The Role of Liquid Crystal Elastomers in Pioneering Biological Applications
Abstract
:1. Introduction
1.1. Overview of Liquid Crystal Elastomers
1.2. LCE Significance in Biological Applications
2. Fundamentals of Liquid Crystal Elastomers
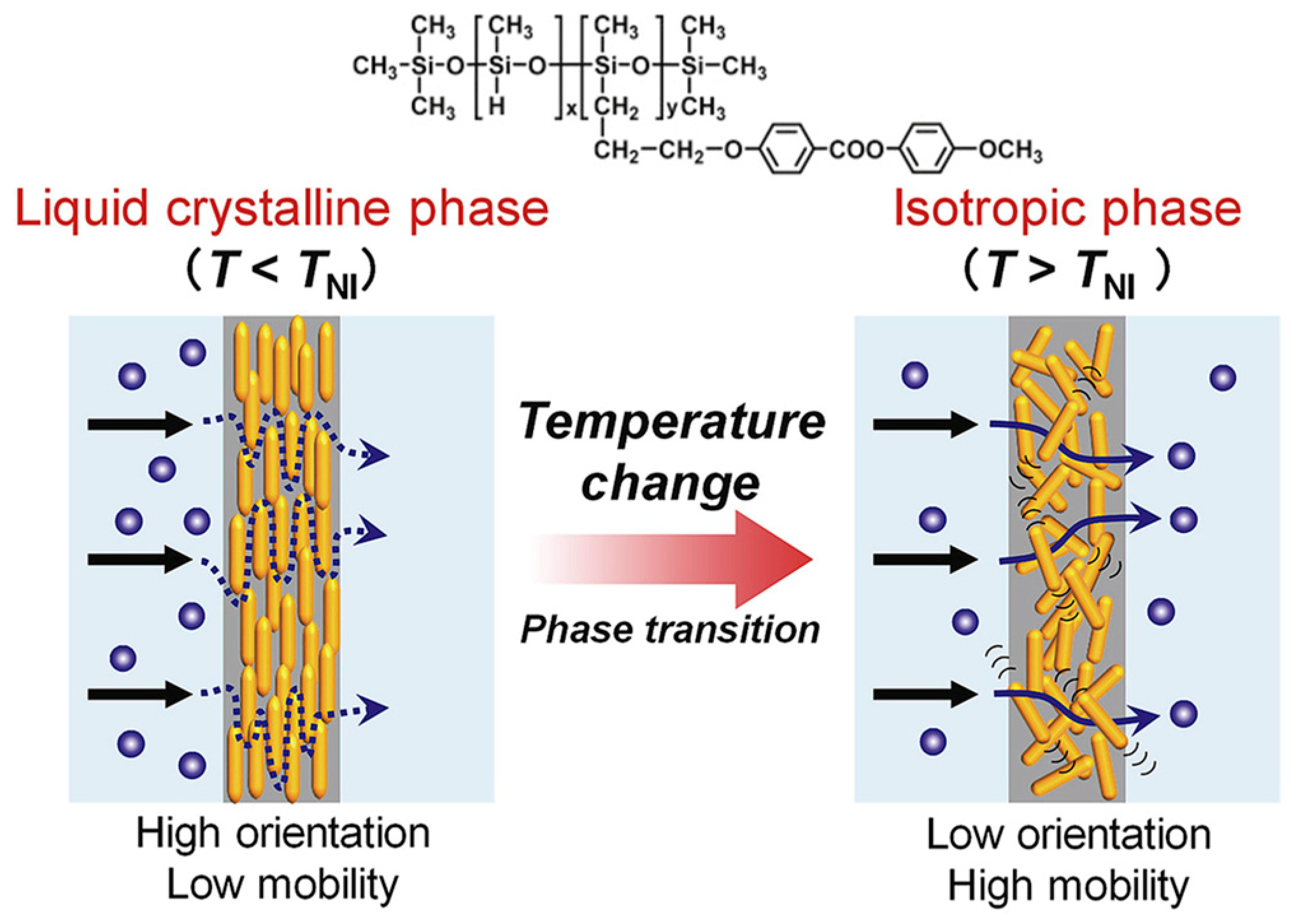
2.1. Chemical and Physical Properties
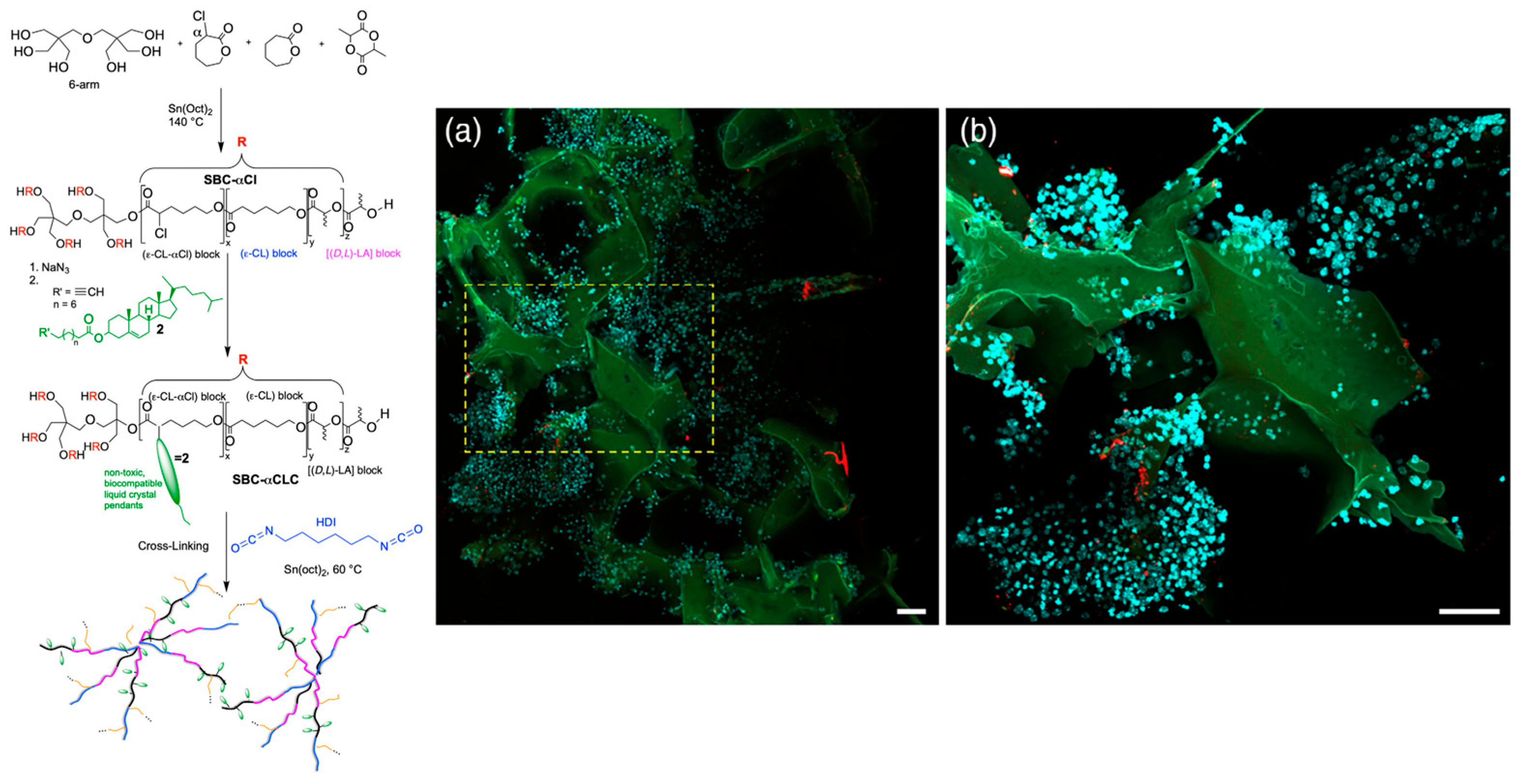
2.2. Synthetic Strategies
3. LCEs in Biomedical Engineering
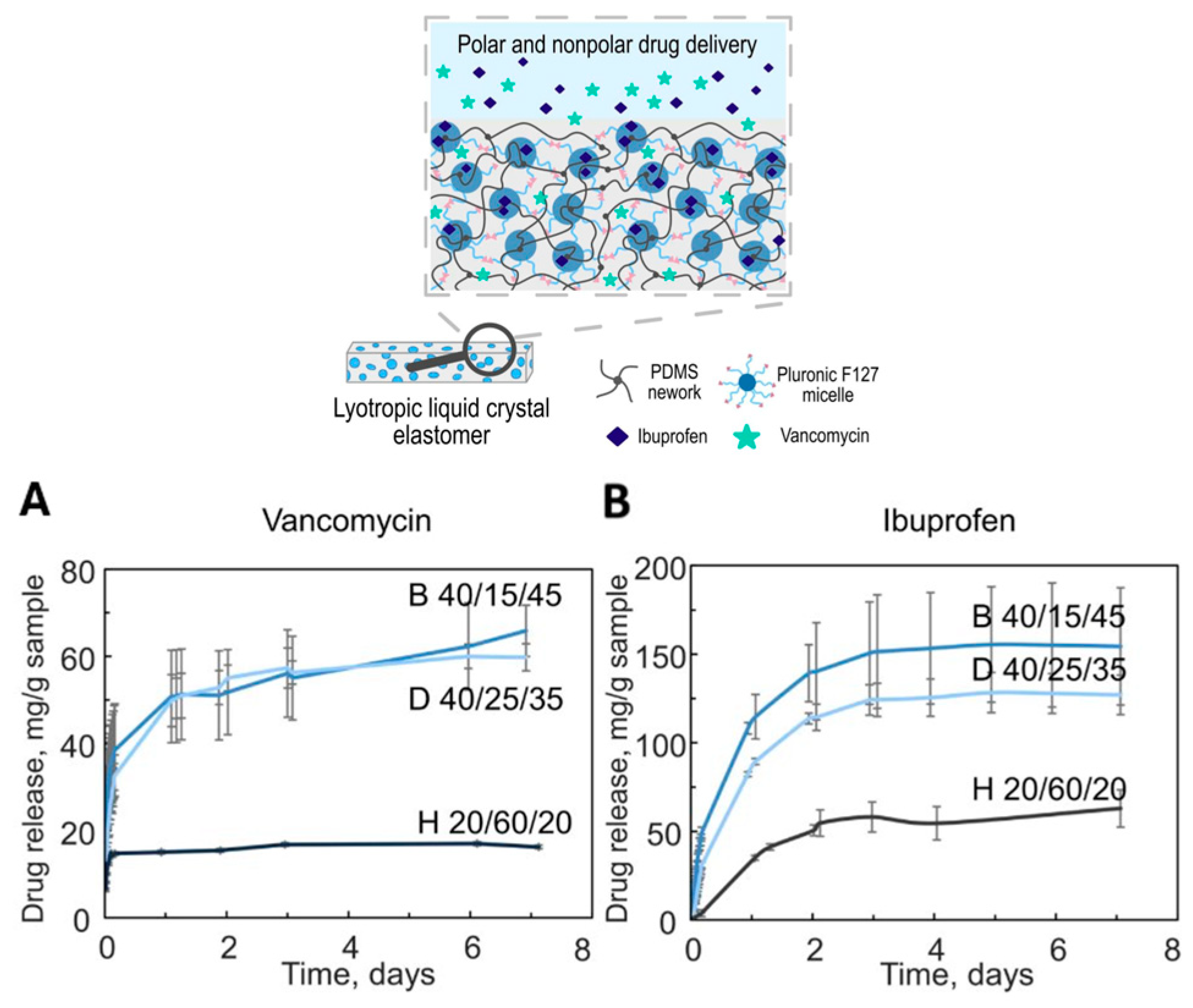
3.1. Drug Delivery Systems
3.2. Soft Robotics
3.3. Tissue Engineering
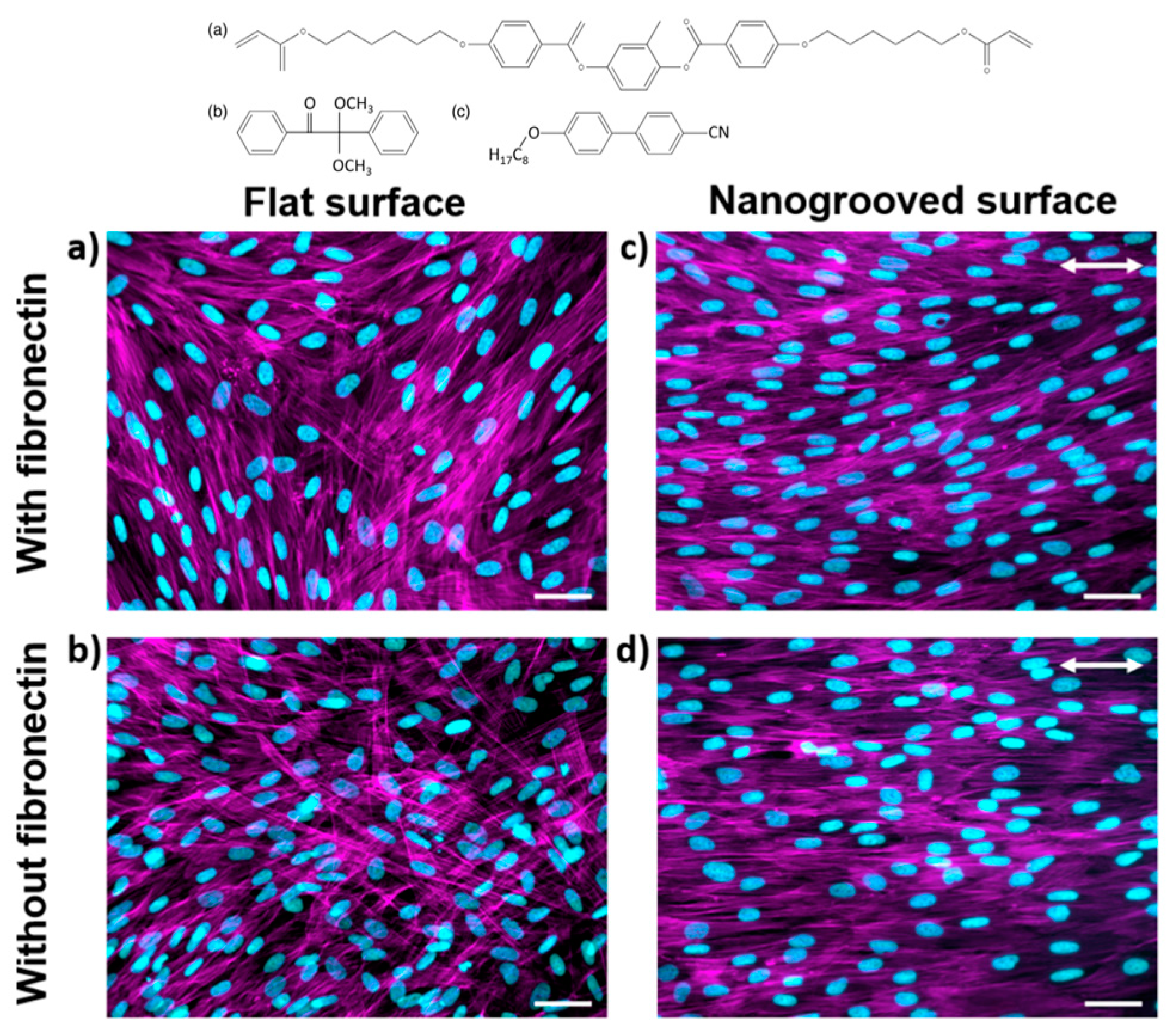
3.3.1. Cell Culture Platforms
3.3.2. Effects of Mechanical Properties of LCEs on Cell Behavior
3.4. Organ-on-a-Chip Applications
4. Biosensing Applications
5. 3D and 4D Printing of LCEs
5.1. 3D Printing
5.2. 4D Printing
6. Challenges and Limitations
7. Conclusions and Future Perspectives
7.1. Material Innovations
7.2. Expanding Biological Applications
Author Contributions
Funding
Conflicts of Interest
References
- Lehmann, O. Über fliessende krystalle. Z. Phys. Chem. 1889, 4, 462–472. [Google Scholar] [CrossRef]
- Reinitzer, F. Beiträge zur Kenntnis des Cholesterins. Monatshefte Chem. 1888, 9, 20. [Google Scholar] [CrossRef]
- Lehmann, O. Les cristaux liquides. J. Phys. Theor. Appl. 1909, 8, 713–735. [Google Scholar] [CrossRef]
- de Gennes, P.G. A semi-fast artificial muscle. Comptes Rendus L’Acad. Sci. Ser. IIB Mech. Phys. Chem. Astron. 1997, 5, 343–348. [Google Scholar] [CrossRef]
- de Gennes, P.G.; Hebert, M.; Kant, R. Artificial muscles based on nematic gels. Macromol. Symp. 1997, 113, 39–49. [Google Scholar] [CrossRef]
- Warner, M.; Terentjev, E. Liquid Crystal Elastomers; Oxford University Press (OUP): Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Saed, M.; Nair, D.P.; Gong, T.; Reed, S.M.; Bowman, C.N. Tailorable and programmable liquid-crystalline elastomers using a two-stage thiol-acrylate reaction. RSC Adv. 2015, 5, 18997–19001. [Google Scholar] [CrossRef]
- Martella, D.; Paoli, P.; Pioner, J.M.; Sacconi, L.; Coppini, R.; Santini, L.; Lulli, M.; Cerbai, E.; Wiersma, D.S.; Poggesi, C.; et al. Liquid Crystalline Networks toward Regenerative Medicine and Tissue Repair. Small 2017, 13, 1702677. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Chen, X.; Kang, I.-K. Advances in Bioinspired and Biomedical Materials Volume 2; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1253. [Google Scholar]
- Barón, M. Definitions of basic terms relating to low-molar-mass and polymer liquid crystals. Pure Appl. Chem. 2001, 73, 845–895. [Google Scholar] [CrossRef]
- Kerkam, K.; Viney, C.; Kaplan, D.; Lombardi, S. Liquid crystallinity of natural silk secretions. Nature 1991, 349, 596–598. [Google Scholar] [CrossRef]
- Willcox, P.J.; Gido, S.P.; Muller, W.; Kaplan, D.L. Evidence of a Cholesteric Liquid Crystalline Phase in Natural Silk Spinning Processes. Macromolecules 1996, 29, 5106–5110. [Google Scholar] [CrossRef]
- Fontana, F.; Bellini, T.; Todisco, M. Liquid Crystal Ordering in DNA Double Helices with Backbone Discontinuities. Macromolecules 2022, 55, 5946–5953. [Google Scholar] [CrossRef]
- Saw, T.B.; Doostmohammadi, A.; Nier, V.; Kocgozlu, L.; Thampi, S.; Toyama, Y.; Marcq, P.; Lim, C.T.; Yeomans, J.M.; Ladoux, B. Topological defects in epithelia govern cell death and extrusion. Nature 2017, 544, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Eggleson, K.K. Stem cell-based therapies: Promises, obstacles, discordance, and the agora. Perspect. Biol. Med. 2012, 55, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Prévôt, M.; Bergquist, L.; Sharma, A.; Mori, T.; Gao, Y.; Bera, T.; Zhu, C.; Leslie, M.; Cukelj, R.; Korley, L.T.J.; et al. New Developments in 3D Liquid Crystal Elastomers Scaffolds for Tissue Engineering: From Physical Template to Responsive Substrate; SPIE: Philadelphia, PA, USA, 2017. [Google Scholar]
- Prévôt, M.E.; Ustunel, S.; Bergquist, L.E.; Cukelj, R.; Gao, Y.; Mori, T.; Pauline, L.; Clements, R.J.; Hegmann, E. Synthesis of Biocompatible Liquid Crystal Elastomer Foams as Cell Scaffolds for 3D Spatial Cell Cultures. J. Vis. Exp. 2017, 122, e55452. [Google Scholar] [CrossRef]
- Ustunel, S.; Prévôt, M.E.; Rohaley, G.A.R.; Webb, C.R.; Yavitt, B.; Freychet, G.; Zhernenkov, M.; Pindak, R.; Schaible, E.; Zhu, C.; et al. Mechanically tunable elastomer and cellulose nanocrystal composites as scaffolds for in vitro cell studies. Mater. Adv. 2021, 2, 464–476. [Google Scholar] [CrossRef]
- Prévôt, M.; Hegmann, E. From Biomaterial, Biomimetic, and Polymer to Biodegradable and Biocompatible Liquid Crystal Elastomer Cell Scaffolds. In Advances in Bioinspired and Biomedical Materials Volume 2; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2017; Volume 1253, pp. 3–45. [Google Scholar]
- Cheng, H.; Hill, P.S.; Siegwart, D.J.; Vacanti, N.; Lytton-Jean, A.K.R.; Cho, S.W.; Ye, A.; Langer, R.; Anderson, D.G. A Novel Family of Biodegradable Poly(ester amide) Elastomers. Adv. Mater. 2011, 23, H95–H100. [Google Scholar] [CrossRef]
- Novak, U.; Kaye, A.H. Extracellular matrix and the brain: Components and function. J. Clin. Neurosci. 2000, 7, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Bartolo, P.; Domingos, M.; Gloria, A.; Ciurana, J. BioCell Printing: Integrated automated assembly system for tissue engineering constructs. CIRP Ann. 2011, 60, 271–274. [Google Scholar] [CrossRef]
- Guo, H.; Saed, M.O.; Terentjev, E.M. Thiol–acrylate side-chain liquid crystal elastomers. Soft Matter 2022, 18, 4803–4809. [Google Scholar] [CrossRef]
- Ustunel, S.; Prévôt, M.E.; Clements, R.J.; Hegmann, E. Cradle-to-cradle: Designing biomaterials to fit as truly biomimetic cell scaffolds—A review. Liq. Cryst. Today 2020, 29, 40–52. [Google Scholar] [CrossRef]
- Ustunel, S.; Sternbach, S.; Prévôt, M.E.; Freeman, E.J.; McDonough, J.A.; Clements, R.J.; Hegmann, E. 3D Co-culturing of human neuroblastoma and human oligodendrocytes, emulating native tissue using 3D porous biodegradable liquid crystal elastomers. J. Appl. Polym. Sci. 2023, 140, e53883. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef] [PubMed]
- Ambulo, C.P.; Burroughs, J.J.; Boothby, J.M.; Kim, H.; Shankar, M.R.; Ware, T.H. Four-dimensional Printing of Liquid Crystal Elastomers. ACS Appl. Mater. Interfaces 2017, 9, 37332–37339. [Google Scholar] [CrossRef] [PubMed]
- Kotikian, A.; Truby, R.L.; Boley, J.W.; White, T.J.; Lewis, J.A. 3D Printing of Liquid Crystal Elastomeric Actuators with Spatially Programed Nematic Order. Adv. Mater. 2018, 30, 1706164. [Google Scholar] [CrossRef] [PubMed]
- Mistry, D.; Traugutt, N.A.; Yu, K.; Yakacki, C.M. Processing and reprocessing liquid crystal elastomer actuators. J. Appl. Phys. 2021, 129, 130901. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Ustunel, S.; Yavitt, B.M.; Freychet, G.; Webb, C.R.; Zhernenkov, M.; Hegmann, E.; Pindak, R. Synchrotron Microbeam Diffraction Studies on the Alignment within 3D-Printed Smectic-A Liquid Crystal Elastomer Filaments during Extrusion. Crystals 2021, 11, 523. [Google Scholar] [CrossRef]
- Sharma, A.; Neshat, A.; Mahnen, C.J.; Nielsen, A.D.; Snyder, J.; Stankovich, T.L.; Daum, B.G.; LaSpina, E.M.; Beltrano, G.; Gao, Y.; et al. Biocompatible, Biodegradable and Porous Liquid Crystal Elastomer Scaffolds for Spatial Cell Cultures. Macromol. Biosci. 2015, 15, 200–214. [Google Scholar] [CrossRef]
- Sharma, A.; Mori, T.; Mahnen, C.J.; Everson, H.R.; Leslie, M.T.; Nielsen, A.d.; Lussier, L.; Zhu, C.; Malcuit, C.; Hegmann, T.; et al. Effects of Structural Variations on the Cellular Response and Mechanical Properties of Biocompatible, Biodegradable, and Porous Smectic Liquid Crystal Elastomers. Macromol. Biosci. 2017, 17, 1600278. [Google Scholar] [CrossRef]
- Prévôt, M.E.; Andro, H.; Alexander, S.L.M.; Ustunel, S.; Zhu, C.; Nikolov, Z.; Rafferty, S.T.; Brannum, M.T.; Kinsel, B.; Korley, L.T.J.; et al. Liquid crystal elastomer foams with elastic properties specifically engineered as biodegradable brain tissue scaffolds. Soft Matter 2018, 14, 354–360. [Google Scholar] [CrossRef]
- Prevot, M.E.; Ustunel, S.; Freychet, G.; Webb, C.R.; Zhernenkov, M.; Pindak, R.; Clements, R.J.; Hegmann, E. Physical Models from Physical Templates Using Biocompatible Liquid Crystal Elastomers as Morphologically Programmable Inks For 3D Printing. Macromol. Biosci. 2023, 23, e2200343. [Google Scholar] [CrossRef]
- Ring, M.E. The history of maxillofacial prosthetics. Plast. Reconstr. Surg. 1991, 87, 174–184. [Google Scholar] [CrossRef]
- Heng, W.; Solomon, S.; Gao, W. Flexible Electronics and Devices as Human–Machine Interfaces for Medical Robotics. Adv. Mater. 2022, 34, 2107902. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C.; Menciassi, A.; Dario, P. Biomedical applications of soft robotics. Nat. Rev. Mater. 2018, 3, 143–153. [Google Scholar] [CrossRef]
- Cianchetti, M.; Laschi, C. Pleasant to the Touch: By Emulating Nature, Scientists Hope to Find Innovative New Uses for Soft Robotics in Health-Care Technology. IEEE Pulse 2016, 7, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Wang, D.; Zhao, S.; Lou, Z.; Shen, G. Wearable Sensors-Enabled Human–Machine Interaction Systems: From Design to Application. Adv. Funct. Mater. 2021, 31, 2008936. [Google Scholar] [CrossRef]
- Yu, H.; Gold, J.I.; Wolter, T.J.; Bao, N.; Smith, E.; Zhang, H.A.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Actuating Liquid Crystals Rapidly and Reversibly by Using Chemical Catalysis. Adv. Mater. 2024, 36, 2309605. [Google Scholar] [CrossRef]
- McEvoy, M.A.; Correll, N. Materials that couple sensing, actuation, computation, and communication. Science 2015, 347, 1261689. [Google Scholar] [CrossRef]
- Hua, Q.; Sun, J.; Liu, H.; Bao, R.; Yu, R.; Zhai, J.; Pan, C.; Wang, Z.L. Skin-inspired highly stretchable and conformable matrix networks for multifunctional sensing. Nat. Commun. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Lagerwall, J.P.F. Embedding intelligence in materials for responsive built environment: A topical review on Liquid Crystal Elastomer actuators and sensors. Build. Environ. 2022, 226, 109714. [Google Scholar] [CrossRef]
- Hussain, M.; Jull, E.I.L.; Mandle, R.J.; Raistrick, T.; Hine, P.J.; Gleeson, H.F. Liquid Crystal Elastomers for Biological Applications. Nanomaterials 2021, 11, 813. [Google Scholar] [CrossRef]
- Herbert, K.M.; Fowler, H.E.; McCracken, J.M.; Schlafmann, K.R.; Koch, J.A.; White, T.J. Synthesis and alignment of liquid crystalline elastomers. Nat. Rev. Mater. 2021, 7, 23–38. [Google Scholar] [CrossRef]
- Resetic, A.; Milavec, J.; Bubnov, A.; Pociecha, D.; Hamplova, V.; Gorecka, E.; Zalar, B.; Domenici, V. New Liquid Crystalline Elastomeric Films Containing a Smectic Crosslinker: Chemical and Physical Properties. Crystals 2023, 13, 96. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yang, S. Multi-functional liquid crystal elastomer composites. Appl. Phys. Rev. 2022, 9, 011301. [Google Scholar] [CrossRef]
- Brannum, M.T.; Steele, A.M.; Venetos, M.C.; Korley, L.T.J.; Wnek, G.E.; White, T.J. Light Control with Liquid Crystalline Elastomers. Adv. Opt. Mater. 2019, 7, 1801683. [Google Scholar] [CrossRef]
- Modes, C.; Warner, M. Shape-programmable materials. Phys. Today 2016, 69, 32–38. [Google Scholar] [CrossRef]
- Babakhanova, G.; Turiv, T.; Guo, Y.; Hendrikx, M.; Wei, Q.H.; Schenning, A.; Broer, D.J.; Lavrentovich, O.D. Liquid crystal elastomer coatings with programmed response of surface profile. Nat. Commun. 2018, 9, 456. [Google Scholar] [CrossRef]
- Pei, Z.; Yang, Y.; Chen, Q.; Terentjev, E.M.; Wei, Y.; Ji, Y. Mouldable liquid-crystalline elastomer actuators with exchangeable covalent bonds. Nat. Mater. 2014, 13, 36–41. [Google Scholar] [CrossRef]
- Ware, T.H.; Perry, Z.P.; Middleton, C.M.; Iacono, S.T.; White, T.J. Programmable Liquid Crystal Elastomers Prepared by Thiol-Ene Photopolymerization. ACS Macro Lett. 2015, 4, 942–946. [Google Scholar] [CrossRef]
- Godman, N.P.; Kowalski, B.A.; Auguste, A.D.; Koerner, H.; White, T.J. Synthesis of Elastomeric Liquid Crystalline Polymer Networks via Chain Transfer. ACS Macro Lett. 2017, 6, 1290–1295. [Google Scholar] [CrossRef]
- Fowler, H.E.; Pearl, H.M.; Hoang, J.D.; White, T.J. Liquid Crystal Elastomers Prepared by Thiol-Ene Photopolymerization Amenable to Surface-Enforced Alignment. Macromolecules 2024, 57, 2619–2627. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Nozawa, I.; Suzuki, Y.; Sato, S.; Sugibayashi, K.; Morimoto, Y. Preparation of thermo-responsive membranes. II. J. Biomed. Mater. Res. 1991, 25, 577–588. [Google Scholar] [CrossRef]
- Chen, K.; Lin, Y.; Lin, S. Thermally on-off switching nylon membrane for controlling drug penetration. Drug Deliv. Syst. 1996, 11, 55–61. [Google Scholar] [CrossRef]
- Inoue, Y.; Atsumi, Y.; Kawamura, A.; Miyata, T. Thermoresponsive liquid crystalline polymer membranes that undergo phase transition at body temperature. J. Membr. Sci. 2019, 588, 117213. [Google Scholar] [CrossRef]
- Stepulane, A.; Ahlgren, K.; Rodriguez-Palomo, A.; Rajasekharan, A.K.; Andersson, M. Lyotropic liquid crystal elastomers for drug delivery. Colloid. Surface B 2023, 226, 113304. [Google Scholar] [CrossRef]
- Küpfer, J.; Finkelmann, H. Nematic liquid single crystal elastomers. Die Makromol. Chem. Rapid Commun. 1991, 12, 717–726. [Google Scholar] [CrossRef]
- Brochu, P.; Pei, Q. Advances in dielectric elastomers for actuators and artificial muscles. Macromol. Rapid Commun. 2010, 31, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, J.A.; Ambulo, C.P.; Lee, H.B.; Kim, S.H.; Naik, V.V.; Haines, C.S.; Aliev, A.E.; Ovalle-Robles, R.; Baughman, R.H.; et al. Intelligently Actuating Liquid Crystal Elastomer-Carbon Nanotube Composites. Adv. Funct. Mater. 2019, 29, 1905063. [Google Scholar] [CrossRef]
- Ambulo, C.P.; Ford, M.J.; Searles, K.; Majidi, C.; Ware, T.H. 4D-Printable Liquid Metal-Liquid Crystal Elastomer Composites. ACS Appl. Mater. Interfaces 2021, 13, 12805–12813. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Jiang, Z.C.; Tong, X.; Zhao, Y. Biomimetic Locomotion of Electrically Powered “Janus” Soft Robots Using a Liquid Crystal Polymer. Adv. Mater. 2019, 31, e1903452. [Google Scholar] [CrossRef] [PubMed]
- Shimoga, G.; Choi, D.-S.; Kim, S.-Y. Bio-Inspired Soft Robotics: Tunable Photo-Actuation Behavior of Azo Chromophore Containing Liquid Crystalline Elastomers. Appl. Sci. 2021, 11, 1233. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Fei, G.; Yu, B.; Tong, X.; Xia, H.; Zhao, Y. Liquid-Crystalline Dynamic Networks Doped with Gold Nanorods Showing Enhanced Photocontrol of Actuation. Adv. Mater. 2018, 30, e1706597. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Lu, H.C.; Lee, X.; Zeng, H.; Priimagi, A. Kirigami-Based Light-Induced Shape-Morphing and Locomotion. Adv. Mater. 2020, 32, e1906233. [Google Scholar] [CrossRef]
- Ma, S.; Li, X.; Huang, S.; Hu, J.; Yu, H. A Light-Activated Polymer Composite Enables On-Demand Photocontrolled Motion: Transportation at the Liquid/Air Interface. Angew. Chem. Int. Ed. Engl. 2019, 58, 2655–2659. [Google Scholar] [CrossRef]
- Li, Y.; Teixeira, Y.; Parlato, G.; Grace, J.; Wang, F.; Huey, B.D.; Wang, X. Three-dimensional thermochromic liquid crystal elastomer structures with reversible shape-morphing and color-changing capabilities for soft robotics. Soft Matter 2022, 18, 6857–6867. [Google Scholar] [CrossRef]
- Zhan, Y.; Broer, D.J.; Liu, D. Perspiring Soft Robotics Skin Constituted by Dynamic Polarity-Switching Porous Liquid Crystal Membrane. Adv. Mater. 2023, 35, e2211143. [Google Scholar] [CrossRef]
- Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2020.
- Martino, F.; Perestrelo, A.R.; Vinarsky, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824. [Google Scholar] [CrossRef]
- Nguyen, A.T.; Sathe, S.R.; Yim, E.K. From nano to micro: Topographical scale and its impact on cell adhesion, morphology and contact guidance. J. Phys. Condens. Matter 2016, 28, 183001. [Google Scholar] [CrossRef]
- Ye, K.; Wang, X.; Cao, L.; Li, S.; Li, Z.; Yu, L.; Ding, J. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015, 15, 4720–4729. [Google Scholar] [CrossRef]
- Tse, J.R.; Engler, A.J. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS ONE 2011, 6, e15978. [Google Scholar] [CrossRef]
- Abadia, A.V.; Herbert, K.M.; White, T.J.; Schwartz, D.K.; Kaar, J.L. Biocatalytic 3D Actuation in Liquid Crystal Elastomers via Enzyme Patterning. ACS Appl. Mater. Interfaces 2022, 14, 26480–26488. [Google Scholar] [CrossRef]
- Babakhanova, G.; Yu, H.; Chaganava, I.; Wei, Q.H.; Shiller, P.; Lavrentovich, O.D. Controlled Placement of Microparticles at the Water-Liquid Crystal Elastomer Interface. ACS Appl. Mater. Interfaces 2019, 11, 15007–15013. [Google Scholar] [CrossRef]
- Babakhanova, G.; Krieger, J.; Li, B.X.; Turiv, T.; Kim, M.H.; Lavrentovich, O.D. Cell alignment by smectic liquid crystal elastomer coatings with nanogrooves. J. Biomed. Mater. Res. A 2020, 108, 1223–1230. [Google Scholar] [CrossRef]
- Turiv, T.; Krieger, J.; Babakhanova, G.; Yu, H.; Shiyanovskii, S.V.; Wei, Q.H.; Kim, M.H.; Lavrentovich, O.D. Topology control of human fibroblast cells monolayer by liquid crystal elastomer. Sci. Adv. 2020, 6, eaaz6485. [Google Scholar] [CrossRef]
- Agrawal, A.; Adetiba, O.; Kim, H.; Chen, H.; Jacot, J.G.; Verduzco, R. Stimuli-responsive liquid crystal elastomers for dynamic cell culture. J. Mater. Res. 2015, 30, 453–462. [Google Scholar] [CrossRef]
- Herrera-Posada, S.; Mora-Navarro, C.; Ortiz-Bermudez, P.; Torres-Lugo, M.; McElhinny, K.M.; Evans, P.G.; Calcagno, B.O.; Acevedo, A. Magneto-responsive liquid crystalline elastomer nanocomposites as potential candidates for dynamic cell culture substrates. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 65, 369–378. [Google Scholar] [CrossRef]
- Khoo, I.C.; Clements, R.J.; McDonough, J.A.; Freeman, E.J.; Korley, L.T.; Cukelj, R.; Leslie, M.T.; Zhu, C.; Bera, T.; Gao, Y.; et al. New developments in 3D liquid crystal elastomers scaffolds for tissue engineering: From physical template to responsive substrate. In Proceedings of the Liquid Crystals XXI, San Diego, CA, USA, 25 August 2017. [Google Scholar]
- Shaha, R.K.; Merkel, D.R.; Anderson, M.P.; Devereaux, E.J.; Patel, R.R.; Torbati, A.H.; Willett, N.; Yakacki, C.M.; Frick, C.P. Biocompatible liquid-crystal elastomers mimic the intervertebral disc. J. Mech. Behav. Biomed. Mater. 2020, 107, 103757. [Google Scholar] [CrossRef]
- Harrison, R.G. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J. Exp. Zool. 2005, 9, 787–846. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Ingber, D.E. Microfluidic organs-on-chips. Nat. Biotechnol. 2014, 32, 760–772. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, N.; Yang, X.; Peng, B.; Jiang, H. New advances in microfluidic flow cytometry. Electrophoresis 2018, 40, 1212–1229. [Google Scholar] [CrossRef]
- Zhang, H.; Chang, H.; Neuzil, P. DEP-on-a-Chip: Dielectrophoresis Applied to Microfluidic Platforms. Micromachines 2019, 10, 423. [Google Scholar] [CrossRef]
- Genkin, M.M.; Sokolov, A.; Lavrentovich, O.D.; Aranson, I.S. Topological Defects in a Living Nematic Ensnare Swimming Bacteria. Phys. Rev. X 2017, 7, 011029. [Google Scholar] [CrossRef]
- Sengupta, A. Topological microfluidics: Present and prospects. Liquid Cryst. Today 2015, 24, 70–80. [Google Scholar] [CrossRef]
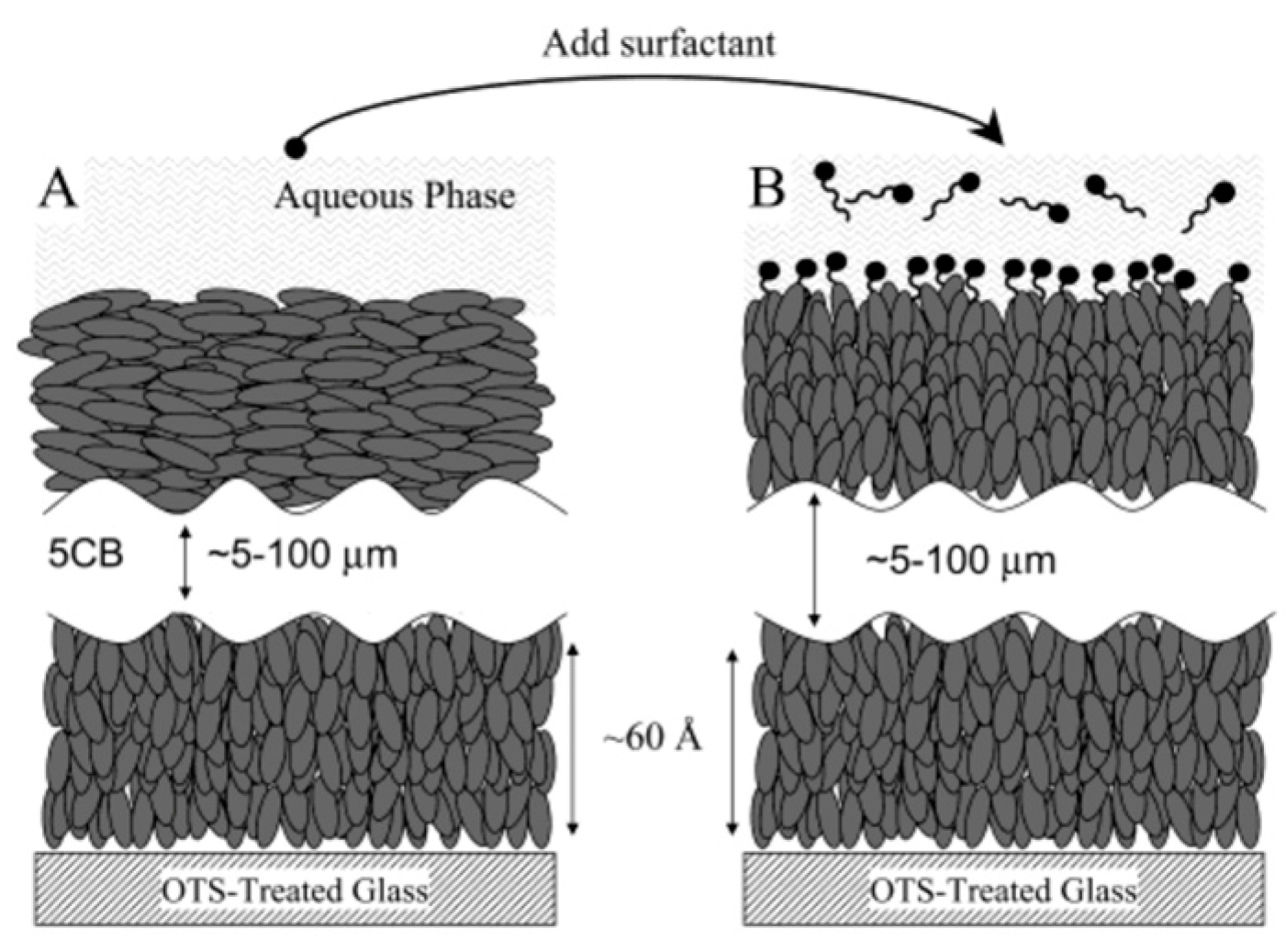
- Gupta, V.K.; Skaife, J.J.; Dubrovsky, T.B.; Abbott, N.L. Optical Amplification of Ligand-ReceptorBinding Using Liquid Crystals. Science 1998, 279, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Brake, J.M.; Abbott, N.L. An experimental system for imaging the reversible adsorption of amphiphiles at aqueous-liquid crystal interfaces. Langmuir 2002, 18, 6101–6109. [Google Scholar] [CrossRef]
- Bedjaoui, L.; Gogibus, N.; Ewen, B.; Pakula, T.; Coqueret, X.; Benmouna, M.; Maschke, U. Preferential solvation of the eutectic mixture of liquid crystals E7 in a polysiloxane. Polymer 2004, 45, 6555–6560. [Google Scholar] [CrossRef]
- Nolan, P.; Tillin, M.; Coates, D. Liquid Crystal Microdroplet Composition in a UV Cured PDLC Film. Mol. Cryst. Liq. Cryst. Lett. 1992, 8, 129–135. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.R.; Shin, J.H.; Park, S.Y. A liquid-crystal-based DNA biosensor for pathogen detection. Sci. Rep. 2016, 6, 22676. [Google Scholar] [CrossRef]
- Xu, Y.; Rather, A.M.; Song, S.; Fang, J.C.; Dupont, R.L.; Kara, U.I.; Chang, Y.; Paulson, J.A.; Qin, R.; Bao, X.; et al. Ultrasensitive and Selective Detection of SARS-CoV-2 Using Thermotropic Liquid Crystals and Image-Based Machine Learning. Cell Rep. Phys. Sci. 2020, 1, 100276. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.-G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Olefsky, J.M. The insulin receptor: Its role in insulin resistance of obesity and diabetes. Diabetes 1976, 25, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
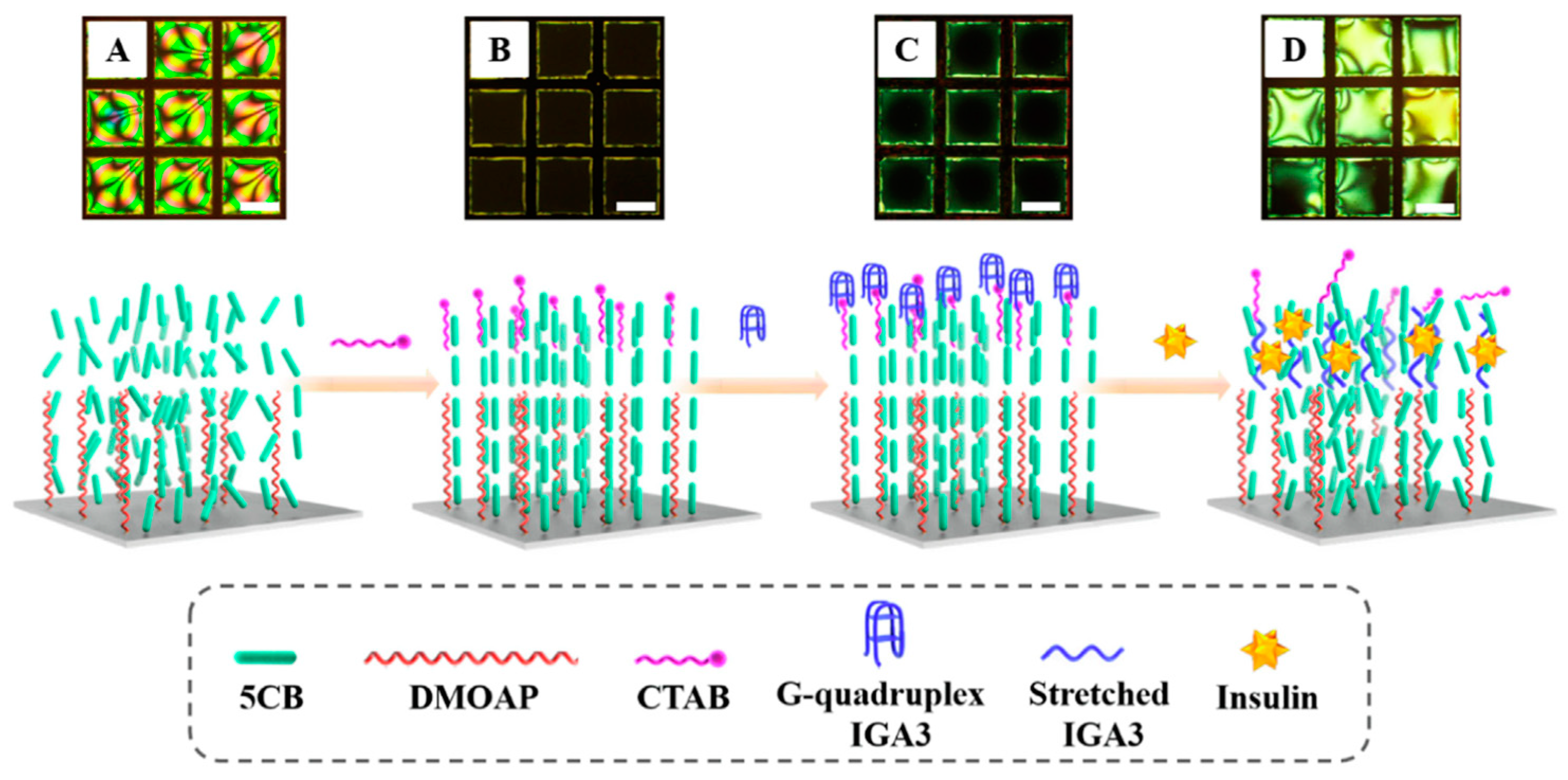
- Chen, J.M.; Liu, Z.P.; Yang, R.Z.; Liu, M.J.; Feng, H.Q.; Li, N.; Jin, M.L.; Zhang, M.M.; Shui, L.L. A liquid crystal-based biosensor for detection of insulin driven by conformational change of an aptamer at aqueous-liquid crystal interface. J. Colloid. Interf. Sci. 2022, 628, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.W.; Roberts, E.; Beck, J.C.; Fiske, B.; Ross, W.; Savica, R.; Van Den Eeden, S.K.; Tanner, C.M.; Marras, C.; Parkinson’s Foundation, P.G. Incidence of Parkinson disease in North America. NPJ Park. Dis. 2022, 8, 170. [Google Scholar] [CrossRef]
- Zheng, Y.; Qu, J.; Xue, F.; Zheng, Y.; Yang, B.; Chang, Y.; Yang, H.; Zhang, J. Novel DNA Aptamers for Parkinson’s Disease Treatment Inhibit α-Synuclein Aggregation and Facilitate its Degradation. Mol. Ther.-Nucleic Acids 2018, 11, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, H.; Zhao, X.; Liao, W.; Zhang, C.X.; Yang, Z. A novel, label-free liquid crystal biosensor for Parkinson’s disease related alpha-synuclein. Chem. Commun. 2020, 56, 5441–5444. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, X.; Liu, F.; Li, H.; Zhang, C.X.; Yang, Z. Simple, rapid and sensitive detection of Parkinson’s disease related alpha-synuclein using a DNA aptamer assisted liquid crystal biosensor. Soft Matter 2021, 17, 4842–4847. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s Disease Facts and Figures, Wiley: Hoboken, NJ, USA, 2023. [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Kemiklioglu, E.; Tuncgovde, E.B.; Ozsarlak-Sozer, G. Development of liquid crystal biosensor for the detection of amyloid beta-42 levels associated with Alzheimer’s disease. J. Biosci. Bioeng. 2021, 132, 88–94. [Google Scholar] [CrossRef]
- Mileson, B.E.; Chambers, J.E.; Chen, W.L.; Dettbarn, W.; Ehrich, M.; Eldefrawi, A.T.; Gaylor, D.W.; Hamernik, K.; Hodgson, E.; Karczmar, A.G.; et al. Common Mechanism of Toxicity: A Case Study of Organophosphorus Pesticides. Toxicol. Sci. 1998, 41, 8–20. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Q.Z.; Guo, Y.X.; Yu, L. A cationic surfactant-decorated liquid crystal sensing platform for simple and sensitive detection of acetylcholinesterase and its inhibitor. Biosens. Bioelectron. 2015, 72, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Q.Z.; Tian, T.T.; Yu, L. Simple and sensitive detection of pesticides using the liquid crystal droplet patterns platform. Sens. Actuat. B-Chem. 2017, 238, 676–682. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.H. Micro-capillary sensor for imaging trypsin activity using confined nematic liquid crystals. J. Mol. Liq. 2016, 222, 596–600. [Google Scholar] [CrossRef]
- Rim, J.; Jang, C.H. Detection of catalase activity with aldehyde-doped liquid crystals confined in microcapillaries. Anal. Biochem. 2018, 560, 19–23. [Google Scholar] [CrossRef]
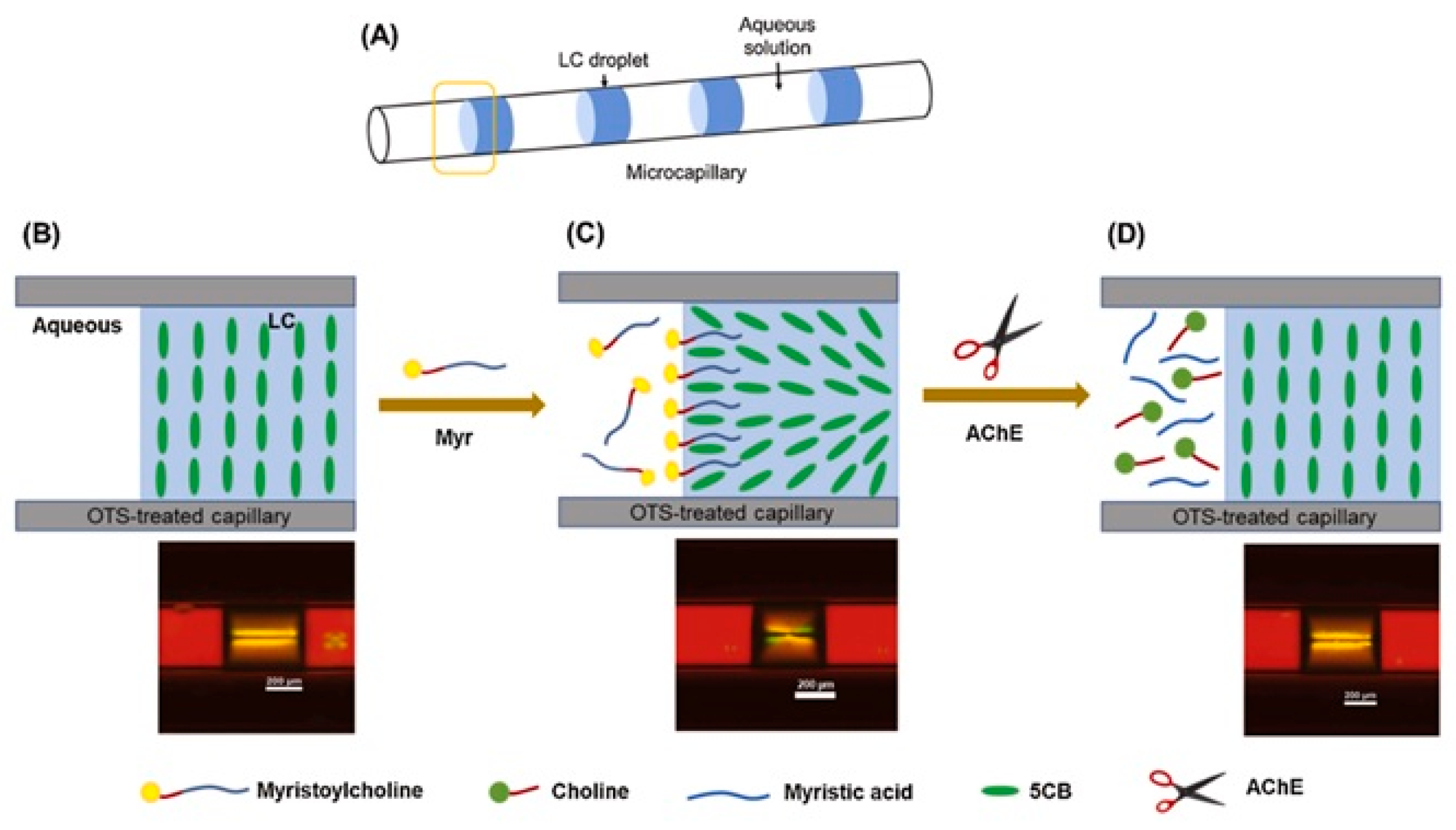
- Nguyen, D.K.; Jang, C.H. An acetylcholinesterase-based biosensor for the detection of pesticides using liquid crystals confined in microcapillaries. Colloids Surf. B Biointerfaces 2021, 200, 111587. [Google Scholar] [CrossRef]
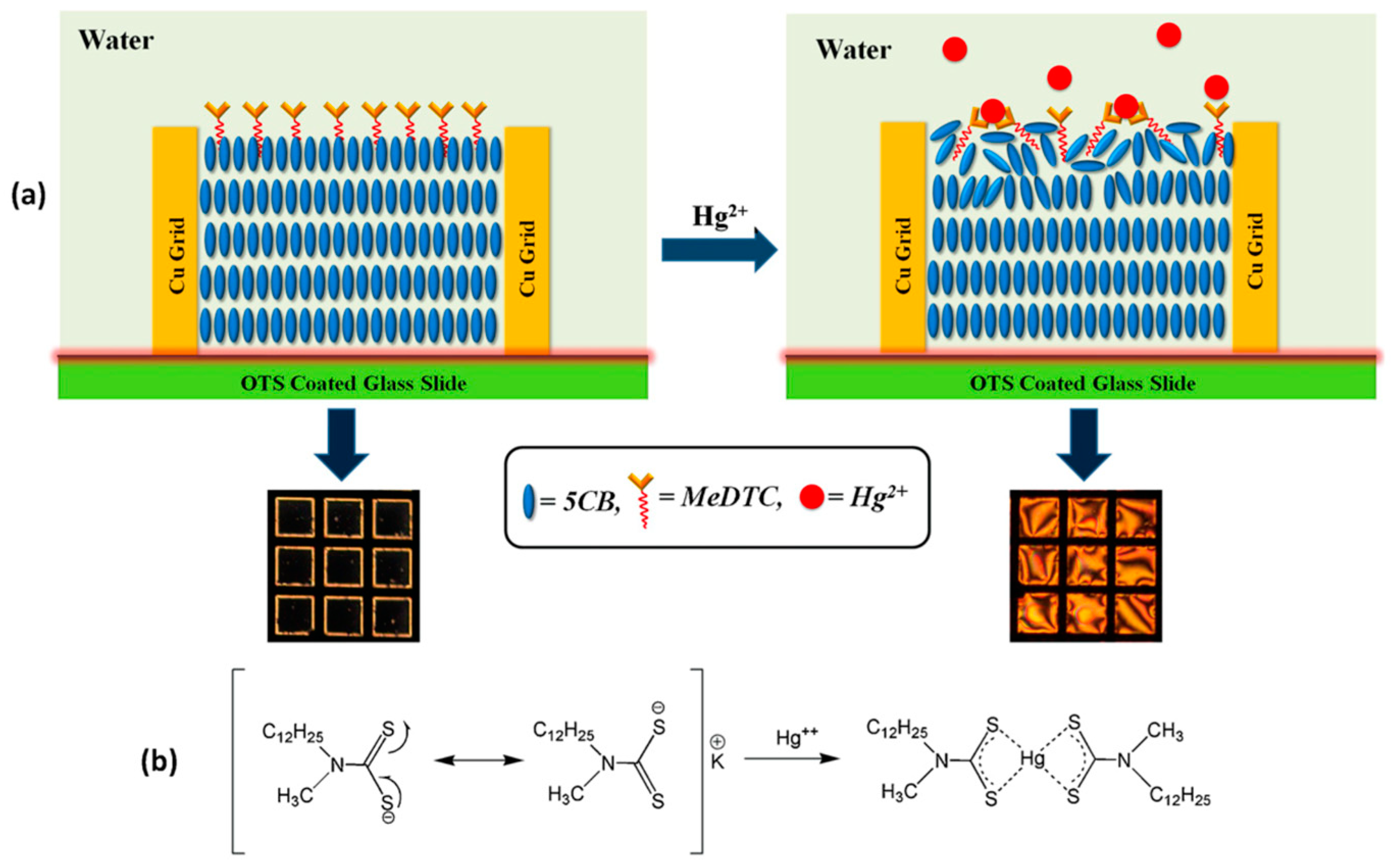
- Singh, S.K.; Nandi, R.; Mishra, K.; Singh, H.K.; Singh, R.K.; Singh, B. Liquid crystal based sensor system for the real time detection of mercuric ions in water using amphiphilic dithiocarbamate. Sens. Actuat. B-Chem. 2016, 226, 381–387. [Google Scholar] [CrossRef]
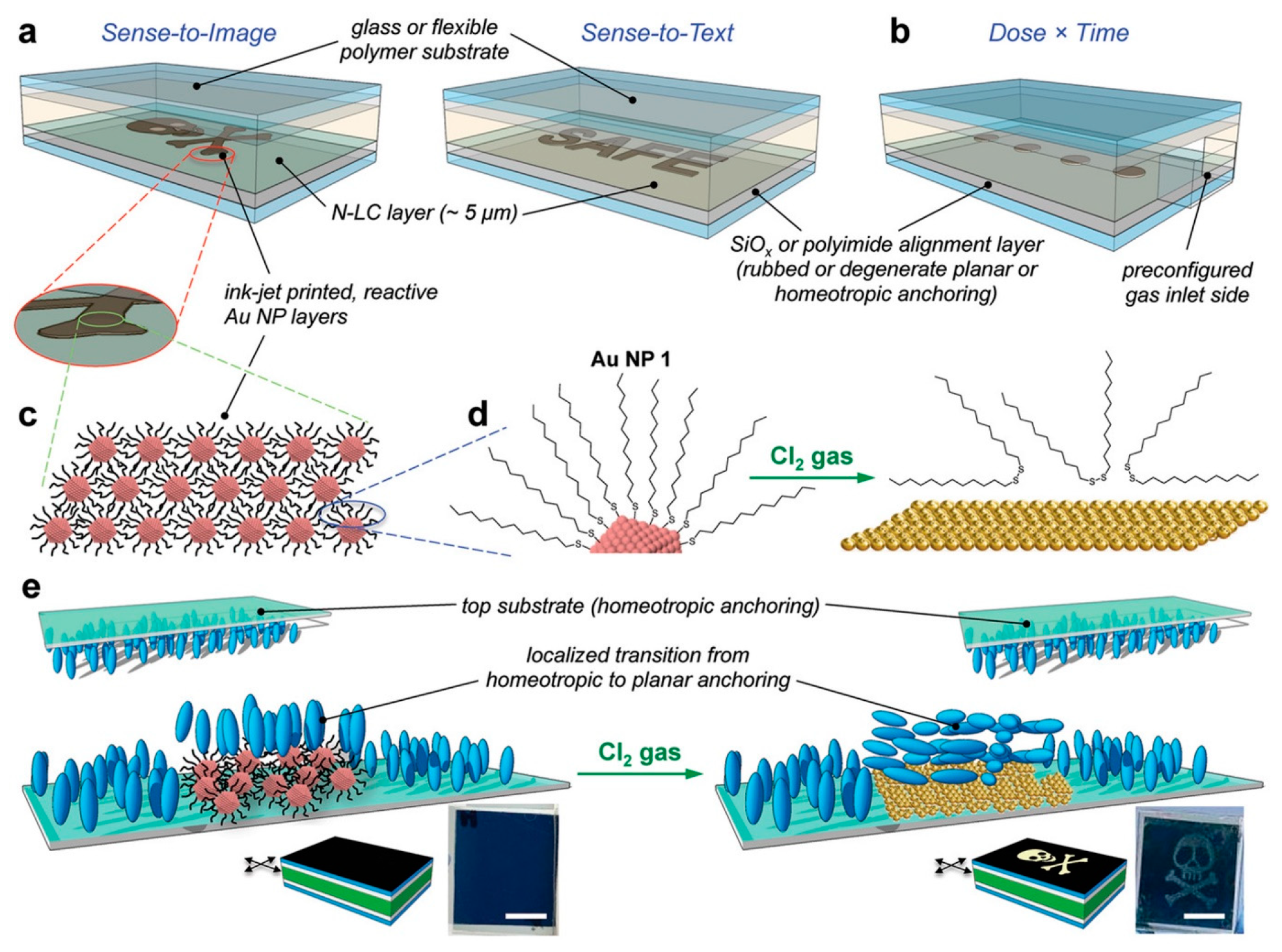
- Prévôt, M.E.; Nemati, A.; Cull, T.R.; Hegmann, E.; Hegmann, T. A Zero-Power Optical, ppt- to ppm-Level Toxic Gas and Vapor Sensor with Image, Text, and Analytical Capabilities. Adv. Mater. Technol. 2020, 5, 2000058. [Google Scholar] [CrossRef]
- Ailincai, D.; Pamfil, D.; Marin, L. Multiple bio-responsive polymer dispersed liquid crystal composites for sensing applications. J. Mol. Liq. 2018, 272, 572–582. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jakli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef]
- Seo, J.M.; Khan, W.; Park, S.Y. Protein detection using aqueous/LC interfaces decorated with a novel polyacrylic acid block liquid crystalline polymer. Soft Matter 2012, 8, 198–203. [Google Scholar] [CrossRef]
- Li, L.; Bai, H.; Dong, X.; Jiang, Y.; Li, Q.; Wang, Q.; Yuan, N.; Ding, J. Flexible Capacitive Sensors Based on Liquid Crystal Elastomer. Langmuir 2023, 39, 12412–12419. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, H.J.; Ahn, H.; Han, W.C.; Yun, H.S.; Choi, Y.S.; Kim, D.S.; Yoon, D.K. Mechanochromic Palettes of Cholesteric Liquid Crystal Elastomers for Visual Signaling. Adv. Opt. Mater. 2024, 12, 2400266. [Google Scholar] [CrossRef]
- Fallah-Darrehchi, M.; Zahedi, P.; Harirchi, P.; Abdouss, M. Performance of Liquid Crystalline Elastomers on Biological Cell Response: A Review. ACS Appl. Polym. Mater. 2023, 5, 1076–1091. [Google Scholar] [CrossRef]
- Shimatani, A.; Hoshi, M.; Oebisu, N.; Iwai, T.; Takada, N.; Nakamura, H. Clinical significance of thermal detection of soft-tissue tumors. Int. J. Clin. Oncol. 2020, 25, 1418–1424. [Google Scholar] [CrossRef]
- Deng, F.; Tang, Q.; Zeng, G.; Wu, H.; Zhang, N.; Zhong, N. Effectiveness of digital infrared thermal imaging in detecting lower extremity deep venous thrombosis. Med. Phys. 2015, 42, 2242–2248. [Google Scholar] [CrossRef]
- Fierheller, M.; Sibbald, R.G. A clinical investigation into the relationship between increased periwound skin temperature and local wound infection in patients with chronic leg ulcers. Adv. Skin Wound Care 2010, 23, 369–379; quiz 380–361. [Google Scholar] [CrossRef]
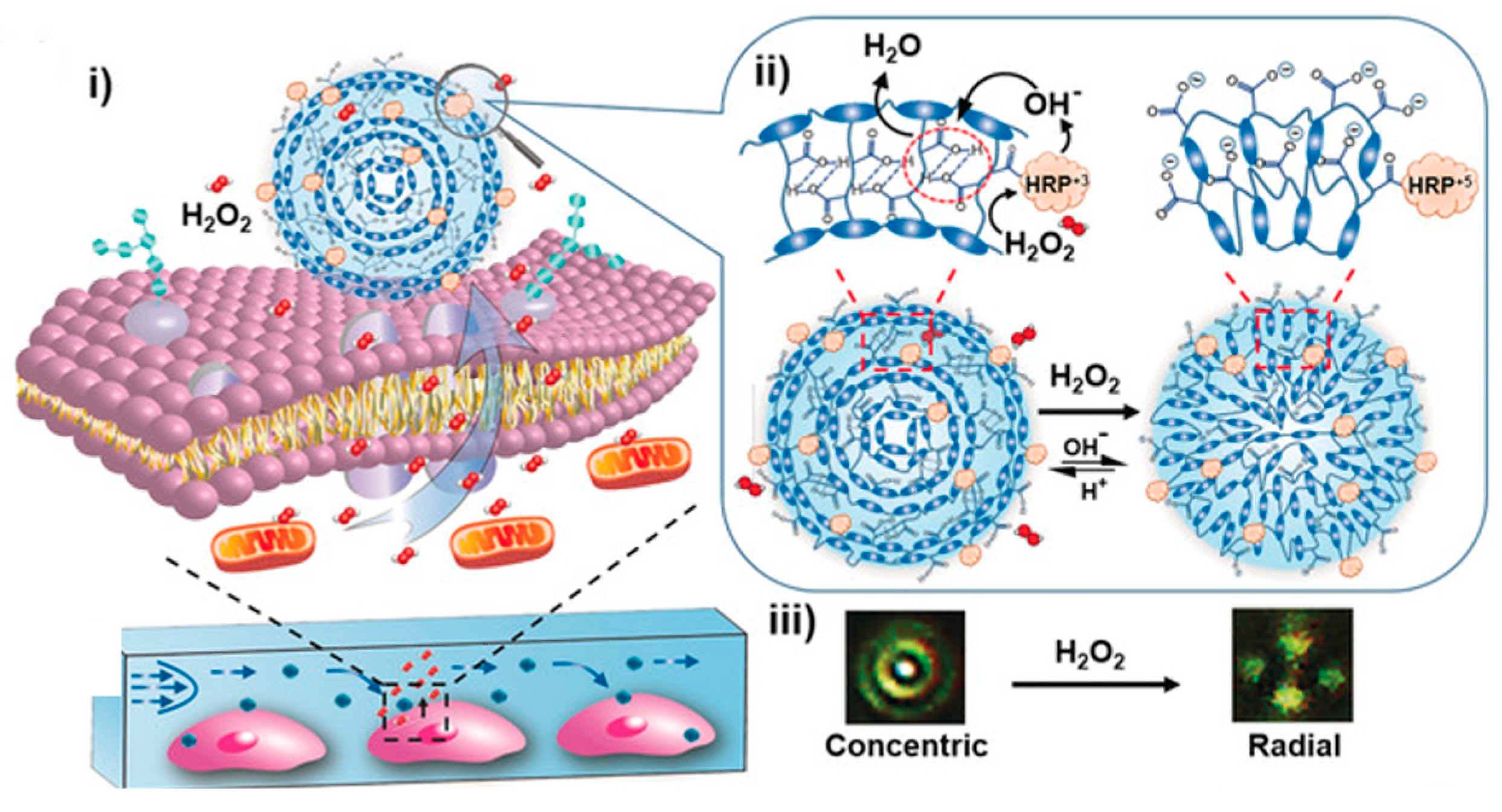
- Li, W.; Khan, M.; Lin, L.; Zhang, Q.; Feng, S.; Wu, Z.; Lin, J. Monitoring H2O2 on the Surface of Single Cells with Liquid Crystal Elastomer Microspheres. Angew. Chem. 2020, 132, 9368–9373. [Google Scholar] [CrossRef]
- Velasco-Abadia, A.; White, T.J.; Schwartz, D.K.; Kaar, J.L. 4D Cumulative Dose Sensing of Malathion Using a Biocatalytic Liquid Crystal Elastomer with Chemical Memory. Sens. Actuators B Chem. 2024, 400, 134877. [Google Scholar] [CrossRef]
- Davidson, E.C.; Kotikian, A.; Li, S.; Aizenberg, J.; Lewis, J.A. 3D Printable and Reconfigurable Liquid Crystal Elastomers with Light-Induced Shape Memory via Dynamic Bond Exchange. Adv. Mater. 2020, 32, e1905682. [Google Scholar] [CrossRef]
- Kotikian, A.; Morales, J.M.; Lu, A.; Mueller, J.; Davidson, Z.S.; Boley, J.W.; Lewis, J.A. Innervated, Self-Sensing Liquid Crystal Elastomer Actuators with Closed Loop Control. Adv. Mater. 2021, 33, e2101814. [Google Scholar] [CrossRef] [PubMed]
- Saed, M.O.; Ambulo, C.P.; Kim, H.; De, R.; Raval, V.; Searles, K.; Siddiqui, D.A.; Cue, J.M.O.; Stefan, M.C.; Shankar, M.R.; et al. Molecularly-Engineered, 4D-Printed Liquid Crystal Elastomer Actuators. Adv. Funct. Mater. 2018, 29, 1806412. [Google Scholar] [CrossRef]
- Lu, X.; Ambulo, C.P.; Wang, S.; Rivera-Tarazona, L.K.; Kim, H.; Searles, K.; Ware, T.H. 4D-Printing of Photoswitchable Actuators. Angew. Chem. Int. Ed. Engl. 2021, 60, 5536–5543. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Tarazona, L.K.; Shukla, T.; Singh, K.A.; Gaharwar, A.K.; Campbell, Z.T.; Ware, T.H. 4D Printing of Engineered Living Materials. Adv. Funct. Mater. 2021, 32, 2106843. [Google Scholar] [CrossRef]
- McDougall, L.; Herman, J.; Huntley, E.; Leguizamon, S.; Cook, A.; White, T.; Kaehr, B.; Roach, D.J. Free-Form Liquid Crystal Elastomers via Embedded 4D Printing. ACS Appl. Mater. Interfaces 2023, 15, 58897–58904. [Google Scholar] [CrossRef]
- Ambulo, C.P.; Tasmim, S.; Wang, S.; Abdelrahman, M.K.; Zimmern, P.E.; Ware, T.H. Processing advances in liquid crystal elastomers provide a path to biomedical applications. J. Appl. Phys. 2020, 128, 140901. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Zhao, Y.; Ye, F.; Shang, L. Liquid Crystal Materials for Biomedical Applications. Adv. Mater. 2023, 35, e2300220. [Google Scholar] [CrossRef]
- Barnes, M.; Cetinkaya, S.; Ajnsztajn, A.; Verduzco, R. Understanding the effect of liquid crystal content on the phase behavior and mechanical properties of liquid crystal elastomers. Soft Matter 2022, 18, 5074–5081. [Google Scholar] [CrossRef]
- Ustunel, S.; Pandya, H.; Prevot, M.E.; Pegorin, G.; Shiralipour, F.; Paul, R.; Clements, R.J.; Khabaz, F.; Hegmann, E. A Molecular Rheology Dynamics Study on 3D Printing of Liquid Crystal Elastomers. Macromol. Rapid Commun. 2024, 45, e2300717. [Google Scholar] [CrossRef]
- Zhang, W.; Nan, Y.; Wu, Z.; Shen, Y.; Luo, D. Photothermal-Driven Liquid Crystal Elastomers: Materials, Alignment and Applications. Molecules 2022, 27, 4330. [Google Scholar] [CrossRef]
- Ford, M.J.; Ambulo, C.P.; Kent, T.A.; Markvicka, E.J.; Pan, C.; Malen, J.; Ware, T.H.; Majidi, C. A multifunctional shape-morphing elastomer with liquid metal inclusions. Proc. Natl. Acad. Sci. USA 2019, 116, 21438–21444. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Y.; Hu, W.; Soon, R.H.; Davidson, Z.S.; Sitti, M. Liquid Crystal Elastomer-Based Magnetic Composite Films for Reconfigurable Shape-Morphing Soft Miniature Machines. Adv. Mater. 2021, 33, e2006191. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Mu, T.; Liu, Y.; Leng, J. Harnessing the power of carbon fiber reinforced liquid crystal elastomer composites for high-performance aerospace materials: A comprehensive investigation on reversible transformation and shape memory deformation. Compos. Part A Appl. Sci. Manuf. 2024, 177, 107943. [Google Scholar] [CrossRef]
- Mistry, D.; Connell, S.D.; Mickthwaite, S.L.; Morgan, P.B.; Clamp, J.H.; Gleeson, H.F. Coincident molecular auxeticity and negative order parameter in a liquid crystal elastomer. Nat. Commun. 2018, 9, 5095. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.J.; Reynolds, M.; Raistrick, T.; Berrow, S.R.; Jull, E.I.L.; Reshetnyak, V.; Mistry, D.; Gleeson, H.F. Controlling the optical properties of transparent auxetic liquid crystal elastomers. Macromolecules 2024, 57, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Mazaev, A.V.; Ajeneza, O.; Shitikova, M.V. Auxetic materials: Classification, mechanical properties and applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 747, 012008. [Google Scholar] [CrossRef]
- Ebrahimi, M.S.; Noruzi, M.; Hamzehei, R.; Etemadi, E.; Hashemi, R. Revolutionary auxetic intravascular medical stents for angioplasty applications. Mater. Des. 2023, 235, 112393. [Google Scholar] [CrossRef]
- Amigo-Melchior, A.; Finkelmann, H. A concept for bifocal contact- or intraocular lenses: Liquid single crystal hydrogels (“LSCH”). Polym. Adv. Technol. 2002, 13, 363–369. [Google Scholar] [CrossRef]
- Foster, L.; Peketi, P.; Allen, T.; Senior, T.; Duncan, O.; Alderson, A. Application of auxetic foam in sports helmets. Appl. Sci. 2018, 8, 354. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiralipour, F.; Nik Akhtar, Y.; Gilmor, A.; Pegorin, G.; Valerio-Aguilar, A.; Hegmann, E. The Role of Liquid Crystal Elastomers in Pioneering Biological Applications. Crystals 2024, 14, 859. https://doi.org/10.3390/cryst14100859
Shiralipour F, Nik Akhtar Y, Gilmor A, Pegorin G, Valerio-Aguilar A, Hegmann E. The Role of Liquid Crystal Elastomers in Pioneering Biological Applications. Crystals. 2024; 14(10):859. https://doi.org/10.3390/cryst14100859
Chicago/Turabian StyleShiralipour, Faeze, Yeganeh Nik Akhtar, Ashley Gilmor, Gisele Pegorin, Abraham Valerio-Aguilar, and Elda Hegmann. 2024. "The Role of Liquid Crystal Elastomers in Pioneering Biological Applications" Crystals 14, no. 10: 859. https://doi.org/10.3390/cryst14100859





