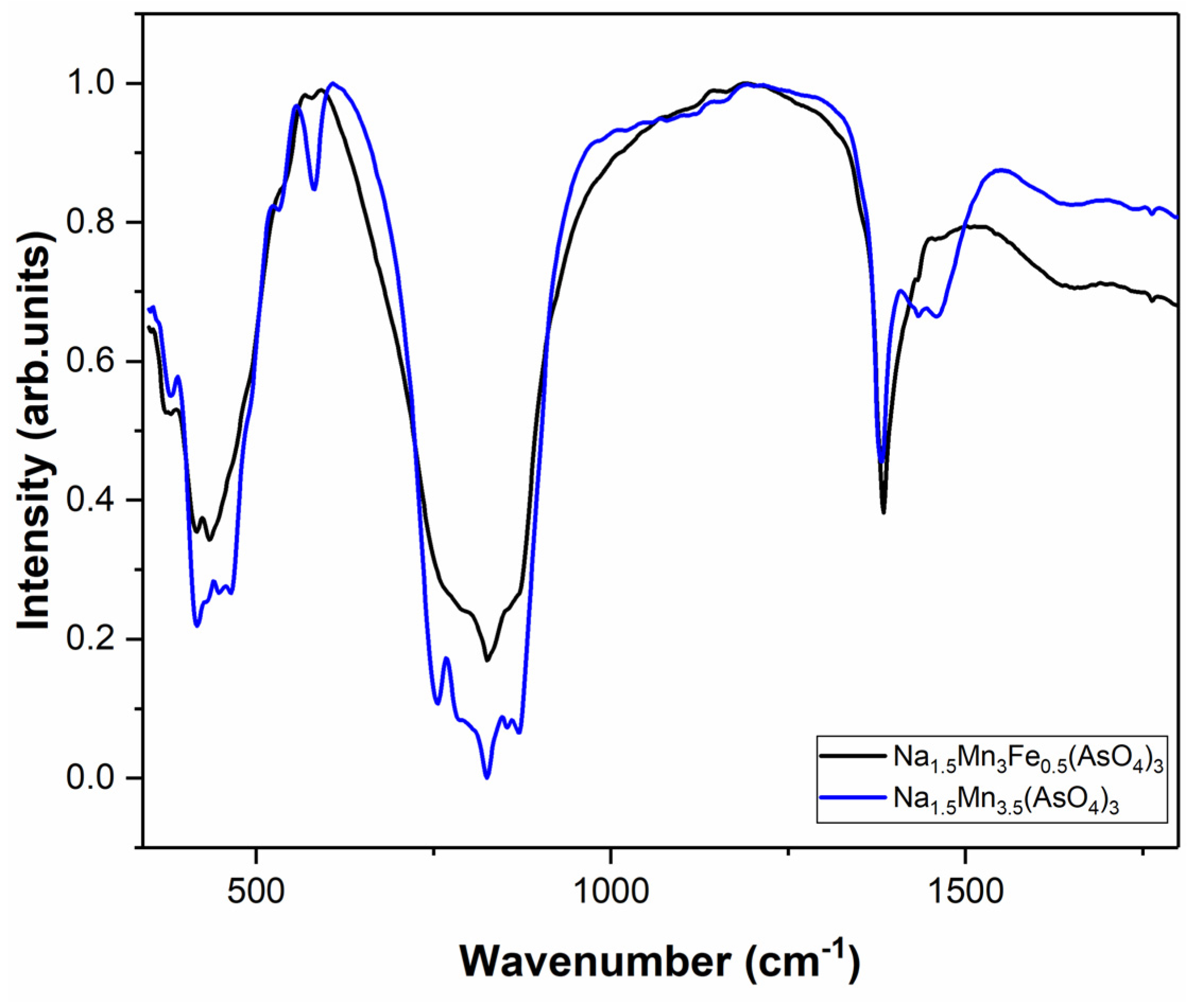
3.4. Infrared Spectroscopy Study
3.5. Raman Spectroscopy Study
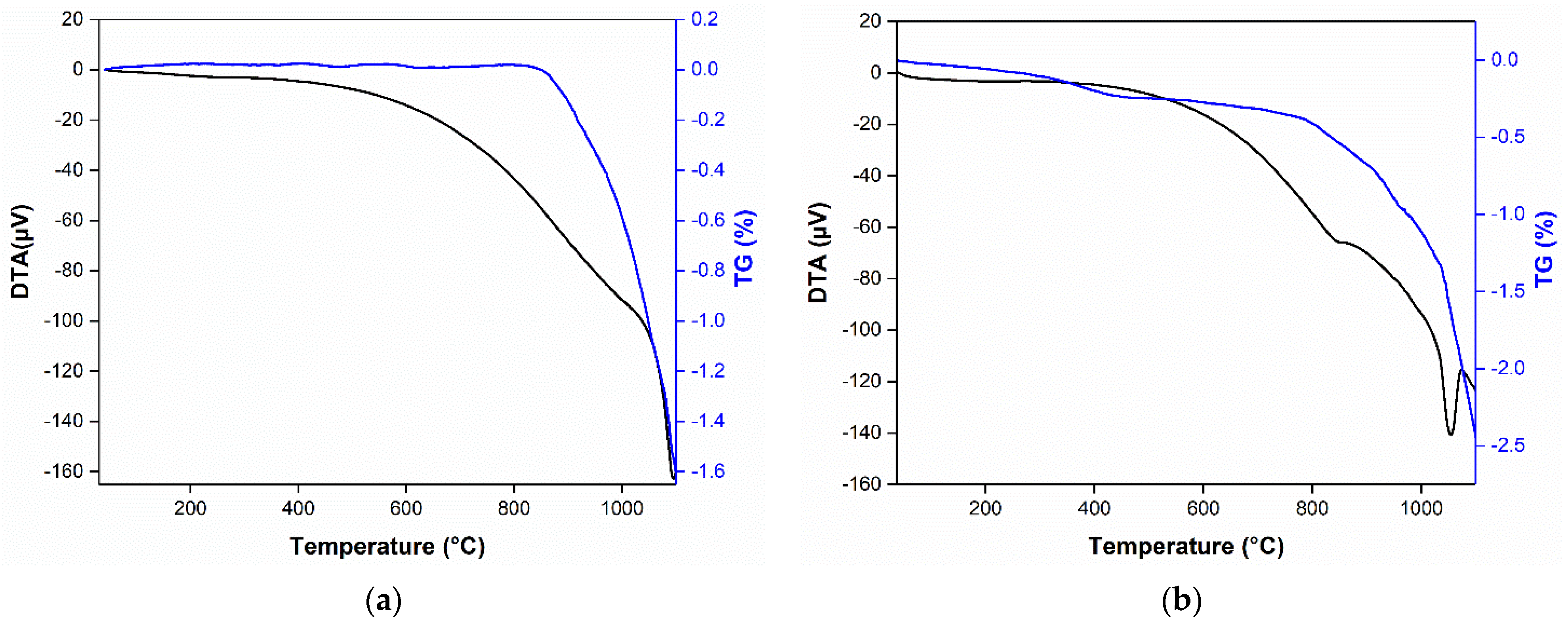
3.6. Thermal Analysis
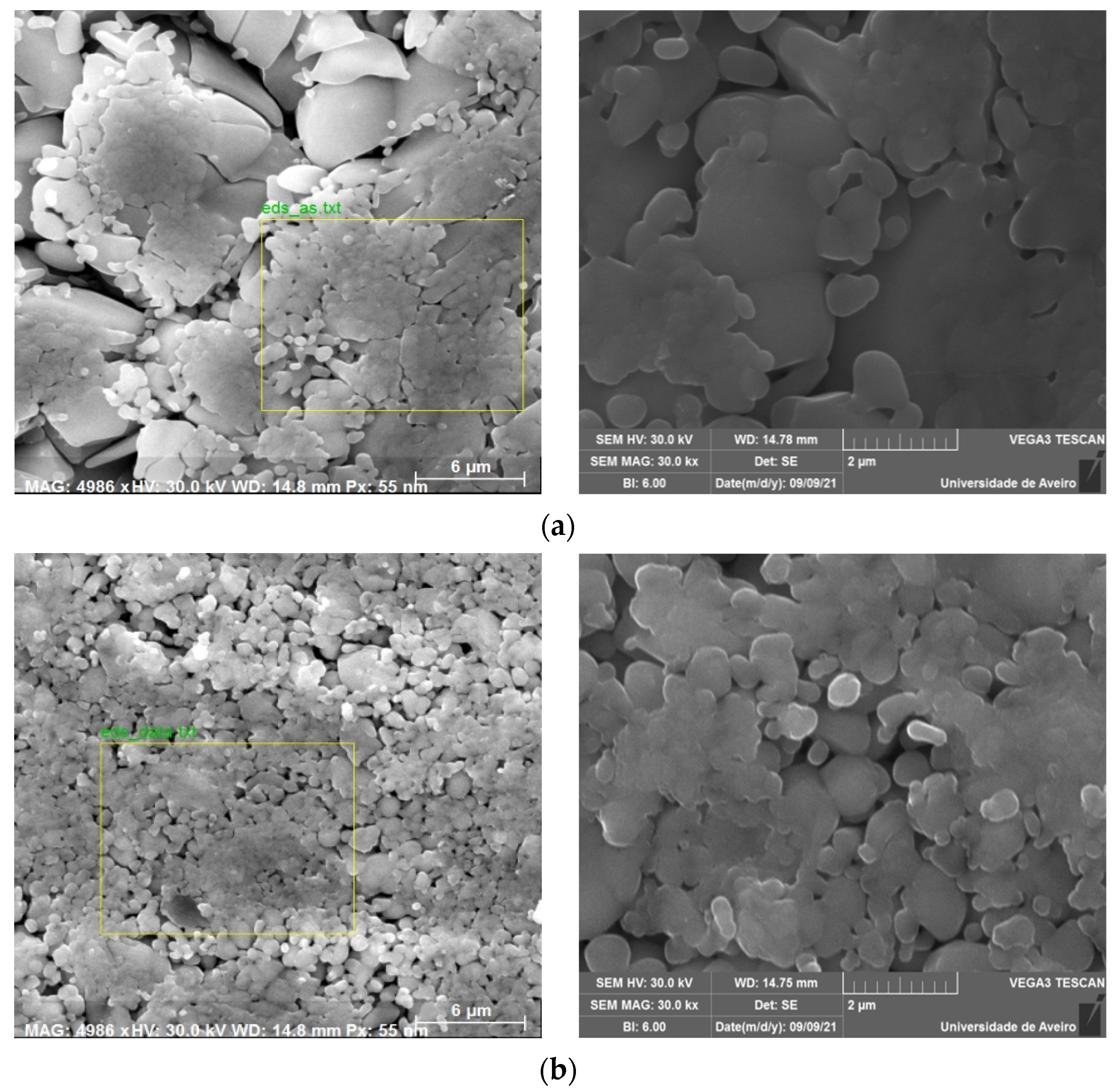
3.7. Morphological Analysis
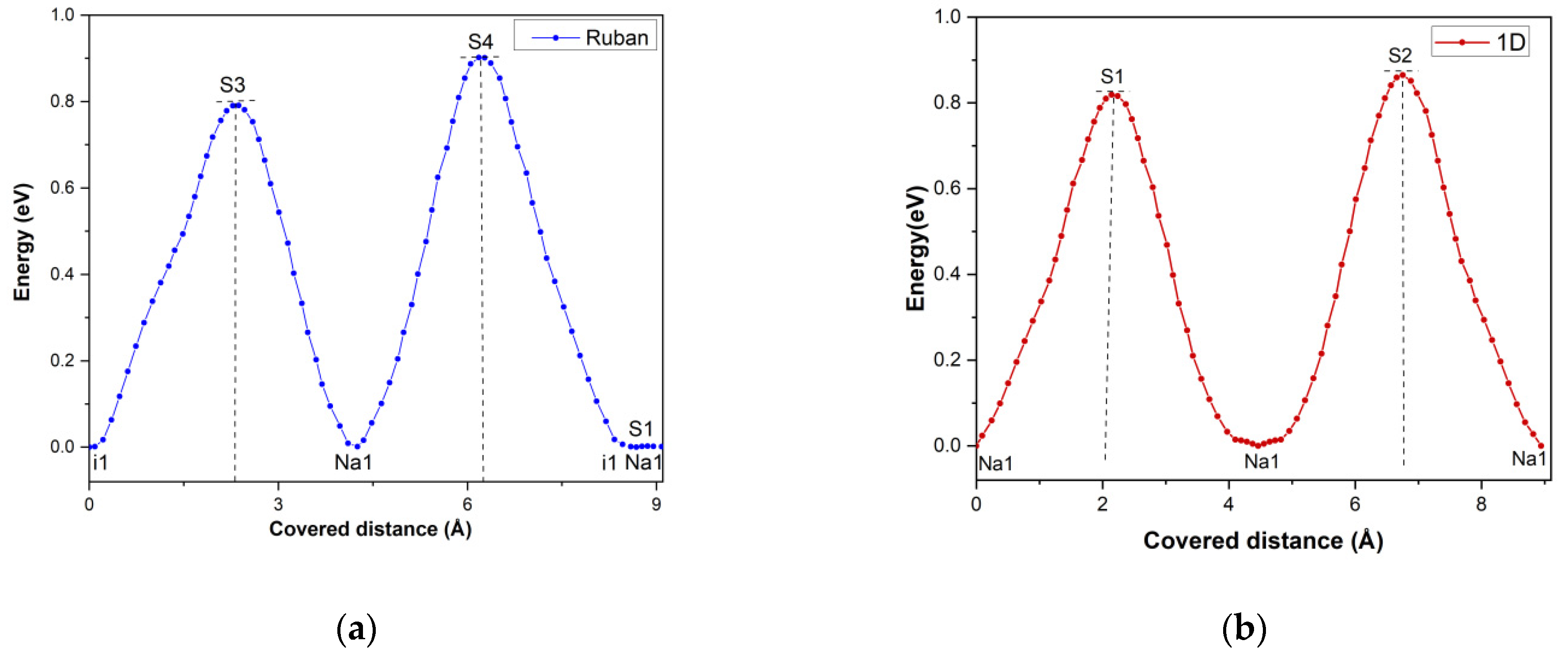
3.8. Na+ Pathways Transport Simulation
3.9. Electrical Measurements
3.9.1. Impedance Analysis
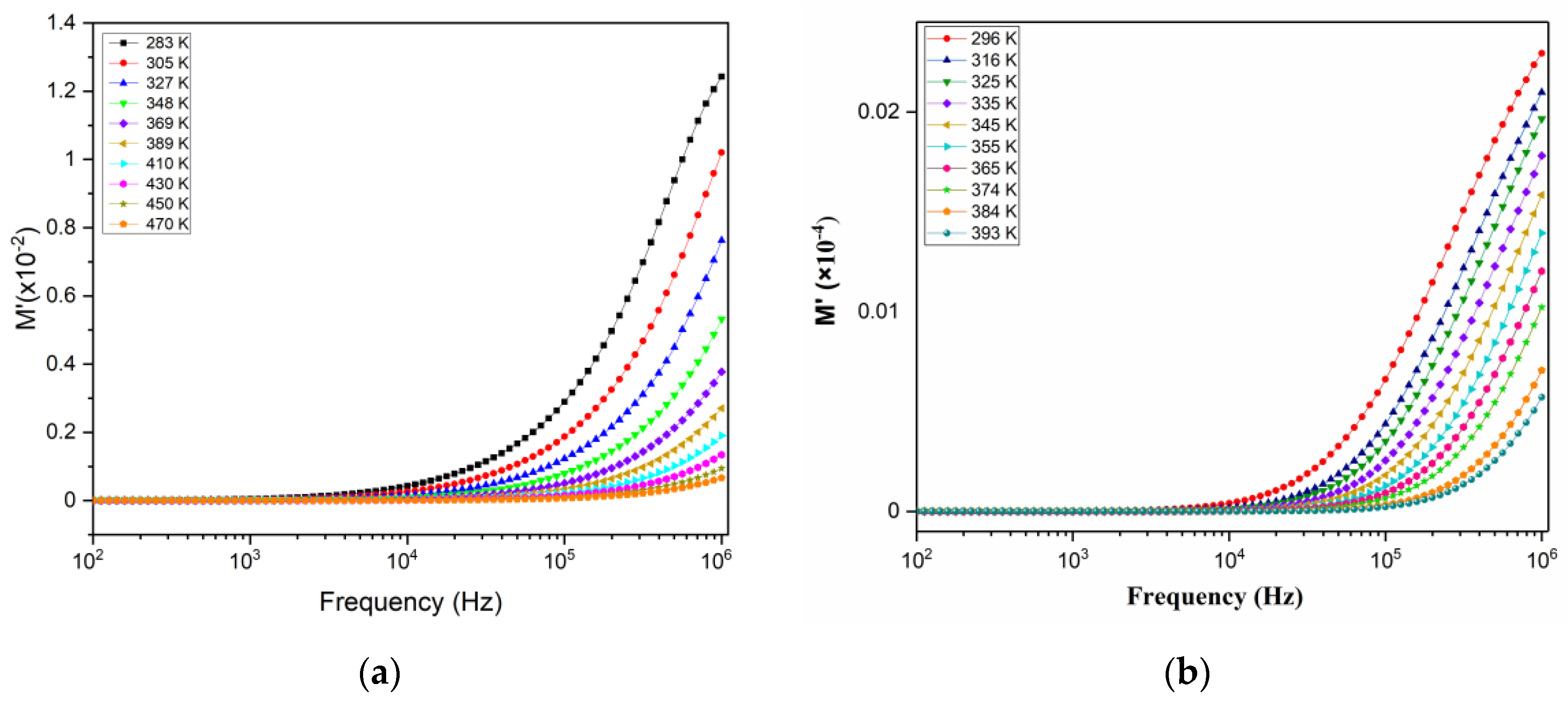
3.9.2. Modulus Analysis
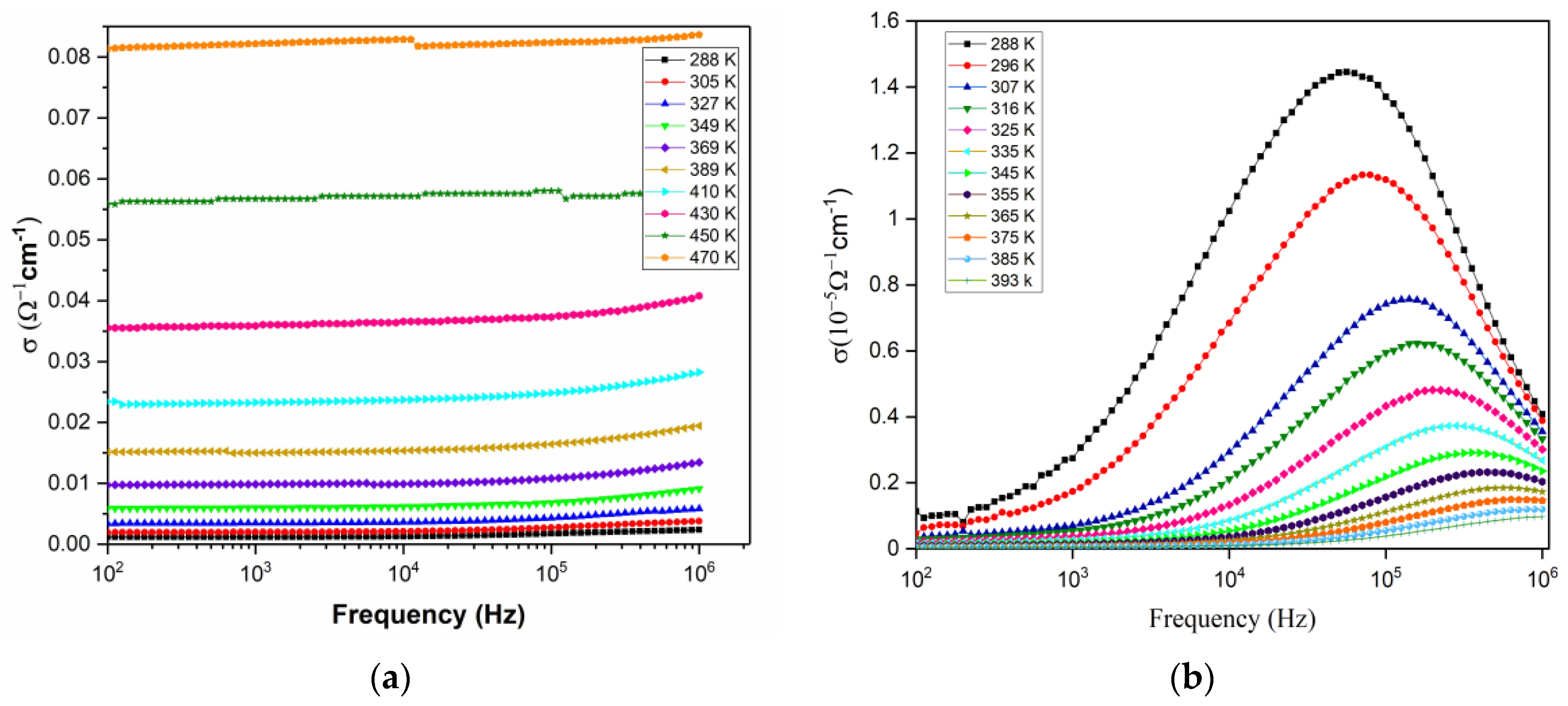
3.9.3. AC Conductivity Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imai, H.; Kamiya, Y.; Okuhara, T. Selective Oxidation of N-Butane over Nanosized Crystallites of (VO)2P2O7 Synthesized by an Exfoliation-Reduction Process of VOPO4·2H2O in a Mixture of 2-Butanol and Ethanol. J. Catal. 2007, 251, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Jouini, A.; Gâcon, J.C.; Ferid, M.; Trabelsi-Ayadi, M. Luminescence and Scintillation Properties of Praseodymium Poly and Diphosphates. Opt. Mater. 2003, 24, 175–180. [Google Scholar] [CrossRef]
- Herle, P.S.; Ellis, B.; Coombs, N.; Nazar, L.F. Nano-Network Electronic Conduction in Iron and Nickel Olivine Phosphates. Nat. Mater. 2004, 3, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Daidouh, A.; Durio, C.; Pico, C.; Veiga, M.L.; Chouaibi, N.; Ouassini, A. Structural and Electrical Study of the Alluaudites (Ag1 − xNax)2FeMn2(PO4)3 (x = 0, 0.5 and 1). Solid State Sci. 2002, 4, 541–548. [Google Scholar] [CrossRef]
- Bdey, S.; Savvin, S.N.; Bourguiba, N.F.; Núñez, P. Synthesis, Crystal Structure and Na+ Transport in Na3La(AsO4)2. J. Solid State Chem. 2022, 305, 122644. [Google Scholar] [CrossRef]
- Masquelier, C.; Patoux, S.; Wurm, C.; Morcrette, M. Polyanion-Based Positive Electrode Materials. In Lithium Batteries; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92675-9_15. [Google Scholar]
- Moore, P.B.; Ito, J. Wyllieite, Na2Fe2+ 2Al[PO4]3, a New Species. Mineral. Rec. 1973, 4, 131–136. [Google Scholar]
- Moore, P.B.; Ito, J. Alluaudites, Wyllieites, Arrojadites: Crystal Chemistry and Nomenclature. Miner. Mag. 1979, 43, 227–235. [Google Scholar] [CrossRef]
- Yang, H.; Downs, R.T.; Gu, X.; Xie, X.; Kobsch, A. Fupingqiuite, IMA 2016-087. CNMNC Newsletter No. 35. Miner. Mag. 2017, 81, 209–213. [Google Scholar]
- Frigui, W.; Zid, M.F.; Driss, A. Wyllieite-Type Ag1.09Mn3.46(AsO4)3. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, i40–i41. [Google Scholar] [CrossRef] [Green Version]
- Frigui, W.; Zid, M.F.; Driss, A. Synthesis and Physico-Chemical Study of Na0.5K0.65Mn3.43(AsO4)3 Compound. Jordan J. Chem. 2011, 6, 295–305. [Google Scholar]
- Marzouki, R.; Ben Smida, Y.; Avdeev, M.; Alghamdi, M.M.; Zid, M.F. Synthesis, Structure and Na+ Migration Pathways of New Wylleite-Type Na1.25Co2.187Al1.125(AsO4)3. Mater. Res. Express 2019, 6, 126313. [Google Scholar] [CrossRef]
- Bdey, S.; Bourguiba, N.F.; Savvin, S.N.; Núñez, P. Na3Bi(AsO4)2: Synthesis, Crystal Structure and Ionic Conductivity. J. Solid State Chem. 2019, 272, 189–197. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The High Score Suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Wong, L.L.; Adams, S. SoftBV—A Software Tool for Screening the Materials Genome of Inorganic Fast Ion Conductors. Acta Crystallogr. Sect. B Struct. Sci. 2019, 75, 18–33. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Teixeira, S.S.; Graça, M.P.F.; Lucas, J.; Valente, M.A.; Soares, P.I.P.; Lança, M.C.; Vieira, T.; Silva, J.C.; Borges, J.P.; Jinga, L.I.; et al. Nanostructured LiFe5O8 by a Biogenic Method for Applications from Electronics to Medicine. Nanomaterials 2021, 11, 193. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Ferreira da Silva, M.G.; Sombra, A.S.B.; Valente, M.A. Electric and Dielectric Properties of a SiO2-Na2O-Nb2O5 Glass Subject to a Controlled Heat-Treatment Process. Phys. B Condens. Matter 2007, 396, 62–69. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Prezas, P.R.; Costa, M.M.; Valente, M.A. Structural and Dielectric Characterization of LiNbO3 Nano-Size Powders Obtained by Pechini Method. J. Solgel. Sci. Technol. 2012, 64, 78–85. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Ferreira da Silva, M.G.; Valente, M.A. NaNbO3 Crystals Dispersed in a B2O3 Glass Matrix—Structural Characteristics versus Electrical and Dielectrical Properties. Solid State Sci. 2009, 11, 570–577. [Google Scholar] [CrossRef]
- Toby, B.H. EXPGUI, a Graphical User Interface for GSAS. J. Appl. Crystallogr. 2001, 34, 210–213. [Google Scholar] [CrossRef] [Green Version]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef] [Green Version]
- Adams, S. Structural Science Relationship between Bond Valence and Bond Softness of Alkali Halides and Chalcogenides. Acta Crystallogr. Sect. B Struct. Sci. 2001, B57, 278–287. [Google Scholar] [CrossRef]
- Brown, I.D.; Shannon, R.D. Empirical Bond-strength–Bond-length Curves for Oxides. Acta Crystallogr. Sect. A 1973, 29, 266–282. [Google Scholar] [CrossRef]
- Yakubovich, O.V.; Massa, W.; Gavrilenko, P.G.; Dimitrova, O.V. The Crystal Structure of a New Synthetic Member in the Wyllieite Group: Na1.265Mn2+2.690Mn3+0.785(PO4)3. Eur. J. Mineral. 2005, 17, 741–748. [Google Scholar] [CrossRef]
- Ayed, B.; Krifa, M.; Haddad, A. Na1.72Mn3.28(AsO4)3. Acta Crystallogra. Sect C Cryst. Struct. Commun. 2002, 58, i98–i100. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Crystallogr. Sect. B Struct. Sci. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Ouaatta, S.; Assani, A.; Saadi, M.; El Ammari, L. Crystal Structure of a New Tripotassium Hexanickel Iron Hexaphosphate. Acta Crystallogr. Sect. E Struct. Rep. Online 2019, 75, 402–404. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley: Hoboken, NJ, USA, 2009; ISBN 978-0-471-74493-1. [Google Scholar]
- Augsburger, M.S.; Juri, M.A.; Pedregosa, J.C.; Mercader, R.C. Crystal Data and Spectroscopic Studies of NaNiFe2(AsO4)3. J. Solid State Chem. 1992, 101, 66–70. [Google Scholar] [CrossRef]
- Frost, R.L.; Čejka, J.; Sejkora, J.; Plášil, J.; Bahfenne, S.; Palmer, S.J. Raman Microscopy of the Mixite Mineral BiCu6(AsO4)3(OH)6·3H2O from the Czech Republic. J. Raman Spectrosc. 2009, 41, 566–570. [Google Scholar] [CrossRef]
- Khorari, S.; Rulmont, A.; Cahay, R.; Tarte, P. Structure of the Complex Arsenates NaCa2M2+2(AsO4)3 (M2+ = Mg, Ni, Co): First Experimental Evidence of a Garnet-Alluaudite Reversible Polymorphism. J. Solid State Chem. 1995, 118, 267–273. [Google Scholar] [CrossRef]
- Khorari, S.; Rulmont, A.; Tarte, P. Alluaudite-Like Structure of the Arsenate Na3In2(AsO4)3. J. Solid State Chem. 1997, 134, 31–37. [Google Scholar] [CrossRef]
- Kawabe, Y.; Yabuuchi, N.; Kajiyama, M.; Fukuhara, N.; Inamasu, T.; Okuyama, R.; Nakai, I.; Komaba, S. Synthesis and Electrode Performance of Carbon Coated Na2FePO4F for Rechargeable Na Batteries. Electrochem. Commun. 2011, 13, 1225–1228. [Google Scholar] [CrossRef]
- Rajah, F.; Graia, M.; Mhiri, T. Synthesis, Crystal Structure, and Characterization of Na7Cu4(AsO4)5. Phosphorus Sulfur Silicon Relat. Elem. 2013, 188, 1244–1253. [Google Scholar] [CrossRef]
- Priestner, M.; Singer, G.; Weil, M.; Kremer, R.K.; Libowitzky, E. Synthesis, Structural, Magnetic and Thermal Properties of Mn2As2O5, the First Pyro-Arsenite of a First-Row Transition Metal. J. Solid State Chem. 2019, 277, 209–215. [Google Scholar] [CrossRef]
- Behera, B.; Nayak, P.; Choudhary, R.N.P. Impedance Spectroscopy Study of NaBa2V5O15 Ceramic. J. Alloys Compd. 2007, 436, 226–232. [Google Scholar] [CrossRef]
- Costa, M.M.; Pires, G.F.M.; Terezo, A.J.; Graa, M.P.F.; Sombra, A.S.B. Impedance and Modulus Studies of Magnetic Ceramic Oxide Ba2Co2Fe12O22(Co2Y) Doped with Bi2O3. J. Appl. Phys. 2011, 110, 034107. [Google Scholar] [CrossRef]
- Sohn, R.S.T.M.; Macêdo, A.A.M.; Costa, M.M.; Mazzetto, S.E.; Sombra, A.S.B. Studies of the Structural and Electrical Properties of Lithium Ferrite (LiFe5O8). Phys. Scr. 2010, 82, 055702. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, B.P.; Choudhary, R.N.P.; Thakur, A.K. Characterization of Electrical Properties of Pb-Modified BaSnO3 Using Impedance Spectroscopy. Mater. Chem. Phys. 2006, 99, 150–159. [Google Scholar] [CrossRef]
- Lily; Kumari, K.; Prasad, K.; Choudhary, R.N.P. Impedance Spectroscopy of (Na0.5Bi0.5)(Zr0.25Ti0.75)O3 Lead-Free Ceramic. J. Alloys Compd. 2008, 453, 325–331. [Google Scholar] [CrossRef]
- Lu, W.; Jiang, S.; Zhou, D.; Gong, S. Structural and Electrical Properties of Ba(Sn,Sb)O3 Electroceramics Materials. Sens. Actuators A 2000, 80, 35–37. [Google Scholar] [CrossRef]
- Iben Nassar, K.; Rammeh, N.; Teixeira, S.S.; Graça, M.P.F. Physical Properties, Complex Impedance, and Electrical Conductivity of Double Perovskite LaBa0.5Ag0.5FeMnO6. J. Electron. Mater. 2022, 51, 370–377. [Google Scholar] [CrossRef]
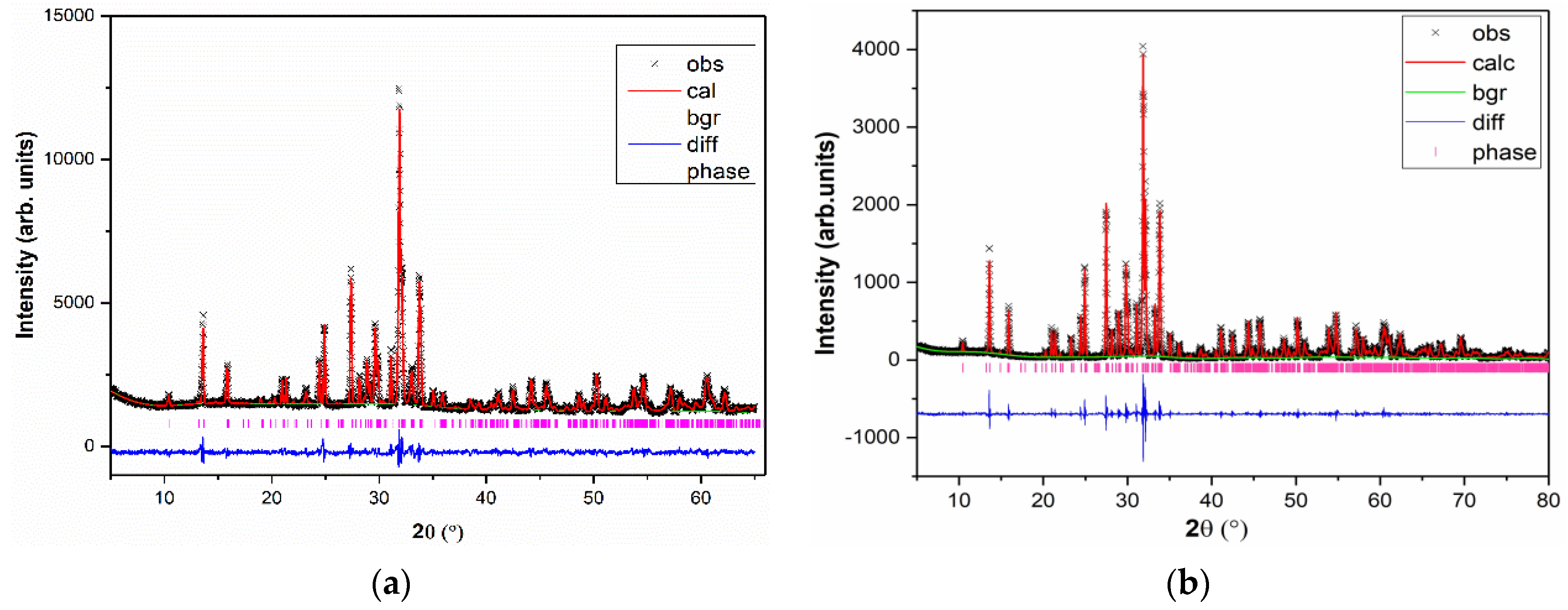
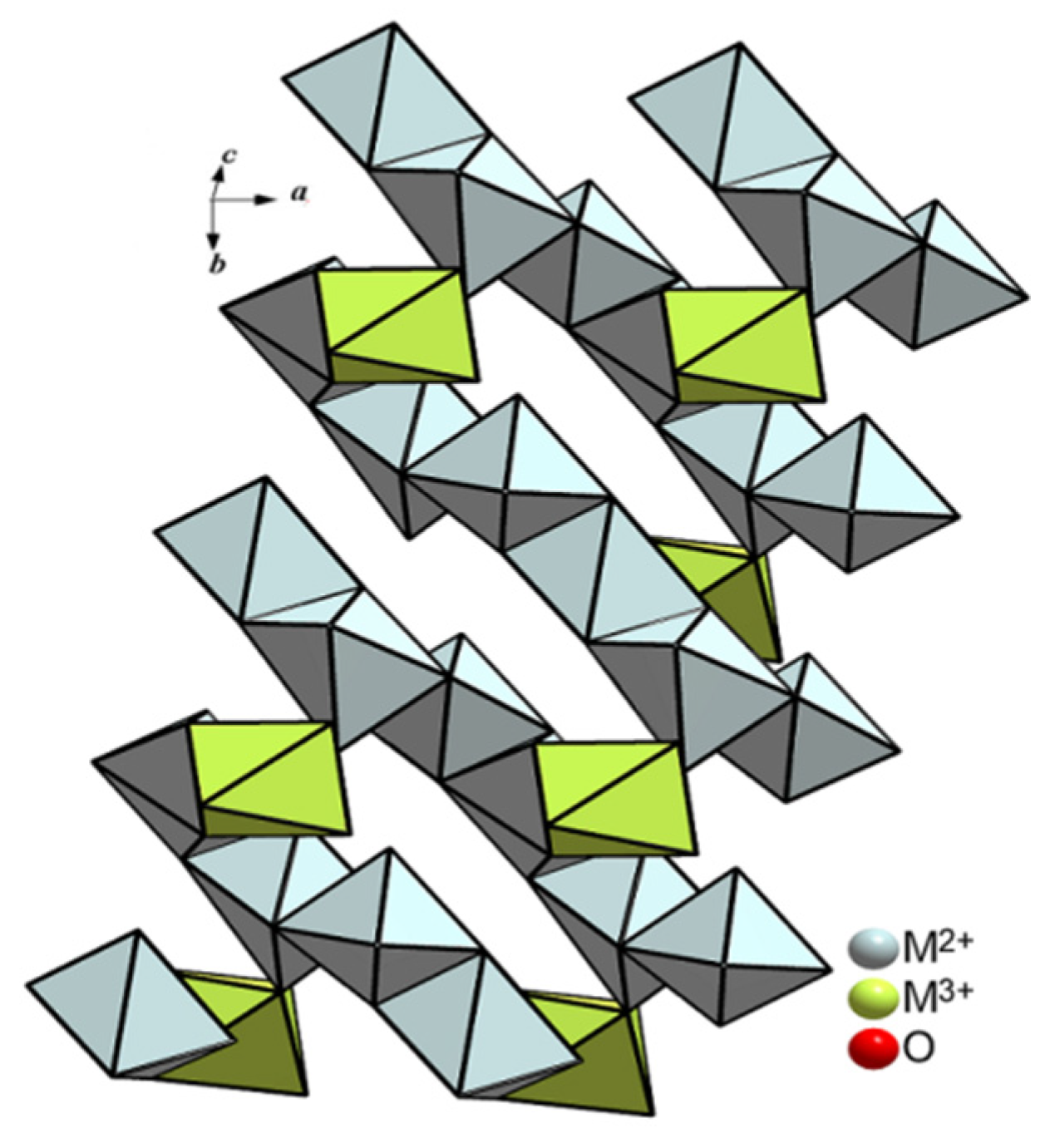
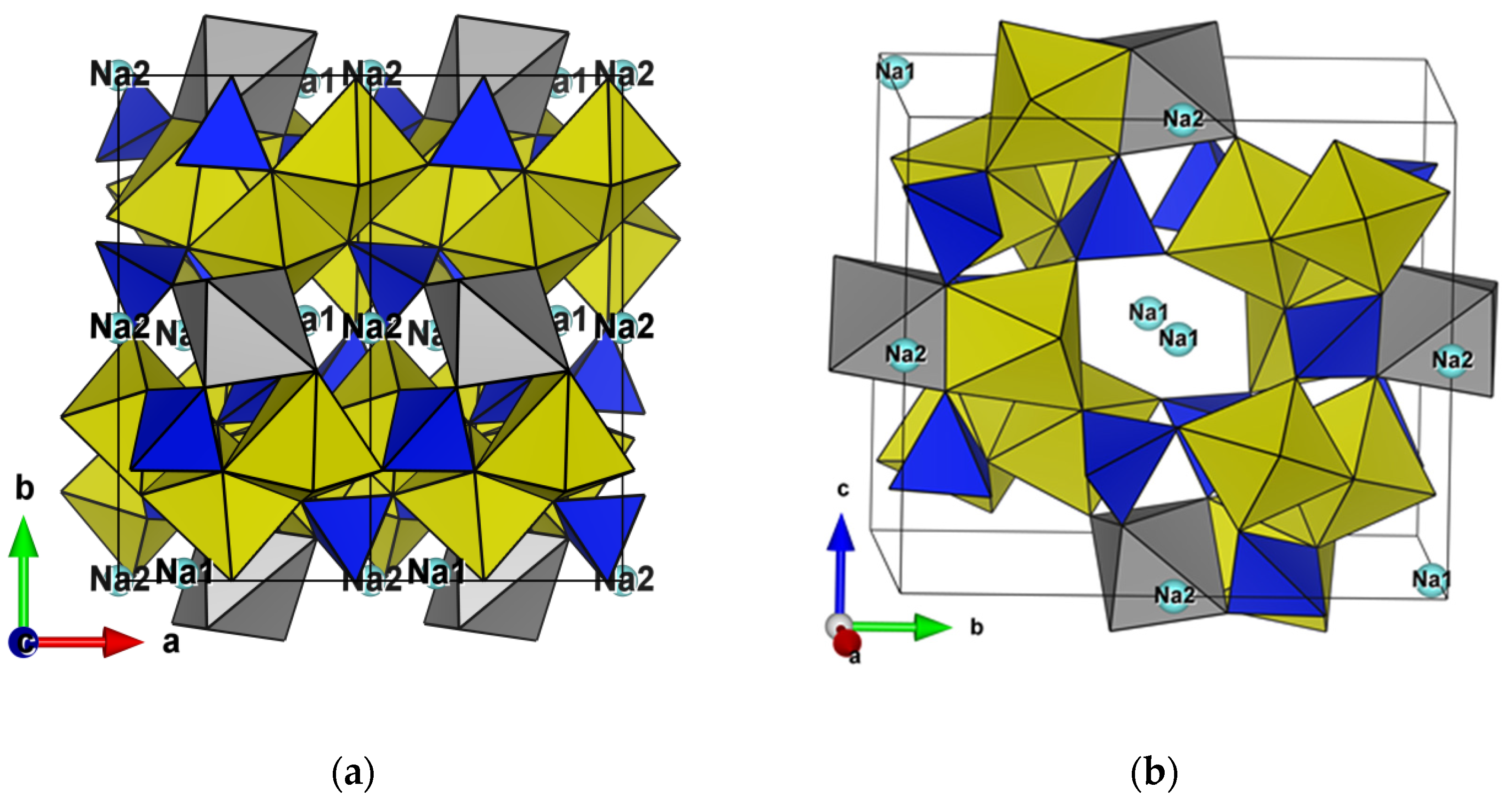



| Crystal Data | ||
|---|---|---|
| Formula | Na1.5MnII3MnIII0.5(AsO4)3 | Na1.5MnII3FeIII0.5(AsO4)3 |
| Molar mass (g/mol) | 643.53 | 643.98 |
| Cristal system, S.G, Z | Monoclinic, P21/c, 4 | |
| a, b, c (Å) ; β (°) | 6.78344 (9), 12.93830 (15), 11.22825 (19); 98.5374 (19) | 6.76723 (12), 12.9864 (3), 11.256; 98.8636 (13) |
| V (Å3) | 974.54 (2) | 977.46 (4) |
| Dx (g·cm−3) | 4.386 | 4.376 |
| Radiation; λ (Å) | Cu Kα (1.54059) | |
| Data Collection | ||
| Temperature (K) | 298(2) | |
| Diffractometer | D8 Bruker | |
| Reflection number | 4613 | 1190 |
| 2θ (°) | 5.028–64.984 | 5–80 |
| Refinement Results | ||
| 0.022 | 0.083 | |
| 0.029 | 0.105 | |
| 0.025 | 0.088 | |
| R(F²) | 0.04407 | 0.05682 |
| 1.464 | 1.440 | |
| Atom | x | y | z | Uiso*/Ueq | Wyckoff Positions |
|---|---|---|---|---|---|
| As1 | 0.5824 (3) | 0.11275 (14) | 0.22827 (18) | 0.0277 (6)* | 4 e |
| As2 | 0.8817 (3) | 0.39458 (14) | 0.26305 (18) | 0.0313 (6)* | 4 e |
| As3 | 0.2272 (3) | 0.28982 (14) | −0.01591 (18) | 0.0114 (6)* | 4 e |
| Mn1 | 0.4139 (3) | 0.35341 (14) | 0.26964 (18) | 0.0216 (6)* | 4 e |
| Mn2 | 0.7199 (3) | 0.23080 (14) | 0.49625 (18) | 0.0092 (6)* | 4 e |
| Mn3 | 0.5 | 0.0 | 0.5 | 0.0491 (6)* | 2 d |
| Mn4 | 0.0827 (3) | 0.16419 (14) | 0.20656 (18) | 0.0217 (6)* | 4 e |
| Na1 | 0.7403 (3) | −0.01431 (14) | 0.00492 (18) | 0.0139 (6)* | 4 e |
| Na2 | 0.0 | 0.5 | 0.0 | 0.0084 (6)* | 2 c |
| O1 | 0.5503 (3) | 0.00107 (14) | 0.15914 (18) | 0.0787 (6)* | 4 e |
| O2 | 0.7718 (3) | 0.17622 (14) | 0.17308 (18) | 0.0301 (6)* | 4 e |
| O3 | 0.6496 (3) | 0.09540 (14) | 0.37900 (18) | 0.0835 (6)* | 4 e |
| O4 | 0.3917 (3) | 0.18877 (14) | 0.19837 (18) | 0.0217 (6)* | 4 e |
| O5 | 0.0884 (3) | 0.33029 (14) | 0.26194 (18) | 0.0381 (6)* | 4 e |
| O6 | 0.2138 (3) | −0.08500 (14) | 0.38092 (18) | 0.0405 (6)* | 4 e |
| O7 | 0.0582 (3) | 0.00566 (14) | 0.15275 (18) | 0.0405 (6)* | 4 e |
| O8 | 0.7251 (3) | 0.34213 (14) | 0.35173 (18) | 0.0746 (6)* | 4 e |
| O9 | 0.4129 (3) | 0.21657 (14) | −0.04974 (18) | 0.0217 (6)* | 4 e |
| O10 | 0.1149 (3) | 0.12330 (14) | 0.37710 (18) | 0.0884 (6)* | 4 e |
| O11 | 0.3420 (3) | 0.38209 (14) | 0.07948 (18) | 0.0217 (6)* | 4 e |
| O12 | 0.0472 (3) | 0.21869 (14) | 0.04507 (18) | 0.0242 (6)* | 4 e |
| Atom | x | y | z | Uiso*/Ueq | Wyckoff Positions |
|---|---|---|---|---|---|
| As1 | 0.5977 (4) | 0.1126 (6) | 0.2306 (6) | 0.0084 (6) | 4 e |
| As2 | 0.890 (1) | 0.3943 (5) | 0.2631 (5) | 0.0228 (5) | 4 e |
| As3 | 0.2385 (2) | 0.2885 (5) | −0.0033 (1) | 0.0138 (1) | 4 e |
| Mn1 | 0.4150 (3) | 0.3469 (3) | 0.2808 (1) | 0.0043 (1) | 4 e |
| Mn2 | 0.7426 (2) | 0.2310 (1) | 0.4986 (2) | 0.0117 (1) | 4 e |
| Fe3 | 0.5 | 0 | 0.5 | 0.07525 | 2 d |
| Mn4 | 0.0797 (8) | 0.1575 (5) | 0.2096 | 0.0168 (9) | 4 e |
| Na1 | 0.7676 (2) | −0.0130 (3) | −0.0003 (6) | 0.0094 | 4 e |
| Na2 | 0 | 0.5 | 0 | 0.04998 | 2 c |
| O1 | 0.5563 (4) | 0.0035 (5) | 0.1576 (7) | 0.0502 (4) | 4 e |
| O2 | 0.7559 | 0.1705 (6) | 0.1537 (4) | 0.0816 (5) | 4 e |
| O3 | 0.6380 (5) | 0.0966 (1) | 0.387 | 0.0184 (6) | 4 e |
| O4 | 0.4055 (6) | 0.1882 (8) | 0.2151 (4) | 0.0092 | 4 e |
| O5 | 0.0942 (6) | 0.3214 (8) | 0.2731 (1) | 0.0336 (5) | 4 e |
| O6 | 0.2627 (7) | −0.0861 (9) | 0.3781 (4) | 0.0636 (7) | 4 e |
| O7 | 0.0497 (3) | −0.0014 (5) | 0.1586 (2) | 0.0086 (9) | 4 e |
| O8 | 0.7278 (9) | 0.3342 (5) | 0.3435 (9) | 0.003 (2) | 4 e |
| O9 | 0.4240 (7) | 0.2137 (9) | −0.0331 (4) | 0.0242 (6) | 4 e |
| O10 | 0.1413 (9) | 0.1352 (8) | 0.3901 (8) | 0.0089 (4) | 4 e |
| O11 | 0.3623 (9) | 0.3826 (7) | 0.0789 (7) | 0.0991 (6) | 4 e |
| O12 | 0.0632 (9) | 0.2151 (9) | 0.0513 (6) | 0.0015 (2) | 4 e |
| BVS Analysis | CHARDI Analysis | |||
|---|---|---|---|---|
| Cation | q(i).sof(i) | Q(i) | CN(i) | ECoN(i) |
| Na1 | 1 | 1.02 | 5 | 5.03 |
| Na2 | 1 | 1.01 | 6 | 5.53 |
| As1 | 5.00 | 4.99 | 4 | 3.92 |
| As2 | 5.00 | 5.21 | 4 | 3.92 |
| As3 | 5.00 | 4.83 | 4 | 3.95 |
| Mn1 | 2.00 | 1.87 | 6 | 5.81 |
| Mn2 | 2.00 | 2.02 | 6 | 5.81 |
| Mn3 | 3.00 | 2.74 | 6 | 5.29 |
| Mn4 | 2.00 | 2.18 | 6 | 5.43 |
| BVS Analysis | CHARDI Analysis | |||
|---|---|---|---|---|
| Cation | q(i).sof(i) | Q(i) | CN(i) | ECoN(i) |
| Na1 | 1 | 0.91 | 5 | 5.18 |
| Na2 | 1 | 1.04 | 6 | 6.39 |
| As1 | 5.00 | 4.98 | 4 | 3.87 |
| As2 | 5.00 | 5.02 | 4 | 3.86 |
| As3 | 5.00 | 4.92 | 4 | 3.94 |
| Fe | 3.00 | 3.19 | 6 | 5.72 |
| Mn1 | 2.00 | 1.94 | 6 | 5.88 |
| Mn2 | 2.00 | 1.88 | 6 | 5.85 |
| Mn3 | 2.00 | 2.22 | 6 | 5.28 |
| Formula | S.G | M(II)-O (Å) | M(III)-O (Å) | Ref. |
|---|---|---|---|---|
| Na1.5Mn3Fe0.5(AsO4)3 | P21/c | 1.959 (5)–2.256 (6) | 1.973 (2)–2.040 (3) | * |
| Na1.265MnII2.690MnIII0.785(PO4)3 | 1.893 (2)–2.334 (2) | 2.171 (6)–2.648 (5) | [25] | |
| Na1.5Mn3Mn0.5(AsO4)3 | 1.9270 (1)–2.341 (4) | 2.193 (2)–2.4489 (19) | * | |
| Na1.25Co2.187Al1.125(AsO4)3 | 1.928–2.151 | 2.170–2.184 | [12] | |
| Na2Fe2IIAl(PO4)3 | 2.089–2.225 | 1.973 | [7] | |
| Ag1.09Mn3.46(AsO4)3 | 2.105 (4)–2.413 (5) | 1.915 (5)–2.186 (6) | [10] | |
| Na0.5K0.65Mn3.43(AsO4)3 | 2.113 (5)–2.46 (5) | 1.954 (5)–2.178 (6) | [11] | |
| NaMnII2.5FeIII0.5 Al0.5(PO4)3 | P21/n | 2.103–2.245 | 1.956–2.025 | [8] |
| K1.5Ni3 Fe0.5(PO4)3 | C2/c | 2.0153 (14)–2.2241 (13) | 2.016 (2)–2.0490 (13) | [28] |
| Na1.72Mn3.28(AsO4)3 | 2.218 (9)–2.360 (8) | 1.990 (12)–2.193 (11) | [26] |
| Compound | Valence Vibrations | Deformation | Vibration M-O | Ref. | ||
|---|---|---|---|---|---|---|
| ν1 | ν3 | ν2 | ν4 | |||
| (AsO4)(III) | 837 | 878 | 349 | 463 | - | [29] |
| Na0.5K0.65Mn3.43(AsO4)3 | 822 | 880 | - | 443 | 564; 420; 669 | [11] |
| BiCu6(AsO4)3(OH)6.3H2O | 848 | 814; 797 | 390; 311 | 553; 529; 494 | - | [30] |
| NaNiFe2(AsO4)3 | 860; 901 | 820; 789; 700 | 447 | 496; 474 | [31] | |
| NaCa2Mg2(AsO4)3 | 950; 750 | 550; 300 | - | [32] | ||
| Na3In2(AsO4)3 | 866 | 934 | 342 | 398 | [33] | |
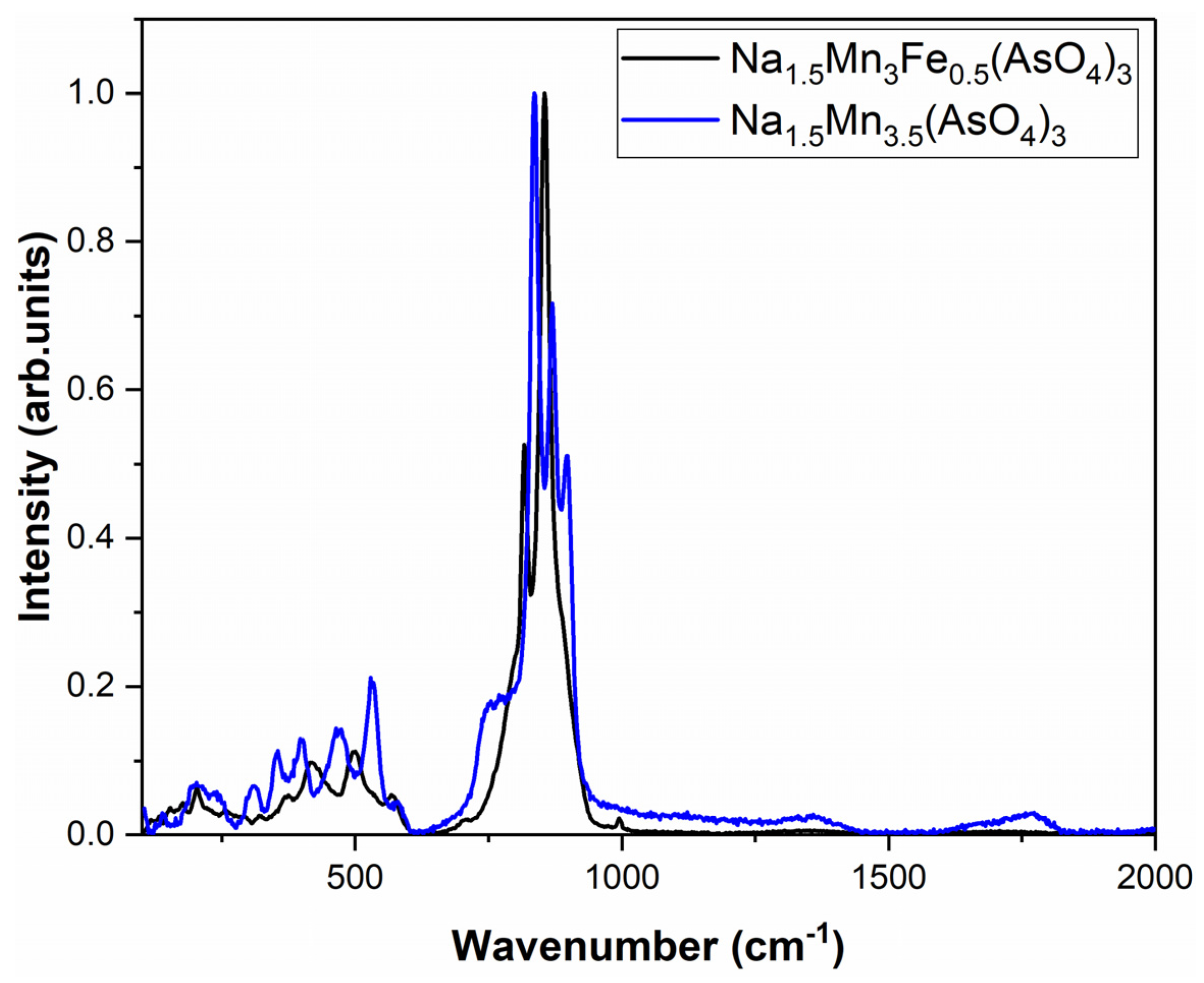
| Na1.5MnII3MnIII0.5(AsO4)3 | 820; 750 | 861 | - | 456; 426 | 538; 581 | * |
| Na1.5MnII3FeIII0.5(AsO4)3 | 825; 889 | 767 | - | 436; 371 | 583 | * |
| Compound | ν1 s | ν3 as | ν2 s | ν4 as | ν5 M(III) | ν5 M(II) | Ref. |
|---|---|---|---|---|---|---|---|
| Na1.5Fe0.5Mn3(AsO4)3 | 854–848–816 | 1000 | 417–496 | 578–569 | (Fe) 374 | (Mn) 256–206 | * |
| Na1.5Mn3.5(AsO4)3 | 838–867–759 | 899 | 403–475 | 529–580 | (Mn) 358–313 | (Mn) 230–192 | * |
| Na7Cu4(AsO4)5 | 813–880 | 902 | 480–485 | 511–527 | (Cu) 403 334 | - | [35] |
| Mn2As2O5 | 820 | 900 | 440 | 580 | - | [36] |
| Material | Na1.5MnII3MnIII0.5(AsO4)3 | Na1.5MnII3FeIII0.5(AsO4)3 | ||
|---|---|---|---|---|
| Element | Experimental | Theoretical | Experimental | Theoretical |
| Manganese | 20.27% | 17.5% | 16.75% | 15% |
| Arsenic | 16.34% | 15% | 17.28% | 15% |
| Silicon | 1.38% | - | - | - |
| Sodium | 9.33% | 7.5% | 8.42% | 7.5% |
| Oxygen | 52.67% | 60% | 54.55% | 60% |
| Iron | - | - | 3% | 2.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezgui, E.; Ouerfelli, N.; Gavinho, S.R.; Carvalho, J.P.F.; Graça, M.P.F.; Teixeira, S.S. Crystal Structure, Ionic Conductivity, Dielectric Properties and Electrical Conduction Mechanism of the Wyllieites Na1.5Mn3.5(AsO4)3 and Na1.5Mn3Fe0.5(AsO4)3. Crystals 2023, 13, 1251. https://doi.org/10.3390/cryst13081251
Rezgui E, Ouerfelli N, Gavinho SR, Carvalho JPF, Graça MPF, Teixeira SS. Crystal Structure, Ionic Conductivity, Dielectric Properties and Electrical Conduction Mechanism of the Wyllieites Na1.5Mn3.5(AsO4)3 and Na1.5Mn3Fe0.5(AsO4)3. Crystals. 2023; 13(8):1251. https://doi.org/10.3390/cryst13081251
Chicago/Turabian StyleRezgui, Eya, Najoua Ouerfelli, S. R. Gavinho, J. P. F. Carvalho, M. P. F. Graça, and S. Soreto Teixeira. 2023. "Crystal Structure, Ionic Conductivity, Dielectric Properties and Electrical Conduction Mechanism of the Wyllieites Na1.5Mn3.5(AsO4)3 and Na1.5Mn3Fe0.5(AsO4)3" Crystals 13, no. 8: 1251. https://doi.org/10.3390/cryst13081251





