On the Polymorphism of Cu2V2O7: Synthesis and Crystal Structure of δ-Cu2V2O7, a New Polymorph
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
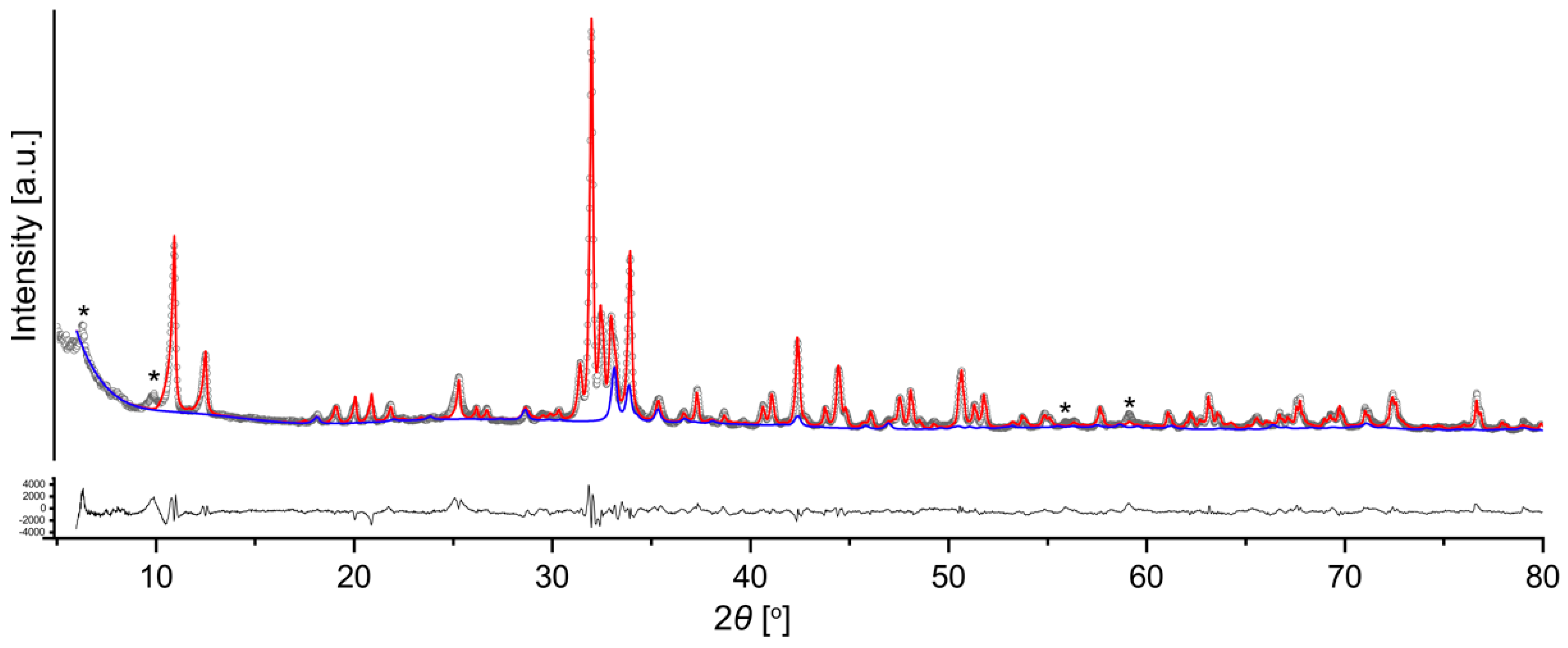
2.2. Powder X-ray Diffraction
2.3. Single-Crystal X-ray Diffraction
3. Results
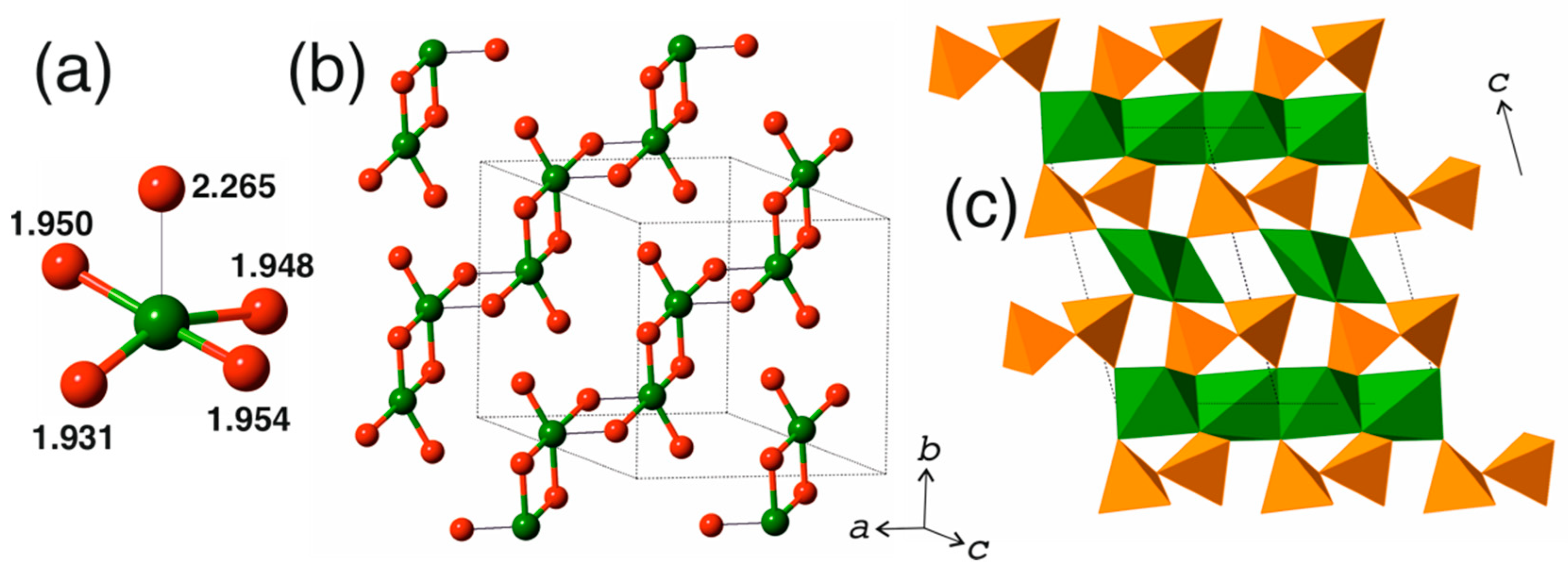
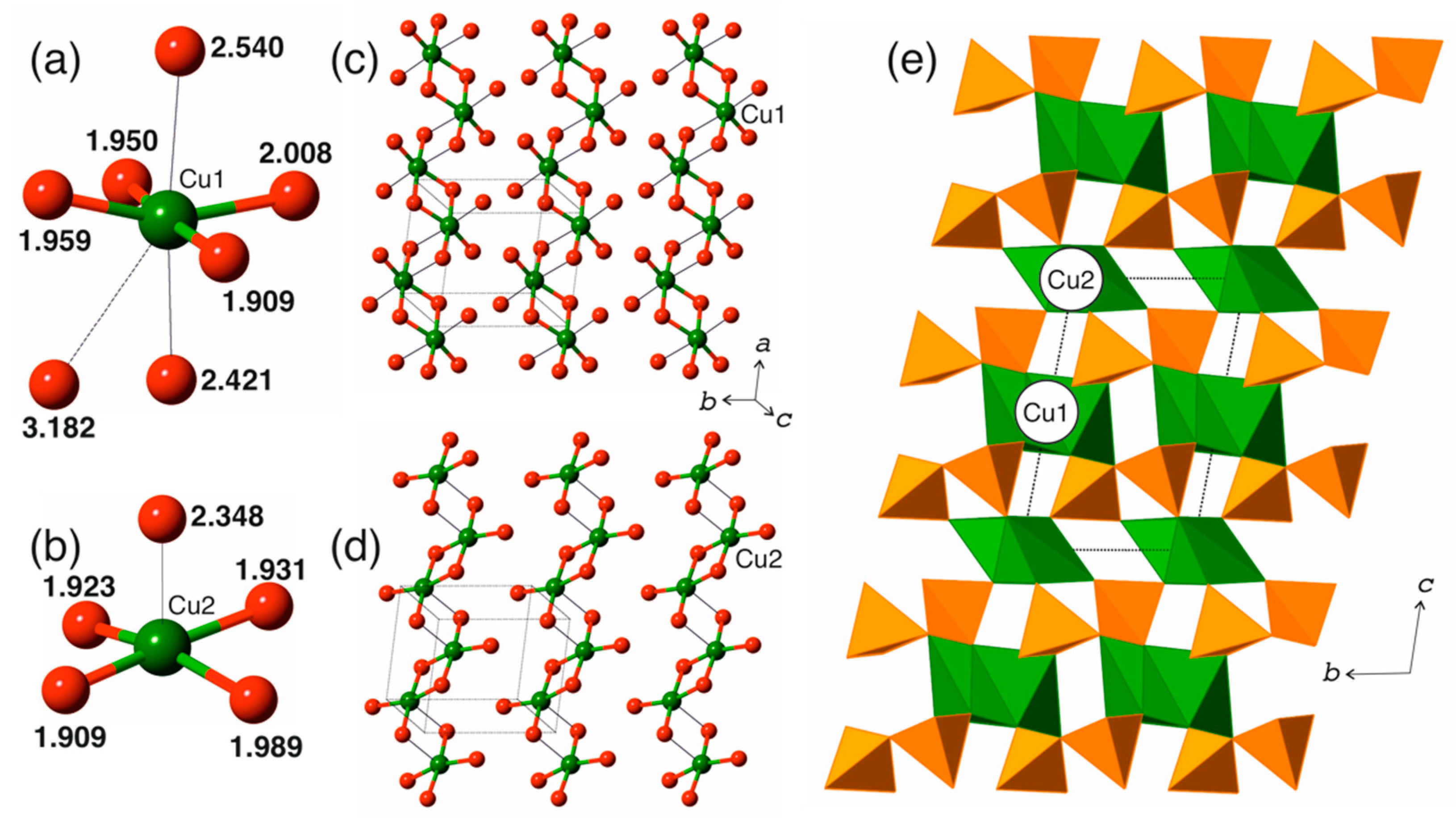
3.1. Cation Coordination
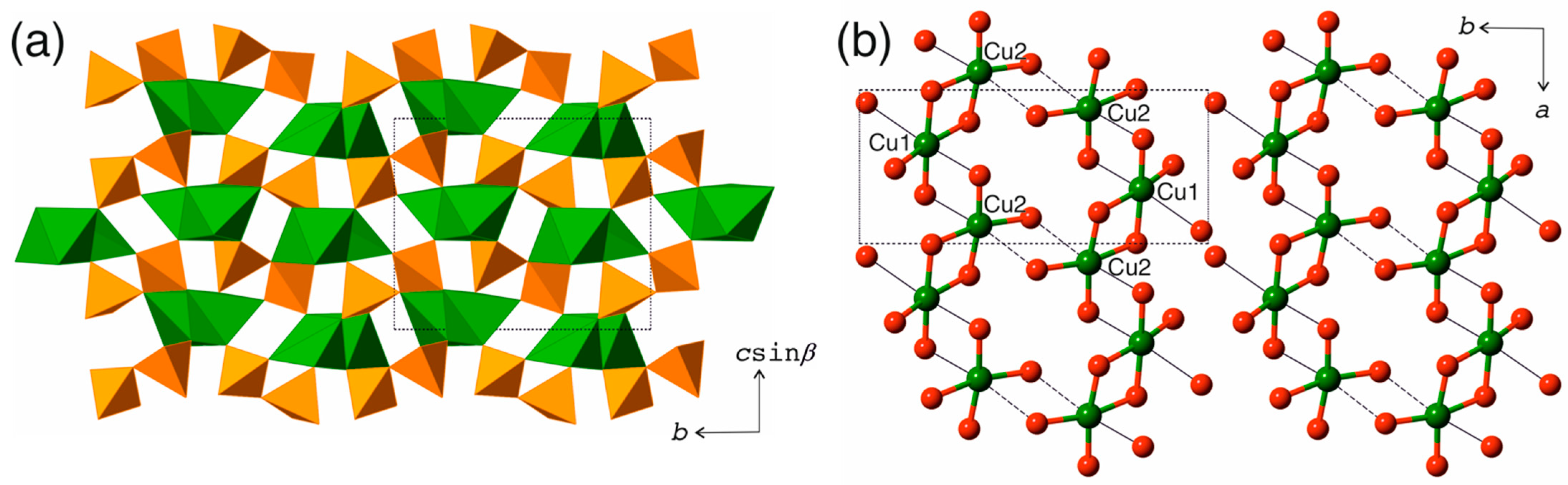
3.2. Structure Description
4. Discussion
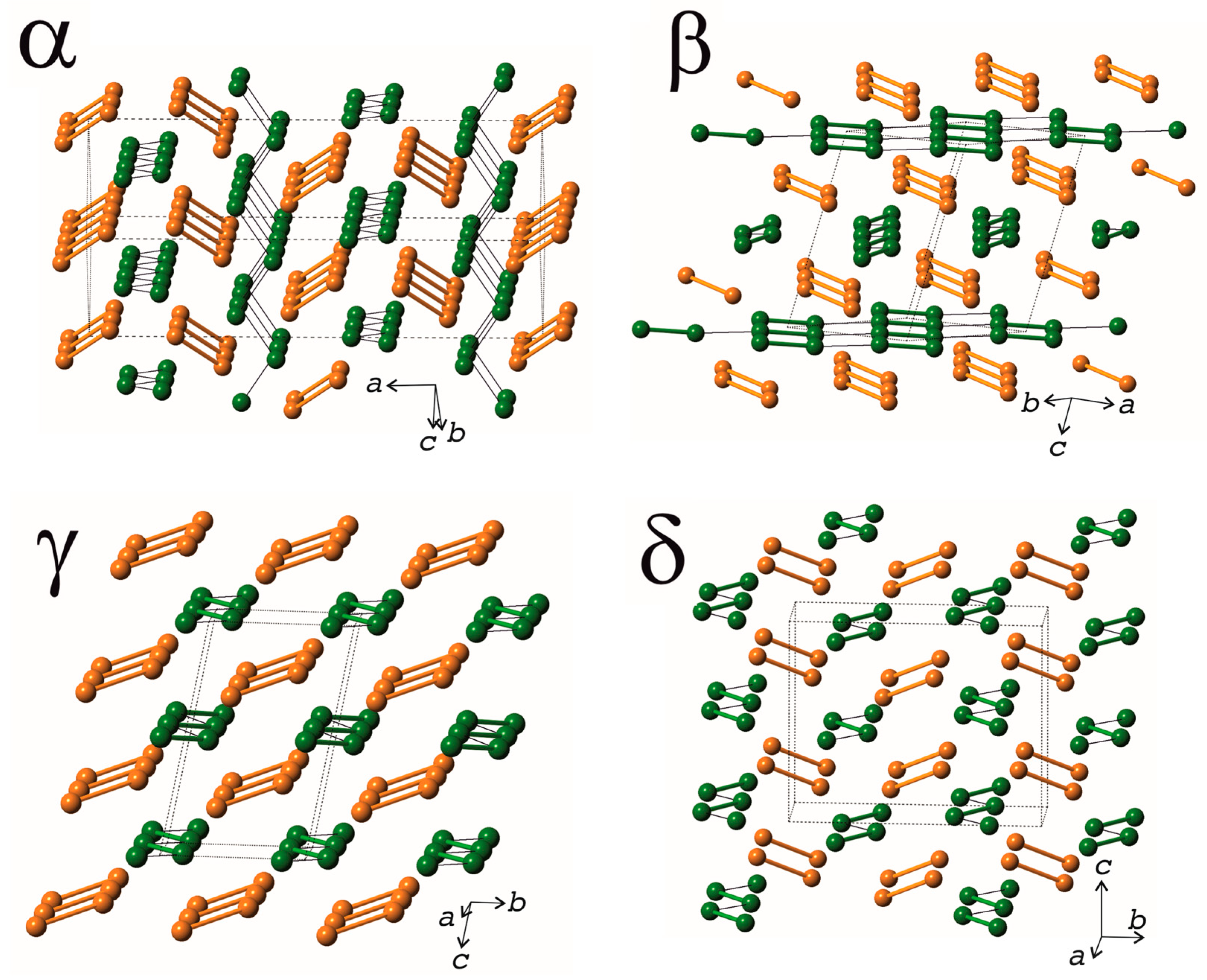
4.1. Structure Comparison of the Cu2V2O7 Polymorphs
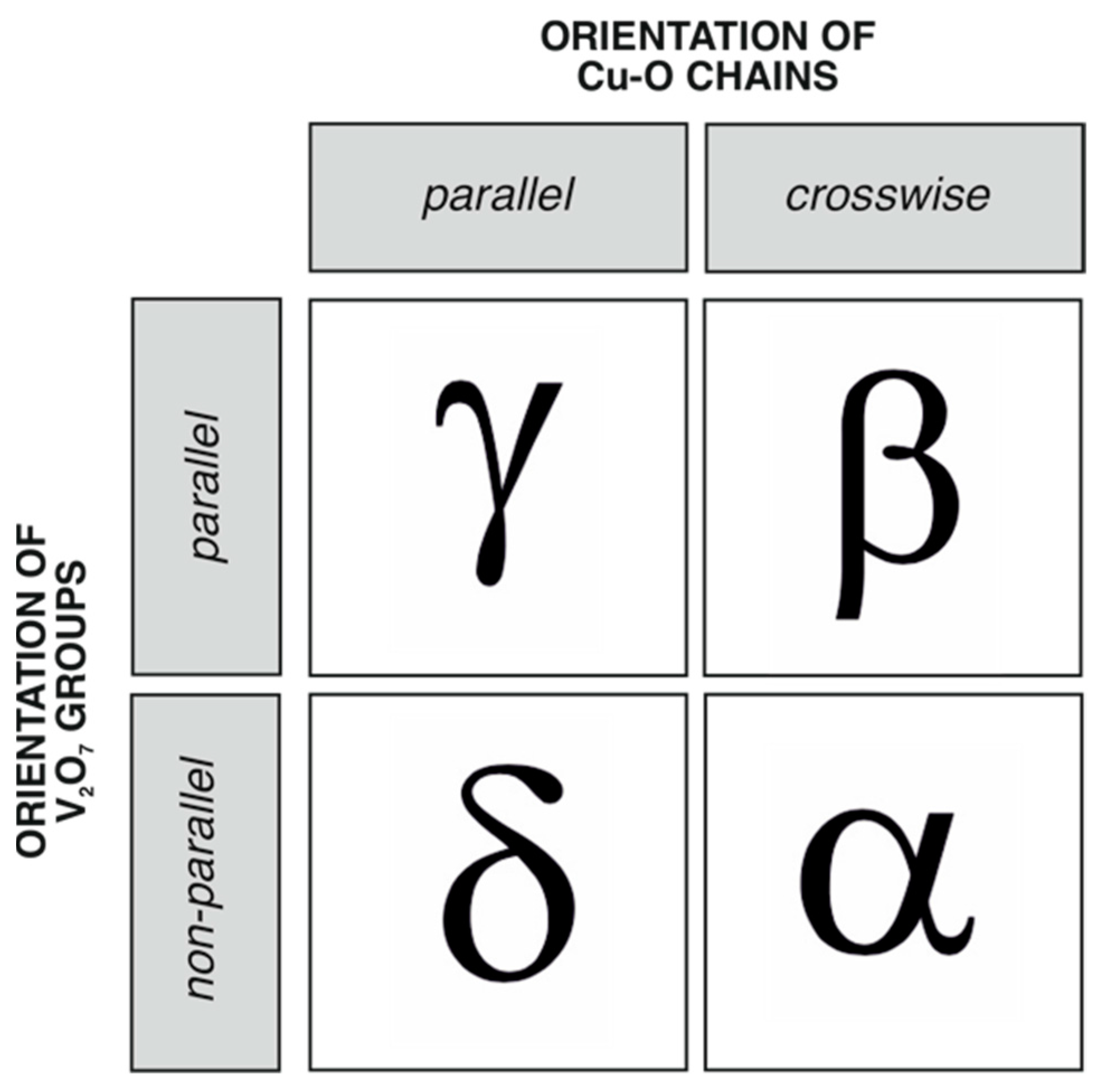
4.2. Stability and Nomenclature of the Cu2V2O7 Polymorphs
4.3. Structural Complexity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, W.; Chemelewski, W.D.; Mabayoje, O.; Xiao, P.; Zhang, Y.; Mullins, C.B. Synthesis and Characterization of CuV2O6 and Cu2V2O7: Two Photoanode Candidates for Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2015, 119, 27220–27227. [Google Scholar] [CrossRef]
- Kim, M.; Joshi, B.; Yoon, H.; Ohm, T.Y.; Kim, K.; Al-Deyab, S.S.; Yoon, S.S. Electrosprayed Copper Hexaoxodivanadate (CuV2O6) and Pyrovanadate (Cu2V2O7) Photoanodes for Efficient Solar Water Splitting. J. Alloys Compds 2017, 708, 444–450. [Google Scholar] [CrossRef]
- Gadiyar, C.; Strach, M.; Schouwink, P.; Loiudice, A.; Buonsanti, R. Chemical Transformations at the Nanoscale: Nanocrystal-Seeded Synthesis of β-Cu2V2O7 with Enhanced Photoconversion Efficiencies. Chem. Sci. 2018, 9, 5658–5665. [Google Scholar] [CrossRef] [PubMed]
- Wiktor, J.; Reshetnyak, I.; Strach, M.; Scarongella, M.; Buonsanti, R.; Pasquarello, A. Sizable Excitonic Effects Undermining the Photocatalytic Efficiency of β-Cu2V2O7. J. Phys. Chem. Lett. 2018, 9, 5698–5703. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Iqbal, T.; Tahir, M.B.; Afsheen, S. A Review on Copper Vanadate-Based Nanostructures for Photocatalysis Energy Production. Int. J. Energy Res. 2019, 43, 9–28. [Google Scholar] [CrossRef]
- Rao, M.P.; Akhila, A.K.; Wu, J.J.; Asiri, A.M.; Anandan, S. Synthesis, Characterization and Adsorption Properties of Cu2V2O7 Nanoparticles. Solid State Sci. 2019, 92, 13–23. [Google Scholar] [CrossRef]
- Guo, W.; Lian, X.; Nie, Y.; Hu, M.; Wu, L.; Gao, H.; Wang, T. Facile Growth of β-Cu2V2O7 Thin Films and Characterization for Photoelectrochemical Water Oxidation. Mater. Lett. 2020, 258, 126842. [Google Scholar] [CrossRef]
- Camargo, L.P.; Lucilha, A.C.; Gomes, G.A.B.; Liberatti, V.R.; Andrello, A.C.; da Silva, P.R.C.; Dall’Antonia, L.H. Copper Pyrovanadate Electrodes Prepared by Combustion Synthesis: Evaluation of Photoelectroactivity. J. Solid State Electrochem. 2020, 24, 1935–1950. [Google Scholar] [CrossRef]
- Song, A.; Chemseddine, A.; Ahmet, I.Y.; Bogdanoff, P.; Friedrich, D.; Abdi, F.F.; Berglund, S.P.; van de Krol, R. Evaluation of Copper Vanadate (β-Cu2V2O7) as a Photoanode Material for Photoelectrochemical Water Oxidation. Chem. Mater. 2020, 32, 2408–2419. [Google Scholar] [CrossRef]
- Muthamizh, S.; Yesuraj, J.; Jayavel, R.; Contreras, D.; Arul Varman, K.; Mangalaraja, R.V. Microwave Synthesis of β-Cu2V2O7 Nanorods: Structural, Electrochemical Supercapacitance, and Photocatalytic Properties. J. Mater. Sci.: Mater. Electr. 2021, 32, 2744–2756. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Surfactant Induced Copper Vanadate (β-Cu2V2O7, Cu3V2O8) for Different Textile Dyes Degradation. Environ. Res. 2022, 211, 112964. [Google Scholar] [CrossRef] [PubMed]
- Moussaid, D.; Khallouk, K.; El Khalfaouy, R.; Tagnaouti Moumnani, F.; Kherbeche, A.; Barakat, A. Solution Combustion Synthesis of β-Cu2V2O7 Nanoparticles: Photocatalytic Degradation of Crystal Violet under UV and Visible Light Illumination. React. Kin. Mech. Catal. 2022, 135, 2797–2812. [Google Scholar] [CrossRef]
- Musuvadhi Babulal, S.; Anupriya, J.; Chen, S.M. Self Assembled Three Dimensional β-Cu2V2O7 Hierarchical Flower Decorated Porous Carbon: An Efficient Electrocatalyst for Flutamide Detection in Biological and Environmental Samples. Chemosphere 2022, 303, 135203. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, L.A.; Vasil’ev, A.N.; Antipov, E.V.; Velikodny, Y.A. Magnetic Properties of Cu2V2O7. Physica B: Cond. Mat. 2000, 284–288, 1459–1460. [Google Scholar] [CrossRef]
- Yashima, M.; Suzuki, R.O. Electronic Structure and Magnetic Properties of Monoclinic β-Cu2V2O7: A GGA+U Study. Phys. Rev. B 2009, 79, 125201. [Google Scholar] [CrossRef]
- Tsirlin, A.A.; Janson, O.; Rosner, H. β-Cu2V2O7: A Spin-1/2 Honeycomb Lattice System. Phys. Rev. B 2010, 82, 144416. [Google Scholar] [CrossRef]
- Janson, O.; Tsirlin, A.A.; Sichelschmidt, J.; Skourski, Y.; Weickert, F.; Rosner, H. Long-Range Superexchange in Cu2A2O7 (A = P, As, V) as a Key Element of the Microscopic Magnetic Model. Phys. Rev. B 2011, 83, 094435. [Google Scholar] [CrossRef]
- Sánchez-Andújar, M.; Yáñez-Vilar, S.; Mira, J.; Biskup, N.; Rivas, J.; Castro-García, S.; Señarís-Rodríguez, M.A. Role of the Magnetic Ordering on the Dielectric Response of M2V2O7 (M = Co and Cu) Divanadates. J. Appl. Phys. 2011, 109, 054106. [Google Scholar] [CrossRef]
- Gitgeatpong, G.; Zhao, Y.; Avdeev, M.; Piltz, R.O.; Sato, T.J.; Matan, K. Magnetic Structure and Dzyaloshinskii-Moriya Interaction in the S = 1/2 Helical-Honeycomb Antiferromagnet a-Cu2V2O7. Phys. Rev. B 2015, 92, 024423. [Google Scholar] [CrossRef]
- Banerjee, A.; Sannigrahi, J.; Bhowal, S.; Dasgupta, I.; Majumdar, S.; Walker, H.C.; Bhattacharyya, A.; Adroja, D.T. Spin Wave Excitations in the Pyrovanadate a-Cu2V2O7. Phys. Rev. B 2016, 94, 144426. [Google Scholar] [CrossRef]
- Bhowal, S.; Sannigrahi, J.; Majumdar, S.; Dasgupta, I. A Comparative Study of Electronic, Structural, and Magnetic Properties of α-, β-, and γ- Cu2V2O7. Phys. Rev. B 2017, 95, 075110. [Google Scholar] [CrossRef]
- Gitgeatpong, G.; Suewattana, M.; Zhang, S.; Miyake, A.; Tokunaga, M.; Chanlert, P.; Kurita, N.; Tanaka, H.; Sato, T.J.; Zhao, Y.; et al. High-Field Magnetization and Magnetic Phase Diagram of a-Cu2V2O7. Phys. Rev. B 2017, 95, 245119. [Google Scholar] [CrossRef]
- Ruan, M.Y.; Ouyang, Z.W.; Sun, Y.C.; Xia, Z.C.; Rao, G.H.; Chen, H.S. Examining Magnetic Models and Anisotropies in β-Cu2V2O7 by High-Frequency ESR. Appl. Magn. Res. 2017, 48, 423–433. [Google Scholar] [CrossRef]
- Kunwar, H.S.; Isha; Yogi, A.K.; De, B.K.; Dwij, V.; Gupta, M.K.; Mittal, R.; Venkatesh, R.; Chaudhary, R.J.; Vedpathak, M.; et al. Raman Scattering of Spin-1/2 Mixed-Dimensionality Antiferromagnetic a-Cu2V2O7. Phys. Rev. B 2024, 109, 054310. [Google Scholar] [CrossRef]
- Sannigrahi, J.; Bhowal, S.; Giri, S.; Majumdar, S.; Dasgupta, I. Exchange-Striction Induced Giant Ferroelectric Polarization in Copper-Based Multiferroic Material a-Cu2V2O7. Phys. Rev. B 2015, 91, 220407. [Google Scholar] [CrossRef]
- Shiomi, Y.; Takashima, R.; Okuyama, D.; Gitgeatpong, G.; Piyawongwatthana, P.; Matan, K.; Sato, T.J.; Saitoh, E. Spin Seebeck Effect in the Polar Antiferromagnet a-Cu2V2O7. Phys. Rev. B 2017, 96, 180414. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wang, J.L.; Ji, C.; Guo, B.X.; Xia, W.S.; Lu, X.M.; Zhu, J.S. Magnetism and Spin-Driven Ferroelectricity in the Multiferroic Material a-Cu2V2O7. Phys. Rev. B 2017, 96, 165132. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Wu, M.; Li, Y.; Feng, D.; Liu, C.; Mao, Y.; Guo, J.; Chao, M.; Liang, E. Negative Thermal Expansion and Electrical Properties of α-Cu2V2O7. J. Eur. Ceram. Soc. 2016, 36, 2761–2766. [Google Scholar] [CrossRef]
- Wang, H.; Yang, M.; Chao, M.; Guo, J.; Gao, Q.; Jiao, Y.; Tang, X.; Liang, E. Negative Thermal Expansion Property of β-Cu2V2O7. Solid State Ionics 2019, 343, 115086. [Google Scholar] [CrossRef]
- Hughes, J.M.; Birnie, R.W. Ziesite, β-Cu2V2O7, a New Copper Vanadate and Fumarole Temperature Indicator. Amer. Miner. 1980, 65, 1146–1149. [Google Scholar]
- Robinson, P.D.; Hughes, J.M.; Malinconico, M.L. Blossite, Alpha -Cu2+2V5+2O7, a New Fumarolic Sublimate from Izalco Volcano, El Salvador. Amer. Miner. 1987, 72, 397–400. [Google Scholar]
- Hughes, J.M.; Brown, M.A. The crystal structure of ziesite, β-Cu2V2O7, a thortveitite-type structure with a non-linear X-O-X inter-tetrahedral bond. N. Jb. Miner. Mh. 1989, 1989, 41–47. [Google Scholar]
- Brisi, C.; Molinari, A. Il sistema ossido ramicoanidride vanadica. Ann. Chim. 1958, 48, 263–269. [Google Scholar]
- Fleury, P. Sur le systéme CuO-V2O5. C.R Acad. Sci. Paris Ser. C 1966, 263, 1375–1377. [Google Scholar]
- Fleury, P. Etudes sur les systémes V2O5-CuO ou Ag2O ou Tl2O3 et sur les combinaisons interoxydes comespondantes. Rev. Chim. Mineral. 1969, 6, 819–851. [Google Scholar]
- Mercurio-Lavaud, D.; Frit, B. Structure cristalline de la variété basse température du pyrovanadate de cuivre: Cu2V2O7 α. Acta Crystallogr. B 1973, 29, 2737–2741. [Google Scholar] [CrossRef]
- Mercurio-Lavaud, D.; Frit, M.B. Structure cristalline de la variété haute température du pyrovanadate de cuivre: Cu2V2O7. C. R. Acad. Sci. Paris 1973, 277C, 1101–1104. [Google Scholar]
- Calvo, C.; Faggiani, R. α Cupric Divanadate. Acta Crystallogr. B 1975, 31, 603–605. [Google Scholar] [CrossRef]
- Clark, G.M.; Garlick, R. Formation and Properties of Copper(II) Divanadate(V). J. Inorg. Nucl. Chem. 1978, 40, 1347–1349. [Google Scholar] [CrossRef]
- Petrova, S.A.; Zakharov, R.G.; Rotermel’, M.V.; Krasnenko, T.I.; Vatolin, N.A. A New High-Temperature Modification of Copper Pyrovanadate. Dokl. Chem. 2005, 400, 30–33. [Google Scholar] [CrossRef]
- Slobodin, B.V.; Surat, L.L.; Samigullina, R.F. Polymorphism in Copper Pyrovanadate. Russ. J. Inorg. Chem. 2009, 54, 797–802. [Google Scholar] [CrossRef]
- Slobodin, B.V.; Samigullina, R.F. Thermoanalytical Study of the Polymorphism and Melting Behavior of Cu2V2O7. Inorg. Mater. 2010, 46, 196–200. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Cherepansky, P.N.; Armbruster, T.; Pankratova, O.Y. Crystal structure of γ-Cu2V2O7 and its comparison to blossite (α-Cu2V2O7) and ziesite (β-Cu2V2O7). Can. Miner. 2005, 43, 671–677. [Google Scholar] [CrossRef]
- Turnbull, R.; González-Platas, J.; Rodríguez, F.; Liang, A.; Popescu, C.; He, Z.; Santamaría-Pérez, D.; Rodríguez-Hernández, P.; Muñoz, A.; Errandonea, D. Pressure-Induced Phase Transition and Band Gap Decrease in Semiconducting β-Cu2V2O7. Inorg. Chem. 2022, 61, 3697–3707. [Google Scholar] [CrossRef] [PubMed]
- Kornyakov, I.V.; Krivovichev, S.V.; Gurzhiy, V.V.; Leoni, M. ε-RbCuCl3, a new polymorph of rubidium copper trichloride: Synthesis, structure and structural complexity. Acta Crystallogr. C 2018, 74, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Rigaku, C. Integrated X-ray Powder Diffraction Software PDXL. Rigaku J. 2010, 26, 23–27. [Google Scholar]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Zagorac, D.; Müller, H.; Ruehl, S.; Zagorac, J.; Rehme, S. Recent developments in Inorganic Crystal Structure Database: Theoretical crystal structure data and related features. J. Appl. Crystallogr. 2019, 52, 918–925. [Google Scholar] [CrossRef]
- Coelho, A.A. TOPAS and TOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef]
- Savariault, J.-M.; Galy, J. Synthesis and structural investigation of a new potassium vanadium oxide bronze: ρ-K0.50V2O5. J. Solid State Chem. 1992, 101, 119–127. [Google Scholar] [CrossRef]
- Martin, F.-D.; Müller-Buschbaum, H. Zur Kenntnis von K4CuV5O15Cl. Zeitschrift Naturforschung B. 1994, 49, 1459–1462. [Google Scholar] [CrossRef]
- Haase, A.; Brauer, G. Vanadium oxychloride. Acta Cryst. 1975, B31, 2521–2522. [Google Scholar] [CrossRef]
- Agilent Technologies. CrysAlisPro; Version 1.171.36.20; Agilent Technologies: Santa Clara, CA, USA, 2012. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Gagné, O.C.; Hawthorne, F.C. Comprehensive Derivation of Bond-Valence Parameters for Ion Pairs Involving Oxygen. Acta Crystallogr. B 2015, 71, 562–578. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states. Proc. Roy. Soc. Ser. A. 1937, 161, 220–235. [Google Scholar]
- Hathaway, B.J. A new look at the stereochemistry and electronic properties of complexes of the copper(II) ion. In Complex Chemistry. Structure and Bonding; Emsley, J., Ernst, R.D., Hathaway, B.J., Warren, K.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 57, pp. 55–118. [Google Scholar]
- Burns, P.C.; Hawthorne, F.C. Static and dynamic Jahn-Teller effects in Cu2+ oxysalt minerals. Can. Mineral. 1996, 34, 1089–1105. [Google Scholar]
- Brown, I.D. The Chemical Bond in Inorganic Chemistry. The Bond-Valence Model; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Rotermel’, M.V.; Krasnenko, T.I.; Petrova, S.A.; Zakharov, R.G. Thermally Activated Transformations in Stable and Metastable Copper(II) Pyrovanadate Polymorphs. Russ. J. Inorg. Chem. 2009, 54, 22–26. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Metastable Crystallization and Structural Complexity of Minerals. Dokl. Earth Sci. 2022, 507, 1040–1043. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Miner. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Krivovichev, V.G.; Hazen, R.M.; Aksenov, S.M.; Avdontceva, M.S.; Banaru, A.M.; Gorelova, L.A.; Ismagilova, R.M.; Kornyakov, I.V.; Kuporev, I.V.; et al. Structural and chemical complexity of minerals: An update. Mineral. Mag. 2022, 86, 183–204. [Google Scholar] [CrossRef]
- Filatov, S.K. General Concept of Increasing Crystal Symmetry with an Increase in Temperature. Crystallogr. Rep. 2011, 56, 953–961. [Google Scholar] [CrossRef]
- Hazen, R.M.; Navrotsky, A. Effects of Pressure on Order-Disorder Reactions. Amer. Miner. 1996, 81, 1021–1035. [Google Scholar] [CrossRef]


| Crystal Data | |
|---|---|
| Chemical formula | Cu2V2O7 |
| Mr | 340.96 |
| Crystal system, space group | Monoclinic, P21/n |
| Temperature (K) | 296 (2) |
| a, b, c (Å) | 5.0679 (3), 11.4222 (7), 9.4462 (6) |
| β (°) | 97.100 (6) |
| V (Å3) | 542.61 (6) |
| Z | 4 |
| µ (mm−1) | 11.05 |
| Crystal size (mm3) | 0.04 × 0.03 × 0.01 |
| Data collection parameters | |
| Diffractometer | Rigaku XtaLAB Synergy-S |
| Radiation type | MoKα |
| Absorption correction | Gaussian |
| Tmin, Tmax | 0.856, 0.943 |
| Nos. of measured, independent and observed [I > 2σ(I)] reflections | 5290, 1818, 1472 |
| Rint | 0.037 |
| (sin θ/λ)max (Å−1) | 0.777 |
| Refinement parameters | |
| R1 [F2 > 2σ(F2)], wR(F2), S | 0.029, 0.059, 1.03 |
| No. of reflections | 1818 |
| No. of parameters | 100 |
| Δρmax, Δρmin (e Å−3) | 0.80, −0.85 |
| Atom | x/a | y/b | z/c | Uiso |
|---|---|---|---|---|
| Cu1 | 0.14483 (7) | 0.30752 (3) | 0.43693 (4) | 0.01026 (10) |
| Cu2 | 0.87981 (7) | 0.65745 (3) | 0.05332 (4) | 0.01229 (10) |
| V1 | 0.63692 (9) | 0.41149 (4) | 0.20780 (5) | 0.00787 (11) |
| V2 | 0.28194 (9) | 0.62031 (4) | 0.34339 (5) | 0.00739 (11) |
| O1 | 0.8207 (4) | 0.31193 (17) | 0.3096 (2) | 0.0114 (4) |
| O2 | 0.8307 (5) | 0.50883 (19) | 0.1396 (3) | 0.0215 (5) |
| O3 | 0.4445 (4) | 0.34412 (17) | 0.0733 (2) | 0.0140 (4) |
| O4 | 0.4170 (4) | 0.48002 (18) | 0.3227 (3) | 0.0164 (5) |
| O5 | 0.5059 (4) | 0.70625 (16) | 0.4489 (2) | 0.0114 (4) |
| O6 | 0.2050 (4) | 0.68621 (16) | 0.1763 (2) | 0.0107 (4) |
| O7 | 0.0105 (4) | 0.60076 (16) | 0.4206 (2) | 0.0112 (4) |
| Cu1—O1 | 1.913 (2) | V1—O1 | 1.692 (2) |
| Cu1—O5 | 1.962 (2) | V1—O2 | 1.665 (2) |
| Cu1—O6 | 1.961 (2) | V1—O3 | 1.689 (2) |
| Cu1—O7 | 1.947 (2) | V1—O4 | 1.825 (2) |
| Cu1—O3 | 2.452 (2) | <V1—O> | 1.718 |
| Cu1—O4 | 2.705 (2) | ||
| <Cu1—O> | 2.157 | V2—O4 | 1.763 (2) |
| V2—O5 | 1.722 (2) | ||
| Cu2—O2 | 1.913 (2) | V2—O6 | 1.748 (2) |
| Cu2—O3 | 1.909 (2) | V2—O7 | 1.650 (2) |
| Cu2—O5 | 1.990 (2) | <V2—O> | 1.721 |
| Cu2—O6 | 1.924 (2) | ||
| Cu2—O1 | 2.480 (2) | ||
| <Cu2—O> | 2.043 |
| Atom | O1 | O2 | O3 | O4 | O5 | O6 | O7 | S |
|---|---|---|---|---|---|---|---|---|
| Cu1 | 0.53 | 0.12 | 0.06 | 0.46 | 0.46 | 0.48 | 2.11 | |
| Cu2 | 0.11 | 0.53 | 0.54 | 0.43 | 0.51 | 2.11 | ||
| V1 | 1.32 | 1.41 | 1.33 | 0.94 | 4.99 | |||
| V2 | 1.10 | 1.22 | 1.14 | 1.47 | 4.93 | |||
| S | 1.96 | 1.94 | 2.09 | 2.10 | 2.11 | 2.11 | 1.95 |
| Phase | Space Group | a, Å | b, Å | c, Å | α, deg | β, deg | γ, deg | V/Z, Å3 | ρcalc, g·cm−3 | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| α | Fdd2 | 20.676 | 8.392 | 6.446 | 90 | 90 | 90 | 139.8 | 4.049 | [31] |
| 20.680 | 8.411 | 6.448 | 90 | 90 | 90 | 140.2 | 4.038 | [36] | ||
| 20.645 | 8.383 | 6.442 | 90 | 90 | 90 | 139.4 | 4.062 | [38] | ||
| β | C2/c | 7.686 | 8.034 | 10.121 | 90 | 110.39 | 90 | 146.5 | 3.865 | [44] |
| 7.689 | 8.029 | 10.107 | 90 | 110.25 | 90 | 146.4 | 3.868 | [32] | ||
| 7.698 | 8.031 | 10.113 | 90 | 110.29 | 90 | 146.6 | 3.861 | [29] | ||
| β’ | C2/c | 7.325 * | 8.214 * | 10.190 * | 90 * | 111.78 * | 90 * | 142.4 * | 3.997 * | [40] |
| γ | 5.080 ** | 5.810 ** | 9.380 ** | 100.00 ** | 97.20 ** | 97.18 ** | 133.7 ** | 4.234 ** | [44] | |
| 5.087 | 5.823 | 9.402 | 99.78 | 97.25 | 97.20 | 134.6 | 4.206 | [43] | ||
| δ | P21/n | 5.068 | 11.422 | 9.446 | 90 | 97.10 | 90 | 135.7 | 4.173 | This work |
| Polymorph | System | Space Group | strIG, Bit/Atom | strIG,total, Bit/Cell |
|---|---|---|---|---|
| α | Orthorhombic | Fdd2 | 2.550 | 56.107 |
| β | Monoclinic | C2/c | 2.550 | 56.107 |
| γ | Triclinic | 3.459 | 76.107 | |
| δ | Monoclinic | P21/n | 3.459 | 152.215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornyakov, I.V.; Krivovichev, S.V. On the Polymorphism of Cu2V2O7: Synthesis and Crystal Structure of δ-Cu2V2O7, a New Polymorph. Crystals 2024, 14, 857. https://doi.org/10.3390/cryst14100857
Kornyakov IV, Krivovichev SV. On the Polymorphism of Cu2V2O7: Synthesis and Crystal Structure of δ-Cu2V2O7, a New Polymorph. Crystals. 2024; 14(10):857. https://doi.org/10.3390/cryst14100857
Chicago/Turabian StyleKornyakov, Ilya V., and Sergey V. Krivovichev. 2024. "On the Polymorphism of Cu2V2O7: Synthesis and Crystal Structure of δ-Cu2V2O7, a New Polymorph" Crystals 14, no. 10: 857. https://doi.org/10.3390/cryst14100857







