Abstract
Alkaline EDTA solution has been previously identified as an effective leaching agent for solubilising rare-earth oxalates. These oxalates are the product of an oxalic acid conversion leach dissolving monazite and redepositing the salt. Pervious work suggested a significant increase in recovery was observed between pH 8 and 10; we have demonstrated that, in an excess of EDTA, this is not the case, and the dissolution is similar. While demonstrating that, at a nominal solid loading of 100 g/L, 0.2 M EDTA solution produced the highest dissolution, elevated solids require an equivalent increase in lixiviant concentration driven by consumption. Very-high-solution concentrations (>50 g/L dissolved TREEs) were achieved at a high solid loading, indicating both that a solution equilibrium is yet to be reached and that a build-up of oxalate in the system (estimated at ~1 M) does not impact the leach efficiency. We have also demonstrated the recycling of EDTA to use in multiple stages as well as the ability to recover oxalate from this solution.
1. Introduction
Rare-earth elements (REEs) indicate 17 metallic elements, including lanthanides, scandium, and yttrium [1,2]. These elements are classified into light rare-earth elements (LREEs) (lanthanum to gadolinium) and heavy rare-earth elements (HREEs) (yttrium and terbium to lutetium). Scandium is not included in the LREE or HREE classification due to its different properties [3]. Depending on the source, they may be further classified to light, middle, and heavy elements. Rare-earth elements (REEs) are pivotal in modern technologies, with extensive applications in high-strength magnets, electronics, renewable energy, and various high-tech industries [4,5]. Their unique physical properties make the products lighter, stronger, and more conductive. Therefore, rare-earth elements are known as “spice” metals in Germany, as “vitamins” in Japan, and MSG in China [2,6,7]. All rare-earth elements are bound to be found in nature, with the exception of promethium, which is produced by the synthetic method. However, their mineable concentrations are less common, making their extraction and processing a technological challenge and an economic imperative. Therefore, these elements have earned misleading names, e.g., “rareness”, because of their low concentration in the ores and not because of their abundance in the earth.
Monazite, a phosphate mineral containing thorium, uranium, and various rare-earth elements such as cerium (Ce), lanthanum (La), praseodymium (Pr), and neodymium (Nd), is one of the primary sources of these elements [8,9]. Although the concentration of REEs in monazite varies from deposit to deposit, the total REO is between 70 wt% and 95 wt% and the thorium concentration between 4 wt% and 12 wt% [10,11]. As monazite is chemically and thermally stable in its crystalline form, it is difficult to dissolve under acidic or alkali conditions. Therefore, the conventional extraction of REEs from monazite involves intensive energy consumption, highly corrosive conditions, and hazardous gas emission, raising substantial environmental and safety concerns [12,13]. Additionally, separation and purification in the downstream stages are more complex and expensive because of the co-extraction of impurities. As a result, there is growing interest in developing more sustainable and less energy-intensive leaching techniques [14,15,16].
Organic acids are generally less harmful to the ecosystem than inorganic acids because of their weaker acidity and generally low toxicity. Similarly, bioleaching microorganisms are less expensive, environmentally friendly, and more efficient, and they require less energy than the conventional methods. These often generate organic acids naturally, which mobilise nutrients. However, these processes are also slower and can be more difficult to operate. The efficient recovery of REEs has long been a subject of debate, especially in the recycling of waste electrical and electronic equipment (WEEE). Numerous investigations have shown that chelating organic acids exhibit a comparable, if not superior, leaching efficiency relative to inorganic acids, particularly in the extraction of valuable metals [17,18]. Several findings concluded that, in terms of organic acids, the release of rare-earth elements increases on the order of no ligand~ salicylate < phthalate < oxalate < citrate [19]. Additionally, according to the same studies, the release of REEs from apatite and monazite in the presence of low-molecular-weight organic acids such as citrate, oxalate, and phthalate is observed to be enhanced relative to inorganic weathering under equivalent conditions [19]. Additionally, REEs release is more pronounced when an aliphatic ligand is present rather than an aromatic ligand, and the dissolution of minerals is typically increased with an increased concentration of the ligand. Other investigations of a variety of organic ligands revealed that citrate generated the most significant increase in mineral dissolution (at least 40 times compared to samples without ligands), while salicylate had no effect or a diminished effect on dissolution. In general, studies found that when the amount of aliphatic ligand is increased, the RE patterns display the lanthanide contraction effect. The sustainable use of rare-earth resources is important for scientific development and human progress. Although current metallurgical processes allow for high-yield rare earths and high product quality, there is a significant drawback to the current method in terms of chemical and energy demand, and these processes have become a large source of low-grade waste.
Ethylenediaminetetraacetic acid (EDTA) is widely recognised for its role as a chelating agent, particularly in its interactions with rare-earth elements (REEs) and other metals such as calcium and manganese, Figure 1 [20,21]. EDTA acts as a hexadentate ligand, using its four carboxyl and two amine groups to form stable coordinate covalent bonds with metal cations. In aqueous solutions, EDTA can exist in seven different forms depending on pH, with H6Y2 + predominant at low pH levels and Y4 prevailing at higher pH levels. This ability to form strong chelation complexes underscores the potential of EDTA in hydrometallurgical processes, particularly in the extraction and separation of valuable metals.
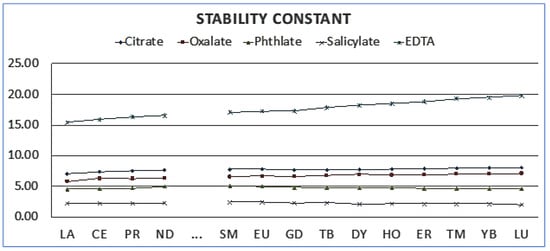
Figure 1.
Stability constant of organic solvents with rare-earth elements.
Research has demonstrated the efficacy of EDTA in various metal extraction processes. For example, Di Palma et al. [22] found that a 0.05 M EDTA solution could efficiently extract copper with a 95% yield and lead with a 98% yield, illustrating its effectiveness as a leaching agent. EDTA-modified oxides have also shown significant adsorption capabilities for heavy metals such as Pb(II), Hg(II), and Cu(II), highlighting their potential in water treatment applications [23]. Further advancements in the field have replaced earlier methods, such as using citric acid, with EDTA, resulting in a more successful elution of lanthanides [24]. Additionally, EDTA has proven effective in leachate recycling, with studies showing the removal of up to 97% Mo, 95% Ni, and 96% V [25].
Research by Liu et al. [26] has highlighted the importance of managing EDTA-Fe(II)-NO and EDTA-Fe(III) concentrations in electrobiofilm reactors, emphasizing the need for precise parameter control to ensure stable operation. Similarly, Zhang et al. [27] and Sun et al. [28] have demonstrated the importance of understanding the interplay between chemical, biological, and electrochemical processes to optimise EDTA performance in environmental and metal recovery applications.
The capacity of EDTA to form stable complexes with metal ions is well documented and plays a pivotal role in improving the extraction of REEs from aqueous solutions. For example, Dupont et al. [29] demonstrated that EDTA-functionalised nanoparticles exhibit a higher affinity for heavier rare-earth ions, due to their smaller ionic radii and higher charge densities, underscoring the importance of selecting the right extractant and concentration for optimal results. Furthermore, complexing agents such as EDTA can significantly improve the separation of rare earths from transition metals, as evidenced by Rout and Binnemans [30], who investigated the solvent extraction of trivalent rare-earth ions. Collectively, these studies suggest that while the effectiveness of EDTA as a chelating agent is well documented, a deeper investigation into its operating conditions is essential to fully harness its potential across diverse applications, including the leaching of REEs.
The dissolution of rare-earth elements (REEs) using EDTA is influenced by several critical parameters, including temperature, solid-to-liquid ratio, EDTA concentration, pH value, and the duration of the leaching process. Each of these parameters can significantly impact the efficiency of REE extraction, making it essential to thoroughly investigate their interactions. Despite the established effectiveness of EDTA, there remains a considerable gap in understanding the specific conditions under which it operates most efficiently. This study addresses this gap by conducting a deeper exploration of the limits of EDTA leaching, with a particular focus on optimising key parameters such as temperature, pH, EDTA concentration, and solid-to-liquid ratio. The novelty of this research lies in its systematic investigation of these underexplored aspects, providing a comprehensive understanding of how to maximise the effectiveness of EDTA in REE extraction. By doing so, this work not only advances the current state of knowledge but also offers a practical framework for optimising EDTA-based leaching processes in industrial applications, ensuring that the use of EDTA is effective and efficient. The findings of this study are expected to not only revolutionise our understanding of the operational aspects of EDTA leaching but also contribute to the development of an environmentally sustainable method for REE extraction in practice.
The recovery of rare-earth elements (REEs) from monazite concentrate involves a multistep process designed to maximise extraction efficiency and purity and to remove imporities. The process begins with the conversion of monazite concentrate to rare-earth oxalate using oxalic acid. The formation of the tricomplex can be characterised by a series of equilibrium reactions, as described by Crouthamel and Martin Jr [31]. The first equilibrium involves the dissolution of the trioxalate complex into a dioxalate complex and an oxalate ion:
The second equilibrium further dissociates the dioxalate complex into a monooxalate complex and another oxalate ion:
Additionally, the solid rare-earth oxalate can dissociate directly into rare-earth ions and oxalate ions:
Overall, the conversion of monazite concentrate to rare-earth oxalate can be summarised by the following reaction:
After the formation of rare-earth oxalate, a subsequent leaching step involves the use of EDTA (ethylenediaminetetraacetic) at a pH of 10 to extract the rare-earth elements. At this pH, EDTA is fully deprotonated (EDTA4−), maximising the chelating efficiency. The general reaction for this leaching process can be represented as follows:
The leaching process can be characterised by a series of equilibrium reactions, where rare-earth elements transition through various coordinated states with EDTA. Initially, REEs form a 1:1 complex with EDTA:
The high pH enhances not only the deprotonation of EDTA but also the stabilisation of the formed complexes, improving the efficiency of the leaching process. The molecular structures of oxalic acids, Figure 2, and EDTA, Figure 3, are shown below:
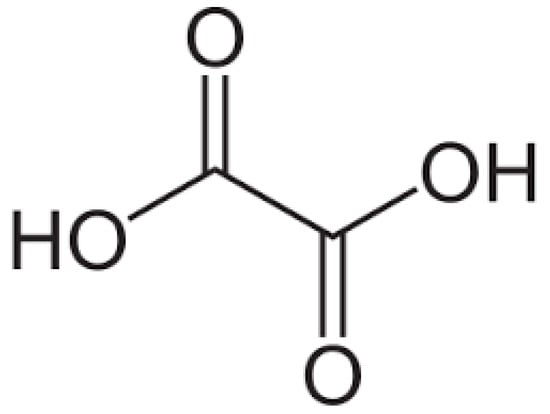
Figure 2.
Molecular structure of oxalic acid.
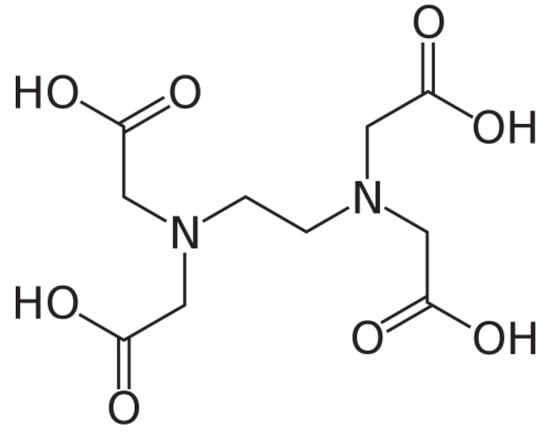
Figure 3.
Molecular structure of EDTA.
2. Experimental
Monazite concentrate was obtained from Lynas Rare Earths Ltd. (Perth, Australia) The concentrate was dried, and the particle size was identified (Figure 4) using a Malvern Mastersizer 3000 Hydro EV. The elemental composition of the monazite concentrate was determined using X-ray fluorescence (XRF) spectroscopy, shown in Table 1. The ethylenediaminetetraacetic acid and other chemicals used were analytical-grade reagents. Sodium hydroxide and sulphuric acid were used to adjust the pH.
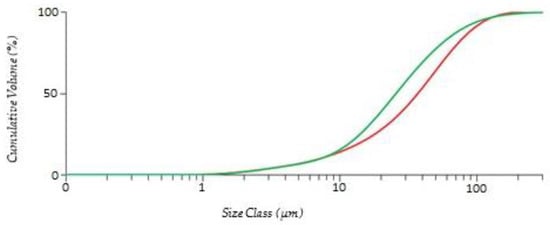
Figure 4.
Distribution of the particle size. Red line is the particle size of monazite concentrates. Green line is the particle size of rare-earth oxalate.

Table 1.
The elemental composition of monazite concentrates.
2.1. Preparation of Rare-Earth-Oxalate-Bearing Feed
Prior to the leaching process, monazite concentrate was subjected to oxalic acid treatment to synthesise rare-earth (RE) oxalates and remove impurities such as phosphorus and iron. The process involves treating the concentrate with 0.8 M oxalic acid in a solid-to-liquid ratio of 0.2, with a pH of 1, and maintaining the mixture at 45 °C for 24 h. During this stage, a selective removal of approximately 60% phosphorus and 10% iron was achieved. The resulting solid residue, mainly consisting of RE oxalates, is enriched with rare-earth elements such as lanthanum (7.71%), cerium (12.40%), praseodymium (1.63%), and neodymium (5.39%), as shown in Table 2, while Figure 5 contains the X-ray diffractogram of the material. This process is crucial for simplifying the subsequent EDTA leaching process by reducing the concentration of interfering ions.

Table 2.
The elemental composition of RE oxalates.
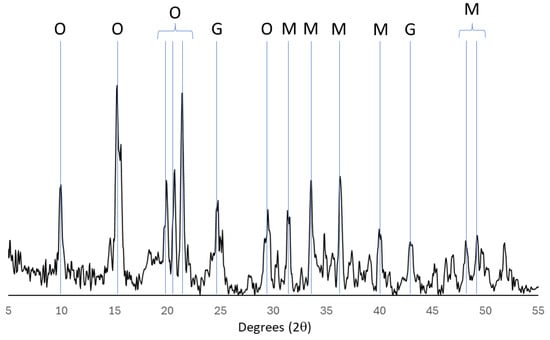
Figure 5.
X-ray diffractogram of oxalic acid leach residue demonstrating characteristic rare-earth oxalate (O) peaks along with the two other predominant primary minerals monazite (M) and Goethite (G).
The oxalic acid stage is indispensable in this process. By directing REEs to their oxalate forms, this step significantly enhances the solubility and reactivity of the elements, thereby facilitating their efficient leaching and extraction in subsequent stages. The experimental data strongly support the necessity of this step, demonstrating that direct leaching without prior conversion to rare-earth oxalate results in markedly inferior recovery efficiencies. Figure 6 below shows the solution concentrations of the elements with EDTA, and the oxalic acid step is omitted.
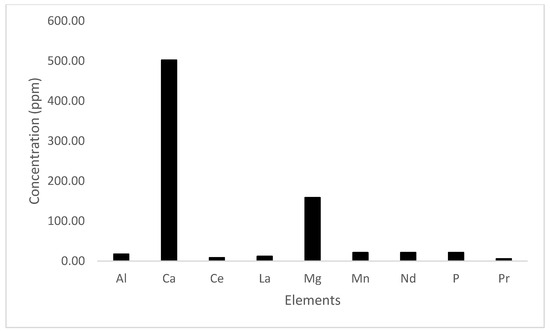
Figure 6.
Dissolution of elements without an oxalic acid step: concentrations (ppm) of various elements recovered from the monazite concentrate directly leached with EDTA without the oxalic acid conversion step. The data show significantly lower recovery efficiencies for key rare-earth elements (Ce, La, Pr, and Nd), highlighting the importance of the oxalic acid step in enhancing the solubility and reactivity of these elements for efficient extraction.
2.2. Leach Testwork
Ethylenediaminetetraacetic acid (EDTA) solutions were prepared by dissolving the appropriate amount of EDTA salts in deionised water, creating solutions ranging from 0.1 M to 1 M. The pH of these solutions was adjusted to 10, using sodium hydroxide.
An extensive series of experiments were conducted to optimise the leaching of rare-earth elements (REEs) using EDTA. Five sets of independent variables were methodically varied: temperature (25 °C to 80 °C), EDTA molarity (ranged from 0.1 M to 1 M), solid-to-liquid ratio (0.1–0.3), pH level (7–13.5), and residence time (up to 6 h). For the temperature-controlled experiments, a precise thermal environment was maintained using Analog Heating Mantle PTHW250 (Melbourne, Australia). The EDTA concentration was methodically adjusted in subsequent tests, keeping all other conditions constant. The solid-to-liquid ratio and pH level were also independently varied to assess their impact on leaching efficiency. Throughout all experiments, a magnetic stirrer set at 550 rpm ensured the homogeneity of the solution. This systematic approach was designed to identify the optimal conditions for maximum REE recovery. The foundational step in the leaching process is the dissolution of rare-earth (RE) oxalate in an EDTA solution. The initial conditions for this stage were established with an EDTA concentration of 0.2 and a solid-to-liquid ratio of 0.1, at 10 pH, and ambient room temperature with a duration of 1 h.
2.3. Sampling and Analysis
The concentration of REEs in leachate was quantified using inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5100 Synchronous Vertical Dual View (Melbourne, Australia)). For solid residue, Rigaku Supermini200 (Tokyo, Japan) wavelength dispersive X-ray fluorescence (XRF) spectroscopy was employed, and powder X-ray diffraction analysis (XRD) was conducted using an Olympus BTX-II diffractometer (Tokyo, Japan). The Olympus Difdata database was used for the identification of monazite (#1-1720) and goethite (#1-4568) seen in this manuscript, while the oxalate and hydroxide species were identified by comparison to salts generated in synthetic systems as no database pattern was available. The efficiency of leaching was calculated on the basis of the concentration of REEs in the leachate relative to their initial concentration.
It should be noted that all solution assays are given in concentration rather than a percentage recovery. This is performed as recovery can be confusing as the maximum achievable value is dependent on the extent of conversion obtained in the oxalic acid stage. As the proportion of conversion is difficult to measure, and there is a disparity in the availability of different rare earths at this stage, we are refraining from discussing calculated recoveries in this article. Also, some tests display exceedingly high-solution concentrations well above those reported in many other systems. This is lost in reporting recovery values.
3. Results and Discussion
The experiments were conducted to determine the dissolution of rare-earth elements using EDTA. Cerium (Ce), lanthanum (La), neodymium (Nd), and praseodymium (Pr) were studied as the most prolific rare-earth elements in the concentrate. The release response of rare-earth elements was evaluated on various operational parameters.
3.1. Temperature
The impact of temperature on the recovery of rare-earth elements (REEs) from rare-earth oxalate can be analysed based on the data presented in Figure 7. The experiments were carried out in various temperatures ranging from 25 °C to 80 °C, while all other variables kept constant; using a S/L of 0.1, EDTA molarity is 0.2 M at 10 pH in 1 h.
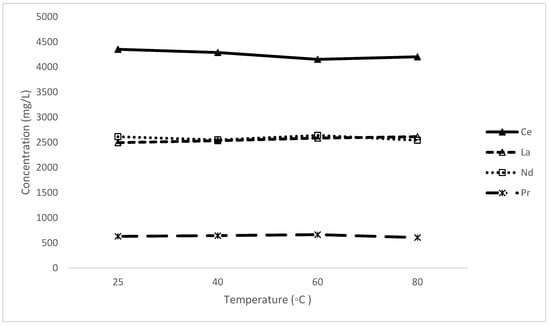
Figure 7.
The effect of temperature on the leaching of rare-earth elements.
The concentration of Ce, La, Nd, and Pr shows a slight variation across the tested temperature rages (25 °C, 40 °C, 60 °C, and 80 °C), indicating that temperature does not significantly impact the leaching process within this range. While the leaching efficiency reached the maximum at room temperature, this value is within experimental error. This suggests that performing the leaching process at room temperature (25 °C) might be as effective as higher temperatures, which can save energy, and, hence, reduce operational costs. This also provides flexibility within a potential operation as the secondary leach can be conducted at the most convenient temperature with respect to its adjacent units.
It is noteworthy that the concentration of Nd in solution is equivalent to that of La across the range of temperature even though its feed concentration is 30% lower in the feed. Upon closer inspection, although all are leached, the proportions of the dissolution of both Nd and Pr are significantly higher than that of Ce and La. This phenomenon may be linked to the slightly higher binding energy of Nd and Pr with the organic molecules used in the two stages or variance in the mineral association of the elements.
3.2. EDTA Concentration
This series of experiments explores the impact of the concentration of EDTA in the leaching reaction. Experiments were conducted at different concentrations of EDTA from 0.1 M to 1 M while all other variables were kept constant; using a S/L 0.1 and pH 10 at room temperature for 1 h (Figure 8).
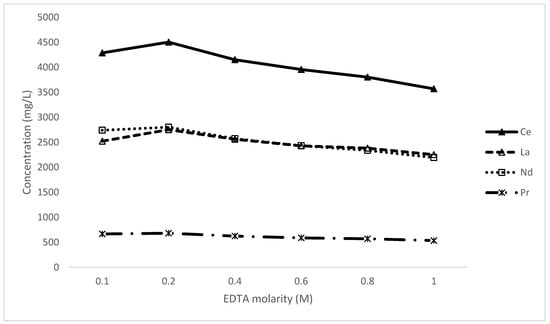
Figure 8.
The impact of EDTA molarity on the leaching of rare-earth elements.
The molarity of EDTA presented a curve with an optimal range of around 0.2 M. While the marginal increase from 0.1 to 0.2 M is logical as the combined dissolved metal concentration is in the order of 0.1 M, thus consuming the lixiviant (based on Equation (5) and 1:1 molar ratio), the decline at higher concentrations is more difficult to rationalise. This phenomenon may be due to a salting-out effect or the formation of more stable solid species; however, it is indicative that a large excess of EDTA is not only expensive but actually detrimental to the performance. Another interesting point of note is that in an EDTA-deficient solution (0.1 M), Nd leached even more preferentially to La than under other conditions, again pointing to a more stable solution complex.
3.3. pH
The pH level is a critical parameter in the leaching efficiency; therefore, the pH of the EDTA solution war varied between 7 and 13.5 while other parameters kept constant (0.2 M of EDTA, S/L:0.1, 1 h residence time at room temperature). Thermodynamic equilibrium analysis demonstrated that the EDTA solution complex is favoured above 7.5 pH [15]. Thus, an alkaline EDTA solution has been proven to be the strongest for releasing rare-earth elements, Figure 9.
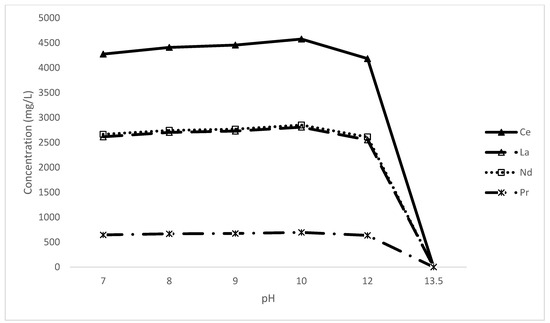
Figure 9.
Presents the pH dependency of REE dissolution.
The leaching efficiency for the extraction of rare-earth elements appears to be relatively unaffected by the pH level within the range of 7 to 12, which is consistent with the findings of other studies [32,33]. As also discussed earlier, this is the window the system favoured. An apparent maximum at pH 10 was observed and thus used for other test work.
Interestingly, the RE oxalate layer has been consumed at the lowest pH without further optimisation. Conversely, a marked decrease in REE concentration is observed at a pH of 13.5. This reduction was anticipated to be attributable to the formation of a hydroxide, precipitating out of solution due to its insolubility in highly alkaline environments. Alternatively, it could remain as an oxalate if the pH was above the effective range of the EDTA itself. The residues of these tests were analysed using XRD to determine whether there were notable differences in the final solids and identify the rare-earth association at pH 13.5 related to the lack of REEs in solution (Figure 10).
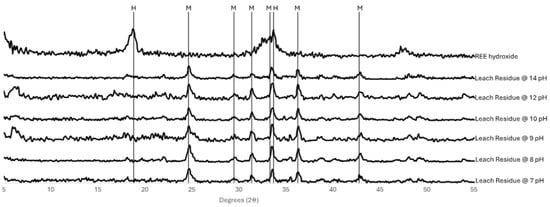
Figure 10.
X-ray diffractograms for the residue samples from the varied pH leach tests and a reference pattern for rare-earth hydroxide. The marked peaks are either labelled M (monazite) or H (hydroxide).
As can be seen, none of the patterns, including that at the highest pH, indicate the formation of a hydroxide. However, nor could an undissolved oxalate compound be identified in the pH 14 pattern. There is in fact little discernible difference between the residues at other pH values that demonstrated high recovery and the pH 14 system that showed no REE dissolution.
3.4. Leaching Time
Previous studies confirmed that the reaction of the EDTA complex is rapid, 5 min. Nevertheless, it is important to examine what happens to the system over time, whether a side reaction occurs and the equilibrium shifts, Figure 11.
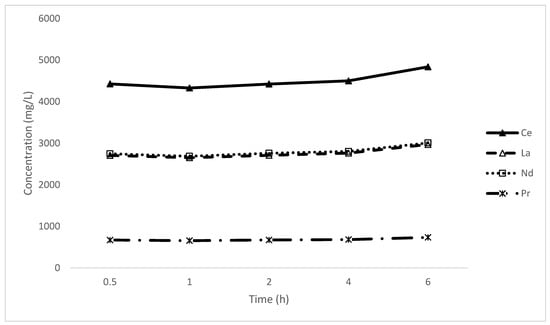
Figure 11.
Leaching of rare-earth elements over time.
The leaching kinetics for the rare-earth elements displayed a constant concentration with the leachate over time, indicating that the leaching process occurs quickly and instantly. This behaviour mirrors observations from previous studies on leaching kinetics, such as the investigation by Palden et al. (2020), which noted that matte-lead leaching achieved maximum efficiency at 6 h, while slag leaching reached its peak at 2 h before stabilisation. Similarly, Forte et al. (2017) and Wojtkowska and Bogacki (2022) have observed rapid leaching reactions when using EDTA [34,35]. Collectively, these findings corroborate the notion that the kinetics of the EDTA leaching reaction tend to be swift, allowing for a rapid and efficient dissolution of targeted metals.
3.5. Solid/Liquid Ratio
The solid/liquid ratio (given as a decimal of the wt/wt ratio) is a key parameter in investigating its impact on the recovery of key rare-earth elements. Experiments were performed with the following content variables: EDTA molarity 0.2 M, pH 10 at room temperature for 1 h, while changing the S/L ratio, Figure 12. During the experiments, it was difficult to go beyond 0.3 S/L ratio as the mixer became thick.
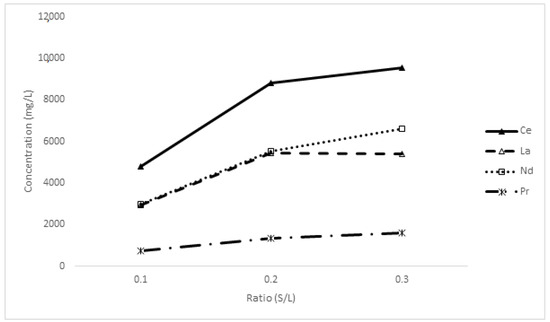
Figure 12.
The relationship between the solid-to-liquid ratio and the dissolution of rare-earth elements.
The results suggest that a comprehensive comparison of the amount of oxalate feed from rare earth and the volume of the EDTA leaching agent are necessary to optimise the leaching process. Given that the molarity of EDTA was kept at 0.2 M throughout the experiments, the recovery of rare-earth elements effectively doubled at 0.2 S/L. In addition to the significantly lower increase in metal dissolution at a ratio of 0.3, this correlates well with the data in Figure 8. Furthermore, the dissolution of Nd and Pr again significantly outperform that of Ce and La in EDTA-deficient conditions.
To further understand the limitations of solid loading under equivalent EDTA concentrations, another set of tests was conducted in which the solid loading was increased simultaneously with the EDTA content. The results are presented in Figure 13 using concentrations equal to the S/L ratio (S/L ratio of 0.6 combined with 0.6 M EDTA solution).
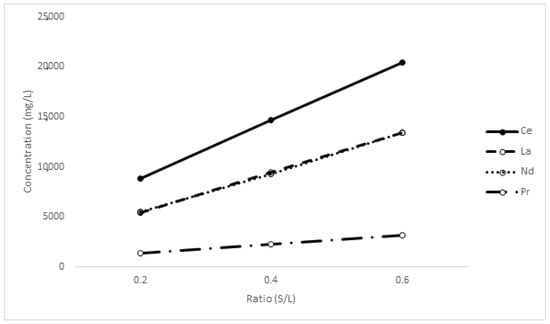
Figure 13.
The recovery of rare-earth elements at different S/L ratios.
Interestingly, the plots are practically linear across the range of solid loadings used. While the increase in metals dissolved from ratios of 0.2 to 0.6 is not exactly three-fold, the results indicate that the leach efficiency, even at extremely high solid loading, remains strong. The kinetics are also still rapid. The decrease could be related to several drivers, the first being the accumulation of oxalate in the solution forcing the equilibrium in the opposite direction, particularly as EDTA is consumed. Another possibility is a disparity in the consumption of EDTA when trebling the solid loading; the same effect observed in Figure 8 at higher EDTA concentrations may be at play (salting out or similar); and, finally, inefficiencies due to the viscosity and bulk of solution could be a contributing factor. In any case, the optimal conditions of the process may actually be at very high solids providing opportunities to recover oxalate from a high-concentration solution in addition to decreasing water demand and footprint.
3.6. EDTA Recycling
To further understand the ability to maximise the usage of EDTA, a second stage of leaching reusing the leach solution once the rare earths are removed was investigated. The process involved multiple leaching and precipitation cycles. Each cycle consisted of a leaching phase, where rare-earth elements (REE) were extracted using EDTA, and a precipitation phase, where REEs were removed from the solution using sodium hydroxide.
In the initial leaching cycle, oxalate residue was leached with a 0.2 M EDTA solution for 1 h at room temperature with magnetic stirring. Subsequently, in the precipitation step, sodium hydroxide was added to the RE-EDTA complex to increase the pH to 14. The solution was then heated to 90 ° C for 1 h.
In the second leaching cycle, the resulting liquid containing EDTA from the previous precipitation step was reused after reconditioning its molarity to 0.2 M. To this, fresh oxalate residue was added, and the mixture was leached under the same conditions as in the initial cycle. Sulphuric acid was added to drop the pH to 12. A second precipitation step was then performed by adding sodium hydroxide to the RE-EDTA complex, followed by heating the solution to 90 °C for 1 h.
The EDTA solution was reconditioned again to revert it to 0.2 M for the third leaching cycle. In this cycle, fresh monazite concentrate was added to the reconditioned EDTA solution and leached under the same conditions as in the earlier cycles. Finally, in the third precipitation step, sodium hydroxide was added to the RE-EDTA complex. The solution concentration of the key REEs throughout this process is provided in Figure 14.
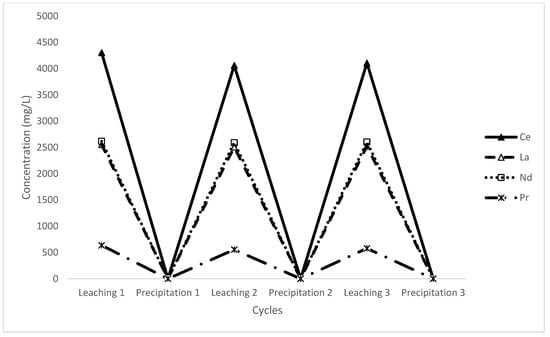
Figure 14.
Concentration of rare-earth elements during leaching and precipitation cycles.
As can be seen, the precipitation was highly effective, almost completely removing the REEs from the solution. The subsequent leach steps demonstrate consistent performance, while slightly reduced concentrations were observed in stages 2 and 3. Due to the solution losses in the solid/liquid separation and the necessary make-up, these lie within uncertainty so may be an artefact rather than a real loss. In any case, the leach remains rapid and effective using recycled lixiviant. This will need to be tested in more depth with further stages and a material balance to understand the physical limitations on the efficiency of recycling the solution.
In addition to the EDTA, higher levels of dissolution through high solid loading or solution recycling generate a large proportion of oxalate ions in the solution. Therefore, a rapid test was conducted to prove the concept of recycling the oxalate out of the EDTA leach solution. Given the low solubility of calcium oxalate, particularly at high pH, CaCl2 was added to the EDTA leach solution following REE removal. This resulted in the precipitation of a white solid, which was analysed via XRD (Figure 15) and identified as whewellite (calcium oxalate). The precipitating agent was not in excess, and this was achieved at a REE precipitation pH and room temperature, indicating that there is potential for recovering and recycling a large proportion of the oxalate in the second stage as well as potentially in the first. These findings provide confidence that, with optimisation, the recycling of the primary reagents will provide the opportunity to add significant value through cost, handling, and storage savings.
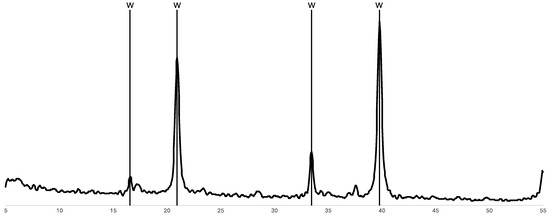
Figure 15.
X-ray diffractogram of precipitate formed upon the addition of calcium to the EDTA leach residue following REE removal.
4. Conclusions
In addition to the reaffirming previous findings regarding the efficacy and rapid nature of the reaction, the present study has identified several key characteristics of the EDTA system in solubilising rare earths following an oxalate-based conversion, while others such as temperature and leach time have no impact. The first thing to note is that the lixiviant concentration is key to maximising recovery. The consumption of EDTA not only leads to a deficit in solution but to a loss of buffering allowing the pH to drop below the equilibrium between the stability of the oxalate salt and the EDTA solution complex. The high purity of the leach solution means that the 1:1 molar ratio of REEs to EDTA is an excellent guide, and an excess of EDTA is required for full dissolution.
Notably, exceedingly high solid loadings can be treated effectively with this system with minimal losses in recovery as long as an appropriate excess of EDTA is present. This suggests that oxalate released into the solution, at least in our test work, demonstrated no impact on the rare-earth dissolution. EDTA was successfully recycled as part of this process demonstrating its potential to have far less impact both on cost and environmental considerations by using less. The high solid loadings and ability to recycle the reagent allow very high levels of oxalate into the solution, and yet this does not appear detrimental to recovery. It provides an opportunity demonstrated here to recover the oxalate from multiple stages.
Author Contributions
A.S.A.A.S.—design, experimentation, analysis, writing, and editing. L.G.D.—project initiation, obtaining funding, supervision, design, analysis, and editing. B.T.—supervision and analysis. All authors have read and agreed to the published version of the manuscript.
Funding
Lynas Rare Earths and the Minerals Research Institute of Western Australia (MRIWA) (Project M541).
Data Availability Statement
Raw data can be made available upon request.
Acknowledgments
The authors would like to thank Lynas Rare Earths and the Minerals Research Institute of Western Australia (Project M541) for their support of the project.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Damhus, T. Nomenclature of inorganic chemistry: IUPAC recommendations 2005. Chem. Int. 2005, 27. [Google Scholar]
- Zepf, V. Rare Earth Elements: A New Approach to the Nexus of Supply, Demand and Use: Exemplified along the Use of Neodymium in Permanent Magnets; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Simandl, G.J. Geology and market-dependent significance of rare earth element resources. Miner. Depos. 2014, 49, 889–904. [Google Scholar] [CrossRef]
- Kim, E.; Osseo-Asare, K. Aqueous stability of thorium and rare earth metals in monazite hydrometallurgy: Eh–pH diagrams for the systems Th–, Ce–, La–, Nd–(PO4)–(SO4)–H2O at 25 °C. Hydrometallurgy 2012, 113, 67–78. [Google Scholar] [CrossRef]
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115. [Google Scholar] [CrossRef]
- Dent, P.C. Rare earth elements and permanent magnets (invited). J. Appl. Phys. 2012, 111, 07A721. [Google Scholar] [CrossRef]
- Klinger, J.M. A historical geography of rare earth elements: From discovery to the atomic age. Extr. Ind. Soc. 2015, 2, 572–580. [Google Scholar] [CrossRef]
- Clavier, N.; Podor, R.; Dacheux, N. Crystal chemistry of the monazite structure. J. Eur. Ceram. Soc. 2011, 31, 941–976. [Google Scholar] [CrossRef]
- Haxel, G. Rare Earth Elements: Critical Resources for High Technology; US Department of the Interior, US Geological Survey: Reston, VA, USA, 2002; Volume 87.
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths; CRC Press: Baton Rouge, LA, USA, 2004. [Google Scholar] [CrossRef]
- Sadri, F.; Nazari, A.M.; Ghahreman, A. A review on the cracking, baking and leaching processes of rare earth element concentrates. J. Rare Earths 2017, 35, 739–752. [Google Scholar] [CrossRef]
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Jordens, A.; Sheridan, R.S.; Rowson, N.A.; Waters, K.E. Processing a rare earth mineral deposit using gravity and magnetic separation. Miner. Eng. 2014, 62, 9–18. [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D.; Browner, R. Treatment of monazite by organic acids I: Solution conversion of rare earths. Hydrometallurgy 2017, 174, 202–209. [Google Scholar] [CrossRef]
- Lazo, D.E.; Dyer, L.G.; Alorro, R.D.; Browner, R. Treatment of monazite by organic acids II: Rare earth dissolution and recovery. Hydrometallurgy 2018, 179, 94–99. [Google Scholar] [CrossRef]
- Vander Hoogerstraete, T.; Blanpain, B.; Van Gerven, T.; Binnemans, K. From NdFeB magnets towards the rare-earth oxides: A recycling process consuming only oxalic acid. RSC Adv. 2014, 4, 64099–64111. [Google Scholar] [CrossRef]
- Erust, C.; Akcil, A.; Bedelova, Z.; Anarbekov, K.; Baikonurova, A.; Tuncuk, A. Recovery of vanadium from spent catalysts of sulfuric acid plant by using inorganic and organic acids: Laboratory and semi-pilot tests. Waste Manag. 2016, 49, 455–461. [Google Scholar] [CrossRef]
- Le, M.N.; Lee, M.S. Leaching of rare metals from spent petroleum catalysts by organic acid solution. Resour. Recycl. 2019, 28, 36–45. [Google Scholar] [CrossRef]
- Goyne, K.W.; Brantley, S.L.; Chorover, J. Rare earth element release from phosphate minerals in the presence of organic acids. Chem. Geol. 2010, 278, 1–14. [Google Scholar] [CrossRef]
- Feng, J.; Wu, X.; Gao, Z.; Sun, W.; Zhou, F.; Chi, R. Leaching Behavior of Rare Earth Elements and Aluminum from Weathered Crust Elution-Deposited Rare Earth Ore with Ammonium Formate Inhibitor. Minerals 2023, 13, 1245. [Google Scholar] [CrossRef]
- Kumari, A.; Sahu, K.K.; Sahu, S.K. Solvent Extraction and Separation of nd, pr and dy from leach liquor of waste NdFeB Magnet using the nitrate form of Mextral® 336At in the presence of aquo-complexing agent EDTA. Metals 2019, 9, 269. [Google Scholar] [CrossRef]
- Di Palma, L.; Ferrantelli, P.; Merli, C.; Biancifiori, F. Recovery of EDTA and metal precipitation from soil flushing solutions. J. Hazard. Mater. 2003, 103, 153–168. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 281, 1–10. [Google Scholar] [CrossRef]
- Cotton, S. Lanthanide and Actinide Chemistry; John Wiley & Sons: New York, NY, USA, 2013. [Google Scholar]
- Marafi, M.; Rana, M.S. Role of EDTA on metal removal from refinery waste catalysts. WIT Trans. Ecol. Environ. 2019, 231, 137–147. [Google Scholar]
- Liu, N.; Li, Y.-Y.; Ouyang, D.-J.; Zou, C.-Y.; Li, W.; Zhao, J.-H.; Li, J.-X.; Wang, W.-J.; Hu, J.-J. Performance and Microbial Community Analysis of an Electrobiofilm Reactor Enhanced by Ferrous-EDTA. ACS Omega 2021, 6, 17766–17775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, J.; Sun, C.; Li, S.; Zhang, D.; Guo, T.; Li, W. Two-stage chemical absorption–biological reduction system for NO removal: System start-up and optimal operation mode. Energy Fuels 2018, 32, 7701–7707. [Google Scholar] [CrossRef]
- Sun, Y.; Garg, S.; Zhang, C.; Lian, B.; Waite, T.D. Kinetic modeling of the anodic degradation of Ni-EDTA complexes: Insights into the reaction mechanism and products. ACS EST Eng. 2023, 4, 105–114. [Google Scholar] [CrossRef]
- Dupont, D.; Brullot, W.; Bloemen, M.; Verbiest, T.; Binnemans, K. Selective uptake of rare earths from aqueous solutions by EDTA-functionalized magnetic and nonmagnetic nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 4980–4988. [Google Scholar] [CrossRef]
- Rout, A.; Binnemans, K. Separation of rare earths from transition metals by liquid–liquid extraction from a molten salt hydrate to an ionic liquid phase. Dalton Trans. 2014, 43, 3186–3195. [Google Scholar] [CrossRef]
- Crouthamel, C.E.; Martin, D., Jr. Solubility of the rare earth oxalates and complex ion formation in oxalate solution. II. Neodymium and cerium (III). J. Am. Chem. Soc. 1951, 73, 569–573. [Google Scholar] [CrossRef]
- Finžgar, N.; Leštan, D. Multi-step leaching of Pb and Zn contaminated soils with EDTA. Chemosphere 2007, 66, 824–832. [Google Scholar] [CrossRef]
- Palden, T.; Machiels, L.; Onghena, B.; Regadío, M.; Binnemans, K. Selective leaching of lead from lead smelter residues using EDTA. RSC Adv. 2020, 10, 42147–42156. [Google Scholar] [CrossRef]
- Forte, F.; Horckmans, L.; Broos, K.; Kim, E.; Kukurugya, F.; Binnemans, K. Closed-loop solvometallurgical process for recovery of lead from iron-rich secondary lead smelter residues. RSC Adv. 2017, 7, 49999–50005. [Google Scholar] [CrossRef]
- Wojtkowska, M.; Bogacki, J. Assessment of trace metals contamination, species distribution and mobility in river sediments using EDTA extraction. Int. J. Environ. Res. Public Health 2022, 19, 6978. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).