The Influence of Substitutional Defects of Transition Metal Elements on the Stability and Thermal Properties of Al at Finite Temperatures: A First-Principles Study
Abstract
:1. Introduction
2. Computational Detail
3. Result and Discussion
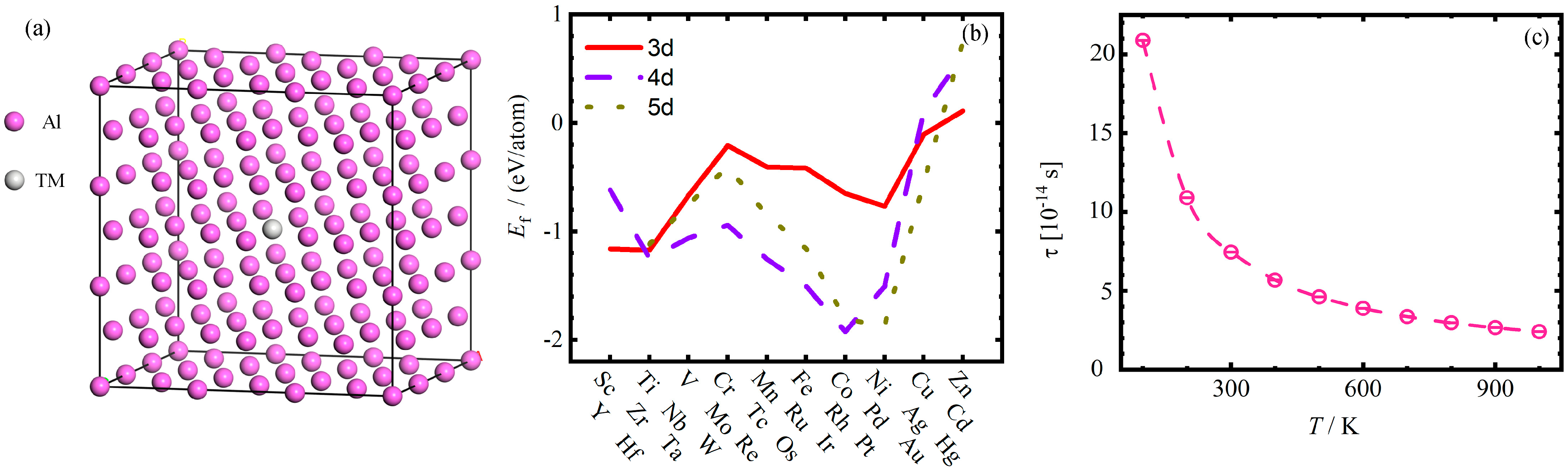
3.1. Defective Formation Energy
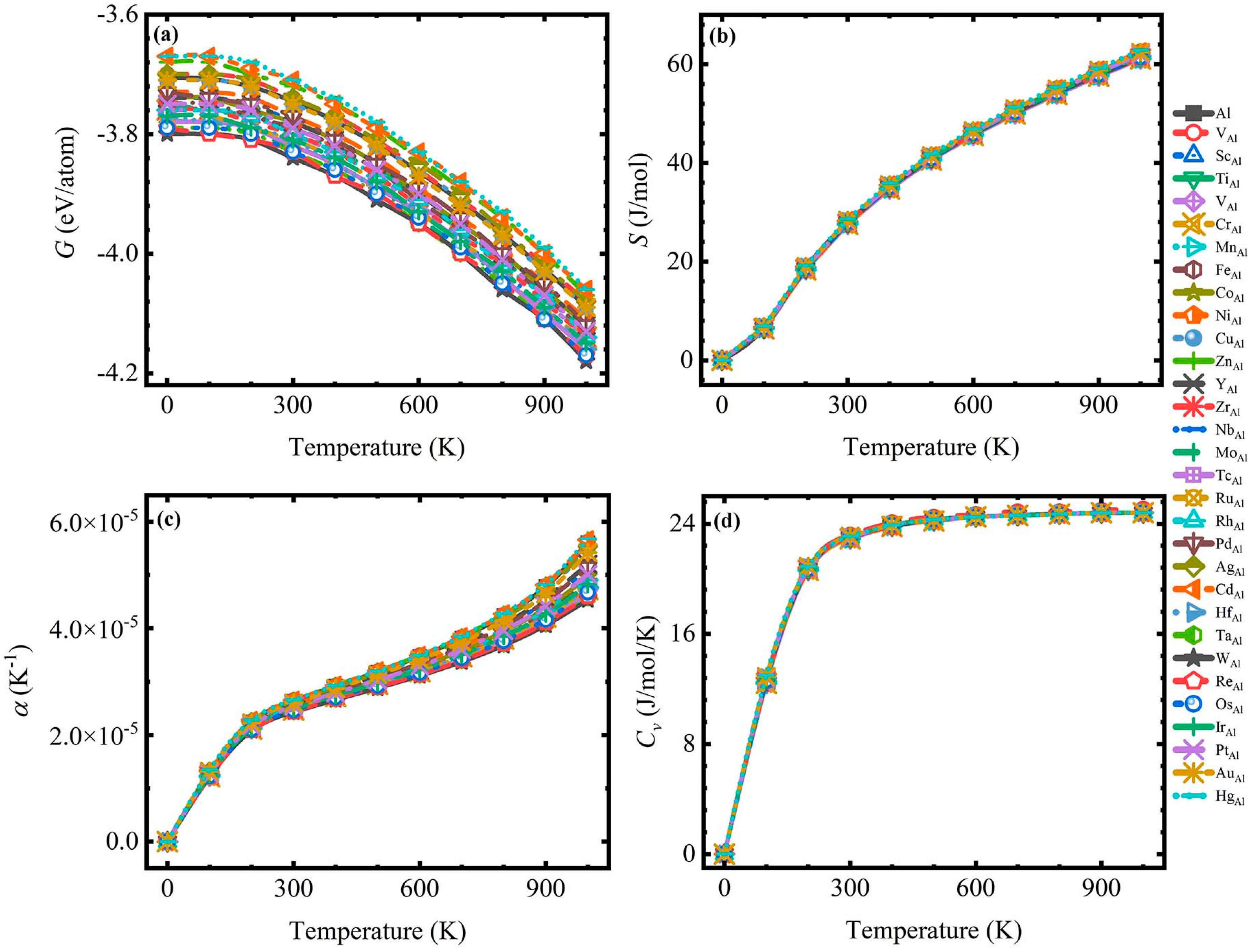
3.2. The Thermodynamic Properties
3.3. Thermal Conductivity
3.4. The Effect of 1 at.% TM Added on Thermal Conductivity
4. Conclusions
- The equilibrium lattice constant a0 of Al-TMAl supercells changes very little, while the defect formation energy of substitution defects TMAl on the Al matrix exhibits a similar “W”-shaped periodic change with the increase in atomic number for 3d–5d TM elements. The Ef of TMAl for both groups IB and IIB is greater than 0, indicating that these two subgroup elements are more difficult to form substitution defects in Al with. It is speculated that the valence electron structures of the elements play a crucial role.
- The thermodynamic property parameters calculated with the Debye theory show that the addition of TM atoms can effectively reduce the thermal expansion coefficient of the material without changing the stability of the Al system, which provides support for the application of Al in the field of high-temperature precision instruments.
- The ETC κe and the TC κ decrease at the temperature range of 100–200 K and then show a small variation. Moreover, because the lattice thermal conductivity κl has little effect, the electron TC κe and total TC of Al-TMAl κ have the same linear increase trends with temperature. But all are slightly lower than that of a pure aluminum matrix.
- At 300 K and 600 K, the total TC κ and the ETC κe for 3d TM increase slowly from Al-ScAl to Al-CuAl and then show a rapid increase to Al-ZnAl with the increase in atomic number. The total TC κ in 4d (Y-Cd) and 5d (Hf-Hg) first drops slowly to Pd and Pt and then rises sharply after reaching the lowest point.
- Finally, we chose to add 1 at.% TM to an Al matrix as L12 s phases and solute atoms at 300 K and 600 K. It was obviously found that the TC of the second-phase-containing Al matrix is slightly lower than that of pure aluminum, while the solute atoms TMAl significantly reduce the thermal conductivity of the Al material. Therefore, the second phase should be favorable and must avoid solid solution atoms in the matrix from the point of view of thermal conductivity.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, K.; Xiong, B.Q.; Fan, Y.Q.; Zhang, Y.A.; Li, Z.H.; Li, X.W.; Wang, F.; Liu, H.W. Transformation and dissolution of second phases during solution treatment of an Al–Zn–Mg–Cu alloy containing high zinc. Rare Met. 2018, 37, 376–380. [Google Scholar] [CrossRef]
- Mulyawan, A.; Terai, T.; Fukuda, T. Interpretation of Fe-rich part of Fe–Al phase diagram from magnetic properties of A2-, B2-, and DO3-phases. J. Alloys Compd. 2020, 834, 155140. [Google Scholar] [CrossRef]
- Oropeza, D.; Hofmann, D.C.; Williams, K.; Firdosy, S.; Bordeenithikasem, P.; Sokoluk, M.; Liese, M.; Liu, J.; Li, X. Welding and additive manufacturing with nanoparticle-enhanced aluminum 7075 wire. J. Alloys Compd. 2020, 834, 154987. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Swaroop, S. Effect of laser shock peening on mechanical and microstructural aspects of 6061-T6 aluminum alloy. J. Mater. Process. Tech. 2020, 282, 116640. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Liu, C.; Zhao, L.; Jiang, W. Structural, elastic, anisotropic and thermodynamic properties of the caged intermetallics RETi2Al20 (RE = La, Ce, Gd and Ho): A first-principles study. Solid State Sci. 2019, 89, 121–129. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Li, Y. Effect of alloying elements on thermal conductivity of aluminum. J. Mater. Res. 2023, 38, 2049–2058. [Google Scholar] [CrossRef]
- Chen, J.K.; Hung, H.Y.; Wang, C.F.; Tang, N.K. Thermal and electrical conductivity in Al–Si/Cu/Fe/Mg binary and ternary Al alloys. J. Mater. Sci. 2015, 50, 5630–5639. [Google Scholar] [CrossRef]
- Gan, J.Q.; Huang, Y.J.; Cheng, W.E.; Jun, D.U. Effect of Sr modification on microstructure and thermal conductivity of hypoeutectic Al−Si alloys. Trans. Nonferrous Met. Soc. China 2020, 30, 2879–2890. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Zhang, B.; Zhang, X.; Ma, M.; Liu, R. Dynamic precipitation-induced simultaneous enhancement of the strength and plasticity of hot-rolled Zr–9Al alloy. J. Alloys Compd. 2020, 829, 154577. [Google Scholar]
- Li, M.; Li, H.; Zhang, Z.; Shi, W.; Liu, J.; Hu, Y.; Wu, Y. Effect of precipitates on properties of cold-rolled Al–Mg–Si–Sc–Zr alloy with higher temperature aging. Mater. Sci. Technol. 2018, 34, 1246–1251. [Google Scholar] [CrossRef]
- Ohara, S.; Chen, G.; Sakamoto, I. Effect of pressure on transport properties of mixed-valence compound YbAl3. J. Alloys Compd. 2001, 323, 632–635. [Google Scholar] [CrossRef]
- Taendl, J.; Orthacker, A.; Amenitsch, H.; Kothleitner, G.; Poletti, C. Influence of the degree of scandium supersaturation on the precipitation kinetics of rapidly solidified Al-Mg-Sc-Zr alloys. Acta Mater. 2016, 117, 43–50. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Qu, F.; Wang, M.; Wang, W.; Bao, Y.; Lai, X. Effects of scandium addition on microstructure, mechanical and thermal properties of cast Be-Al alloy. J. Alloys Compd. 2018, 737, 655–664. [Google Scholar] [CrossRef]
- Suwanpreecha, C.; Pandee, P.; Patakham, U.; Limmaneevichitr, C. New generation of eutectic Al-Ni casting alloys for elevated temperature services. Mater. Sci. Eng. A 2018, 709, 46–54. [Google Scholar] [CrossRef]
- Dorin, T.; Ramajayam, M.; Lamb, J.; Langan, T. Effect of Sc and Zr additions on the microstructure/strength of Al-Cu binary alloys. Mater. Sci. Eng. A 2017, 707, 58–64. [Google Scholar] [CrossRef]
- Tian, T.; Wang, X.; Li, W. Ab initio calculations on elastic properties in L12 structure Al3X and X3Al-type (X=transition or main group metal) intermetallic compounds. Solid State Commun. 2013, 156, 69–75. [Google Scholar] [CrossRef]
- Seidman, D.N.; Marquis, E.A.; Dunand, D.C. Precipitation strengthening at ambient and elevated temperatures of heat-treatable Al(Sc) alloys. Acta Mater. 2002, 50, 4021–4035. [Google Scholar] [CrossRef]
- Iwamura, S.; Miura, Y. Loss in Coherency and Coarsening Behavior of Al3Sc precipitates. Acta Mater. 2004, 52, 591–600. [Google Scholar] [CrossRef]
- Mikhaylovskaya, A.; Mochugovskiy, A.; Levchenko, V.; Tabachkova, N.; Mufalo, W.; Portnoy, V. Precipitation behavior of L12 Al3Zr phase in Al-Mg-Zr alloy. Mater. Charact. 2018, 139, 30–37. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Chen, D.; Wu, Y.; Wang, M.; Ma, N.; Wang, H. First-principles investigation of thermodynamic, elastic and electronic properties of Al3V and Al3Nb intermetallics under pressures. J. Appl. Phys. 2015, 117, 085904. [Google Scholar] [CrossRef]
- Wen, S.P.; Wang, W.; Zhao, W.H.; Wu, X.L.; Gao, K.Y.; Huang, H.; Nie, Z.R. Precipitation hardening and recrystallization behavior of AlMgErZr alloys. J. Alloys Compd. 2016, 687, 143–151. [Google Scholar] [CrossRef]
- Lin, J.D.; Okle, P.; Dunand, D.C.; Seidman, D.N. Effects of Sb micro-alloying on precipitate evolution and mechanical properties of a dilute Al-Sc-Zr alloy. Mater. Sci. Eng. A 2017, 680, 64–74. [Google Scholar] [CrossRef]
- Vo, N.Q.; Dunand, D.C.; Seidman, D.N. Improving aging and creep resistance in a dilute Al–Sc alloy by microalloying with Si, Zr and Er. Acta Mater. 2014, 63, 73–85. [Google Scholar] [CrossRef]
- Clouet, E.; Barbu, A.; Laé, L.; Martin, G. Precipitation kinetics of Al3Zr and Al3Sc in aluminum alloys modeled with cluster dynamics. Acta Mater. 2005, 53, 2313–2325. [Google Scholar] [CrossRef]
- Hyde, K.; Norman, A.; Prangnell, P. The effect of cooling rate on the morphology of primary Al3Sc intermetallic particles in Al–Sc alloys. Acta Mater. 2001, 49, 1327–1337. [Google Scholar] [CrossRef]
- Lee, S.; Utsunomiya, A.; Akamatsu, H.; Neishi, K.; Furukawa, M.; Horita, Z.; Langdon, T. Influence of scandium and zirconium on grain stability and superplastic ductilities in ultrafine-grained Al–Mg alloys. Acta Mater. 2002, 50, 553–564. [Google Scholar] [CrossRef]
- Oku, M.; Shishido, T.; Sun, Q.; Nakajima, K.; Kawazoe, Y.; Wagatsuma, K. Comparison of electronic structures of ScAl3 and ScRh3: X-ray photoelectron spectroscopy and ab initio band calculation. J. Alloys Compd. 2003, 358, 264–267. [Google Scholar] [CrossRef]
- Jahnátek, M.; Krajčí, M.; Hafner, J. Interatomic bonding, elastic properties, and ideal strength of transition metal aluminides: A case study for Al3(V, Ti). Phys. Rev. B 2005, 71, 024101. [Google Scholar] [CrossRef]
- Radmilovic, V.; Ophus, C.; Marquis, E.A.; Rossell, M.D.; Tolley, A.; Gautam, A.; Asta, M.; Dahmen, U. Highly monodisperse core–shell particles created by solid-state reactions. Nat. Mater. 2011, 10, 710–715. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, C.; Huang, H. Mechanical properties of defective L12-Al3X (X= Sc, Lu) phase: A first-principles study. J. Rare Earths 2021, 39, 217–224. [Google Scholar] [CrossRef]
- Hu, W.-C.; Liu, Y.; Li, D.-J.; Zeng, X.-Q.; Xu, C.-S. Mechanical and thermodynamic properties of Al3Sc and Al3Li precipitates in Al–Li–Sc alloys from first-principles calculations. Physica B 2013, 427, 85–90. [Google Scholar] [CrossRef]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Nakamura, M.; Kimura, K. Elastic constants of TiAl3 and ZrAl3 single crystals. J. Mater. Sci. 1991, 26, 2208–2214. [Google Scholar] [CrossRef]
- Umakoshi, Y.; Yamaguchi, M.; Sakagami, T.; Yamane, T. Oxidation resistance of intermetallic compounds Al3Ti and TiAl. J. Mater. Sci. 1989, 24, 1599–1603. [Google Scholar] [CrossRef]
- Aguilar-Virgen, J.; Cabrera, A.; Umemoto, M.; Calderón, H. Compressive mechanical properties of nanostructured intermetallic alloys Al3Ti-X (X = Mn or Fe). Mater. Sci. Forum 2006, 509, 63–68. [Google Scholar] [CrossRef]
- Fu, Y.; Shi, R.; Zhang, J.; Sun, J.; Hu, G. Microstructure and mechanical behavior of a multiphase Al3Ti-based intermetallic alloy. Intermetallics 2000, 8, 1251–1256. [Google Scholar] [CrossRef]
- Guan, R.; Shen, Y.; Zhao, Z.; Wang, X. A high-strength, ductile Al-0.35 Sc-0.2 Zr alloy with good electrical conductivity strengthened by coherent nanosized-precipitates. J. Mater. Sci. Technol. 2017, 33, 215–223. [Google Scholar] [CrossRef]
- Wen, S.P.; Xing, Z.B.; Huang, H.; Li, B.L.; Wang, W.; Nie, Z.R. The effect of erbium on the microstructure and mechanical properties of Al-Mg-Mn-Zr alloy. Mater. Sci. Eng. A 2009, 516, 42–49. [Google Scholar] [CrossRef]
- Xiaoyuan, W.E.; Huang, H.; Ziyong, C.H.; Wei, W.A.; Congying, L.I.; Zuoren, N.I. Microstructure and mechanical properties of Al-Mg-Mn-Zr-Er weld joints filled with Al-Mg-Mn-Zr and Al-Mg-Mn-Zr-Er weld wires. J. Rare Earths 2010, 28, 627–630. [Google Scholar]
- Wen, S.; Gao, K.; Li, Y.; Huang, H.; Nie, Z. Synergetic effect of Er and Zr on the precipitation hardening of Al-Er-Zr alloy. Scr. Mater. 2011, 65, 592–595. [Google Scholar] [CrossRef]
- Koutná, N.; Erdely, P.; Zöhrer, S.; Franz, R.; Du, Y.; Liu, S.; Mayrhofer, P.H.; Holec, D. Experimental chemistry and structural stability of AlNb3 enabled by antisite defects formation. Materials 2019, 12, 1104. [Google Scholar] [CrossRef] [PubMed]
- Valkov, S.; Petrov, P.; Lazarova, R.; Bezdushnyi, R.; Dechev, D. Formation and characterization of Al–Ti–Nb alloys by electron-beam surface alloying. Appl. Surf. Sci. 2016, 389, 768–774. [Google Scholar] [CrossRef]
- Nong, Z.; Zhu, J.; Yang, X.; Cao, Y.; Lai, Z.; Liu, Y. The mechanical, thermodynamic and electronic properties of Al3Nb with DO22 structure: A first-principles study. Physica B 2012, 407, 3555–3560. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, T.; Ma, H.; Li, D.; Ying, C.; Liu, C.; Wang, F. A first principles investigation on the influence of transition-metal elements on the structural, mechanical, and anisotropic properties of CaM2Al20 intermetallics. J. Mol. Graph. Model. 2020, 96, 107509. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Todorova, T.Z.; Zwanziger, J.W. Temperature dependent lattice misfit and coherency of Al3X (X = Sc, Zr, Ti and Nb) particles in an Al matrix. Acta Mater. 2015, 89, 109–115. [Google Scholar] [CrossRef]
- Tzeng, Y.-C.; Chung, C.-Y.; Chien, H.-C. Effects of trace amounts of Zr and Sc on the recrystallization behavior and mechanical properties of Al-4.5 Zn-1.6 Mg alloys. Mater. Lett. 2018, 228, 270–272. [Google Scholar] [CrossRef]
- Krug, M.E.; Mao, Z.; Seidman, D.N.; Dunand, D.C. Comparison between dislocation dynamics model predictions and experiments in precipitation-strengthened Al–Li–Sc alloys. Acta Mater. 2014, 79, 382–395. [Google Scholar] [CrossRef]
- Sheng, B.; Niu, M.; Shao, X. Conductivity and magnetic properties study on doped semiconductor material of 3C-SiC: A first-principle investigation. In Proceedings of the IEEE International Conference on Electric Information and Control Engineering, Wuhan, China, 15–17 April 2011; pp. 5758–5761. [Google Scholar]
- Majid, A.; Rani, N.; Khan, S.U.-D.; Almutairi, Z.A. First principles study of structural, electronic and magnetic properties of transition metals doped SiC monolayers for applications in spintronics. J. Magn. Magn. Mater. 2020, 503, 166648. [Google Scholar] [CrossRef]
- Cao, Z.; Jin, N.; Ye, J.; Du, X.; Liu, Y. First-principles study on the effects of N and Al doping on the mechanical properties and electronic structures of TiC. RSC Adv. 2020, 10, 36295–36302. [Google Scholar] [CrossRef]
- Rajasekar, P.; Umarji, A.M. Effect of Al-doping on suppression of thermal conductivity in Si dispersed β-FeSi2. Intermetallics 2017, 89, 57–64. [Google Scholar] [CrossRef]
- Vandersluis, E.; Lombardi, A.; Ravindran, C.; Bois-Brochu, A.; Chiesa, F.; MacKay, R. Factors influencing thermal conductivity and mechanical properties in 319 Al alloy cylinder heads. Mater. Sci. Eng. A 2015, 648, 401–411. [Google Scholar] [CrossRef]
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
- Maruhn, J.A.; Reinhard, P.G.; Suraud, E. Density Functional Theory; Plenum Press: New York, NY, USA, 2010. [Google Scholar]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Feynman, R.P. Forces in Molecules. Phys. Rev. 1939, 56, 340. [Google Scholar] [CrossRef]
- Madsen, G.K.; Carrete, J.; Verstraete, M.J. BoltzTraP2, a program for interpolating band structures and calculating semi-classical transport coefficients. Comput. Phys. Commun. 2018, 231, 140–145. [Google Scholar] [CrossRef]
- Debye, P. Zur theorie der spezifischen wärmen. Annalen der Physik 1912, 344, 789–839. [Google Scholar] [CrossRef]
- Khenioui, Y.; Boulechfar, R.; Maazi, N.; Ghemid, S. FP-LAPW investigation of Al3(Sc1xTix) alloys properties in L12 and D022 structures. Int. J. Mod. Phys. B 2018, 32, 1850167. [Google Scholar] [CrossRef]
- Tao, X.; Ouyang, Y.; Liu, H.; Zeng, F.; Feng, Y.; Jin, Z. Calculation of the thermodynamic properties of B2 AlRE (RE = Sc, Y, La, Ce–Lu). Physica B 2007, 399, 27–32. [Google Scholar] [CrossRef]
- Fu, H.; Zhao, Z.; Liu, W.; Peng, F.; Gao, T.; Cheng, X. Ab initio calculations of elastic constants and thermodynamic properties of γTiAl under high pressures. Intermetallics 2010, 18, 761–766. [Google Scholar] [CrossRef]
- Zhang, S.B.; Northrup, J.E. Chemical potential dependence of defect formation energies in GaAs: Application to Ga self-diffusion. Phys. Rev. Lett. 1991, 67, 2339. [Google Scholar] [CrossRef]
- Dong, T.H.; Zhang, X.D.; Yang, L.M.; Wang, F. An effect of structural vacancies on the lattice vibration, mechanical, electronic and thermodynamic properties of Cr5BSi3. Chin. Phys. B 2021, 31, 026101. [Google Scholar] [CrossRef]
- Qiu, R.; Lu, H.; Ao, B.; Huang, L.; Tang, T.; Chen, P. Energetics of intrinsic point defects in aluminium via orbital-free density functional theory. Philos. Mag. 2017, 97, 2164–2181. [Google Scholar] [CrossRef]
- Bandyopadhyay, J.; Gupta, K. Low temperature lattice parameters of Al and Al-Zn alloys and Grüneisen parameter of Al. Cryogenics 1978, 18, 54–55. [Google Scholar] [CrossRef]
- Ehrhart, P. Atomic Defects in Metals; Springer: Berlin/Heidelberg, Germany, 1991. [Google Scholar]
- Carling, K.; Wahnström, G.; Mattsson, T.R.; Mattsson, A.E.; Sandberg, N.; Grimvall, G. Vacancies in Metals: From First-Principles Calculations to Experimental Data. Phys. Rev. Lett. 2000, 85, 3862. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.R.; Zeng, Z.Y.; Liu, Z.L.; Cai, L.C.; Jing, F.Q. Elastic anisotropy of ε-Fe under conditions at the Earth’s inner core. Phys. Rev. B 2011, 83, 132102. [Google Scholar] [CrossRef]
- Deng, L.; Liu, X.; Liu, H.; Dong, J. High-pressure phase relations in the composition of albite NaAlSi3O8 constrained by an ab initio and quasi-harmonic Debye model, and their implications. Earth Planet. Sci. Lett. 2010, 298, 427–433. [Google Scholar] [CrossRef]
- Errandonea, D.; Kumar, R.S.; Gracia, L.; Beltran, A.; Achary, S.N.; Tyagi, A.K. Experimental and theoretical investigation of ThGeO4 at high pressure. Phys. Rev. B 2009, 80, 094101. [Google Scholar] [CrossRef]
- Chen, Y.; Hammerschmidt, T.; Pettifor, D.; Shang, J.-X.; Zhang, Y. Influence of vibrational entropy on structural stability of Nb–Si and Mo–Si systems at elevated temperatures. Acta Mater. 2009, 57, 2657–2664. [Google Scholar] [CrossRef]
- Ayad, M.; Belkharroubi, F.; Boufadi, F.Z.; Khorsi, M.; Zoubir, M.K.; Ameri, M.; Ameri, I.; Al-Douri, Y.; Bidai, K.; Bensaid, D. First-principles calculations to investigate magnetic and thermodynamic properties of new multifunctional full-Heusler alloy Co2TaGa. Indian J. Phys. 2020, 94, 767–777. [Google Scholar] [CrossRef]
- Hadji, S.; Bouhemadou, A.; Haddadi, K.; Cherrad, D.; Khenata, R.; Bin-Omran, S.; Al-Douri, Y. Elastic, electronic, optical and thermodynamic properties of Ba3Ca2Si2N6 semiconductor: First-principles predictions. Phys. B Condens. Matter 2020, 589, 412213. [Google Scholar] [CrossRef]
- Anderson, O. Determination and some uses of isotropic elastic constants of polycrystalline aggregates using single-crystal data. In Physical Acoustics; Elsevier: Amsterdam, The Netherlands, 1965; pp. 43–95. [Google Scholar]
- Mallick, P.K. Materials, Design and Manufacturing for Lightweight Vehicles; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Takahashi, K.; Kuwahara, H.; Kawasaki, N.; Obata, T.; Sugawa, E. Enhancement of Thermal Contact Conductance Between Metal Surfaces in an Induction Motor. J. Enhanc. Heat Transf. 2001, 8, 201–213. [Google Scholar] [CrossRef]
- Jain, A.; McGaughey, A.J.H. Thermal transport by phonons and electrons in aluminum, silver, and gold from first principles. Phys. Rev. B 2016, 93, 081206. [Google Scholar] [CrossRef]
- Jossou, E.; Malakkal, L.; Szpunar, B.; Oladimeji, D.; Szpunar, J.A. A first principles study of the electronic structure, elastic and thermal properties of UB2. J. Nucl. Mater. 2017, 490, 41–48. [Google Scholar] [CrossRef]
- Togo, A.; Chaput, L.; Tanaka, I.; Hug, G. First-principles phonon calculations of thermal expansion in Ti3SiC2, Ti3AlC2, and Ti3GeC2. Phys. Rev. B 2010, 81, 174301. [Google Scholar] [CrossRef]
- Wang, Y.; Ohishi, Y.; Kurosaki, K.; Muta, H. First-principles calculation study of Mg2XH6 (X = Fe, Ru) on thermoelectric properties. Mater. Res. Express 2019, 6, 085536. [Google Scholar] [CrossRef]
- Slack, G.A. Nonmetallic crystals with high thermal conductivity. J. Phys. Chem. Solids 1973, 34, 321–335. [Google Scholar] [CrossRef]
- Madsen, G.K.; Singh, D.J. BoltzTraP. A code for calculating band-structure dependent quantities. Comput. Phys. Commun. 2006, 175, 67–71. [Google Scholar] [CrossRef]
- Yadav, M.K.; Sanyal, B. First principles study of thermoelectric properties of Li-based half-Heusler alloys. J. Alloys Compd. 2015, 622, 388–393. [Google Scholar] [CrossRef]
- Mubashir, S.; Butt, M.K.; Yaseen, M.; Iqbal, J.; Iqbal, M.; Murtaza, A.; Laref, A. Pressure induced electronic, optical and thermoelectric properties of cubic BaZrO3: A first principle Calculations. Opt. Int. J. Light Electron Opt. 2021, 239, 166694. [Google Scholar] [CrossRef]
- Remil, G.; Zitouni, A.; Bouadjemi, B.; Houari, M.; Abbad, A.; Benstaali, W.; Cherid, S.; Matougui, M.; Lantri, T.; Bentata, S. A potential full Heusler thermoelectric material CO2ZrZ (Z =Al, Si, Ga and Sn) in low temperature: An Ab-initio investigation. Solid State Commun. 2021, 336, 114422. [Google Scholar] [CrossRef]
- Brandt, R.; Neuer, G. Electrical Resistivity and Thermal Conductivity of Pure Aluminum and Aluminum Alloys up to and above the Melting Temperature. Int. J. Thermophys. 2007, 28, 1429–1446. [Google Scholar] [CrossRef]
- Powell, R.W. Correlation of metallic thermal and electrical conductivities for both solid and liquid phases. Int. J. Heat Mass Transf. 1965, 8, 1033–1045. [Google Scholar] [CrossRef]
- De Lang, H.N.; van Kempen, H.; Wyder, P. The lattice thermal conductivity of very pure aluminium. J. Phys. F Met. Phys. 1978, 8, L39. [Google Scholar] [CrossRef]
- Ahmad, S.; Mahanti, S.D. Energy and temperature dependence of relaxation time and Wiedemann-Franz law on PbTe. Phys. Rev. B 2010, 81, 165203. [Google Scholar] [CrossRef]
- Choi, G.; Kim, H.S.; Lee, K.; Park, S.H.; Cha, J.; Chung, I.; Lee, W.B. Study on thermal conductivity and electrical resistivity of Al-Cu alloys obtained by Boltzmann transport equation and first-principles simulation: Semi-empirical approach. J. Alloys Compd. 2017, 727, 1237–1242. [Google Scholar] [CrossRef]
- Wen, B.; Feng, X. Thermal conductivity of metal from first principles calculations and its application in aluminum. J. Yanshan Univ. 2015, 39, 298–306. [Google Scholar]


| Systems | Ef | a0 |
|---|---|---|
| Al | -- | 4.039; 4.039 [66]; 3.983 [66]; 4.032 [67] |
| VAl | 0.68; 0.67 [66]; 0.73 [66]; 0.67 [68] 0.54 [69]; 0.70 [69] | 4.035 |
| ScAl | −1.16 | 4.045 |
| TiAl | −1.17 | 4.038 |
| VAl | −0.67 | 4.035 |
| CrAl | −0.21 | 4.032 |
| MnAl | −0.41 | 4.032 |
| FeAl | −0.41 | 4.032 |
| CoAl | −0.65 | 4.031 |
| NiAl | −0.76 | 4.033 |
| CuAl | −0.10 | 4.035 |
| ZnAl | 0.11 | 4.038 |
| YAl | −0.62 | 4.052 |
| ZrAl | −1.25 | 4.044 |
| NbAl | −1.06 | 4.039 |
| MoAl | −0.94 | 4.036 |
| TcAl | −1.26 | 4.033 |
| RuAl | −1.50 | 4.032 |
| RhAl | −1.92 | 4.033 |
| PdAl | −1.51 | 4.036 |
| AgAl | 0.10 | 4.039 |
| CdAl | 0.63 | 4.045 |
| HfAl | −1.12 | 4.043 |
| TaAl | −0.75 | 4.039 |
| WAl | −0.40 | 4.035 |
| ReAl | −0.85 | 4.033 |
| OsAl | −1.16 | 4.032 |
| IrAl | −1.81 | 4.033 |
| PtAl | −1.87 | 4.034 |
| AuAl | −0.55 | 4.039 |
| HgAl | 0.74 | 4.045 |
| κl | κe | κ | |
|---|---|---|---|
| Al | 7.38 4.83 [93] | 223.61 | 230.99 190.90 [93]; 240.00 [89] |
| VAl | 7.48 | 178.95 | 186.43 |
| ScAl | 7.71 | 176.13 | 183.85 |
| TiAl | 8.14 | 181.60 | 189.75 |
| VAl | 8.22 | 177.43 | 185.65 |
| CrAl | 8.20 | 178.41 | 186.61 |
| MnAl | 8.15 | 176.13 | 184.28 |
| FeAl | 8.06 | 178.67 | 186.73 |
| CoAl | 7.91 | 177.65 | 185.57 |
| NiAl | 7.69 | 183.41 | 191.10 |
| CuAl | 7.44 | 184.12 | 191.55 |
| ZnAl | 7.28 | 205.54 | 212.82 |
| YAl | 7.53 | 161.69 | 169.21 |
| ZrAl | 8.04 | 158.06 | 166.09 |
| NbAl | 8.24 | 158.30 | 166.54 |
| MoAl | 8.30 | 162.37 | 170.67 |
| TcAl | 8.25 | 153.81 | 162.06 |
| RuAl | 8.12 | 150.51 | 158.62 |
| RhAl | 7.92 | 153.73 | 161.65 |
| PdAl | 7.65 | 140.95 | 148.60 |
| AgAl | 7.33 | 144.98 | 152.30 |
| CdAl | 7.21 | 194.94 | 202.15 |
| HfAl | 7.97 | 175.14 | 183.10 |
| TaAl | 8.21 | 174.88 | 183.09 |
| WAl | 8.28 | 162.70 | 170.98 |
| ReAl | 8.20 | 158.10 | 166.30 |
| OsAl | 8.11 | 151.66 | 159.76 |
| IrAl | 7.92 | 142.88 | 150.80 |
| PtAl | 7.67 | 115.51 | 123.19 |
| AuAl | 7.34 | 127.02 | 134.36 |
| HgAl | 7.09 | 191.10 | 198.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, T.; Lin, L.; Ruan, Z.; Fan, T.; Wu, Y.; Chen, D. The Influence of Substitutional Defects of Transition Metal Elements on the Stability and Thermal Properties of Al at Finite Temperatures: A First-Principles Study. Crystals 2024, 14, 35. https://doi.org/10.3390/cryst14010035
Ye T, Lin L, Ruan Z, Fan T, Wu Y, Chen D. The Influence of Substitutional Defects of Transition Metal Elements on the Stability and Thermal Properties of Al at Finite Temperatures: A First-Principles Study. Crystals. 2024; 14(1):35. https://doi.org/10.3390/cryst14010035
Chicago/Turabian StyleYe, Tuo, Lan Lin, Zixiong Ruan, Touwen Fan, Yuanzhi Wu, and Dongchu Chen. 2024. "The Influence of Substitutional Defects of Transition Metal Elements on the Stability and Thermal Properties of Al at Finite Temperatures: A First-Principles Study" Crystals 14, no. 1: 35. https://doi.org/10.3390/cryst14010035
APA StyleYe, T., Lin, L., Ruan, Z., Fan, T., Wu, Y., & Chen, D. (2024). The Influence of Substitutional Defects of Transition Metal Elements on the Stability and Thermal Properties of Al at Finite Temperatures: A First-Principles Study. Crystals, 14(1), 35. https://doi.org/10.3390/cryst14010035





