Abstract
Three new cationic complexes, [Cu4Tb2(H2L)4(NO3)4(H2O)3](NO3)2·5.5H2O·2MeOH (1), [Cu4Ho2(H2L)4(NO3)4(H2O)3](NO3)2·7.5H2O (2), and [Cu4Er2(H2 L)4(NO3)4(H2O)3](NO3)2·7H2O·3MeOH (3), were synthesized and studied using elemental and TG/DTG/DSC analyses, single-crystal X-ray diffraction, and magnetic measurements. The structure analysis showed that 1–3 crystallize as (NO3)-bridged compounds and that the lanthanide(III) ion acts as a joint connecting two [CuH2L] coordination units. In each heterotrinuclear unit, an asymmetry in the degree of planarity of the bridging CuO2Ln fragments is observed. The CuII ions are five- and six-coordinate, with distorted square pyramidal and octahedral geometry, respectively, whereas the LnIII ions are nine-coordinate. The solvates 1–3 are stable at room temperature, and their desolvation process is consistent with the loss of water and/or methanol molecules. The temperature dependence of the magnetic susceptibility and the field-dependent magnetization indicate the weak ferromagnetic interaction between the paramagnetic centers CuII and TbIII/HoIII 1 and 2.
1. Introduction
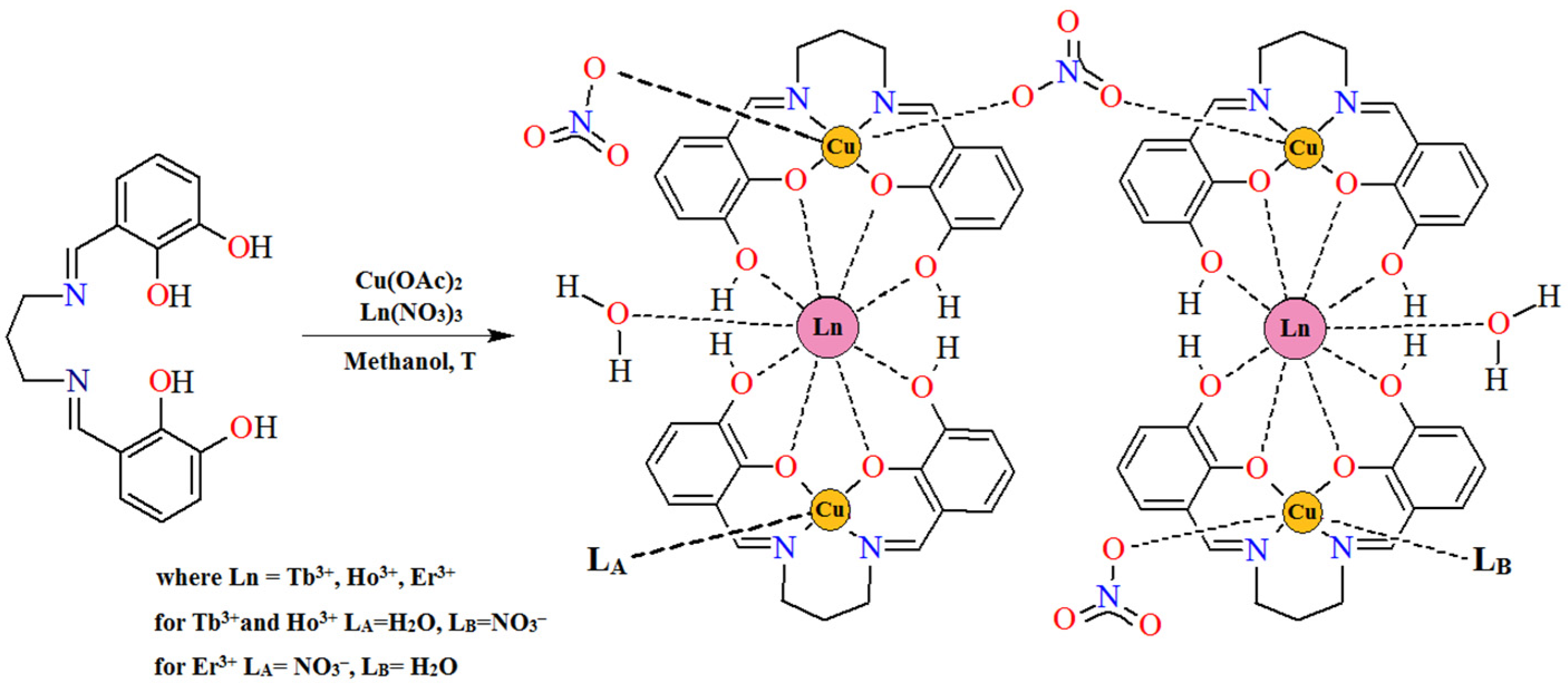
The synthesis of the new homo- and heteronuclear compounds has attracted a great deal of interest from the research community. [1,2,3,4,5,6]. Polydentate Schiff bases (Figure 1) as ligands are essential tools for the synthesis of coordination compounds, which are widely studied due to their interesting structure and potential applications. The coordination diversity of the Schiff base favors this process and provides the possibility to form different complexes with various metals ratios [7] (Figure 2 and Figure 3). Different synthesis conditions also facilitate this process. One of the main factors is the choice of solvent. The most popular solvents, ethanol (EtOH), methanol (MeOH), or acetonitrile (ACN), play a crucial role in the synthesis and crystallization processes. They lead to the obtaining of compounds with different crystal structures and compositions, which are connected to their physio-chemical properties. Moreover, the crystal structures are determined by the type of metal ions used, the nature and position of the ligands, and the methods of the synthesis (a stepwise reaction or a one-pot reaction) [8,9,10,11].
By analyzing the coordination abilities of Schiff bases in terms of the possibility of attaching single metal ions, it can be observed that most Schiff bases formed in the reaction of diamines and their derivatives with o-hydroxyaromatic aldehydes/ketones coordinate a 3d ion in the tetradentate cavity of N2O2. It is possible to obtain mono- [12,13], di- [14,15,16,17,18], tri- [19,20], tetra- [16,21], and even hexanuclear [22] complexes (Figure 2). In the homodinuclear compounds, the metal:ligand ratio can be 2:1, 2:2, 3:2. In the second case, the central ions most often connect to the symmetric units of the ligand through the deprotonated hydroxyl groups of the Schiff base. In the homotrinuclear complexes, the central ions are arranged linearly and are alternately connected to each other by a bridging element, which may be an acetate ion, for example [19,20].
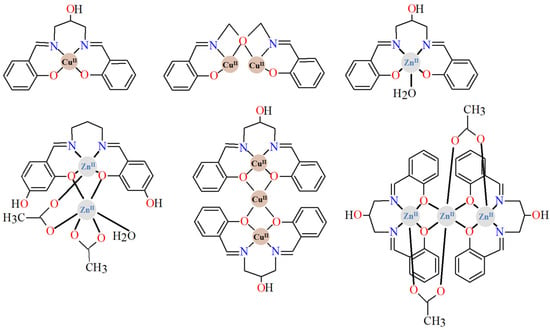
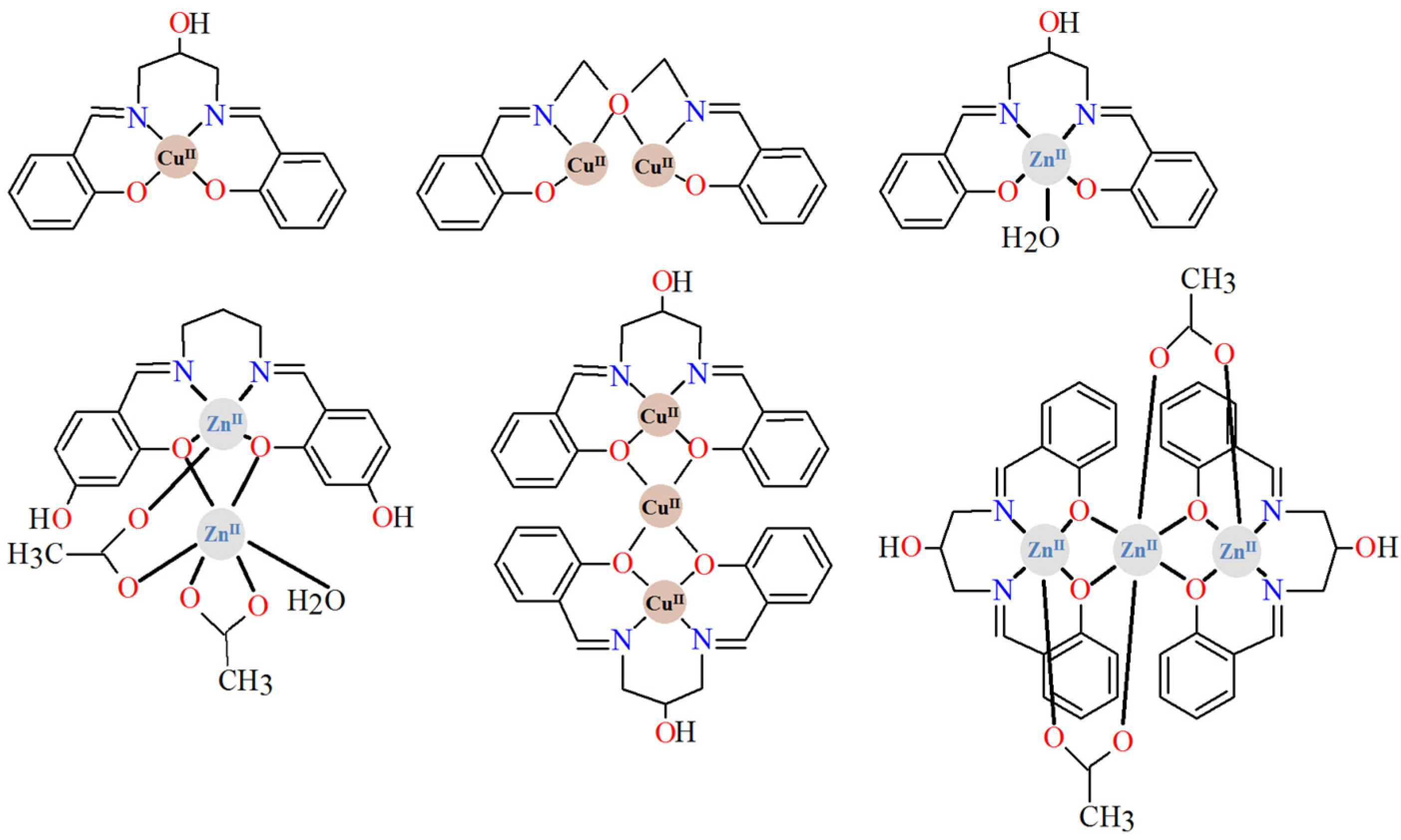

Figure 1.
Examples of Schiff base ligands [7,23].
Figure 1.
Examples of Schiff base ligands [7,23].


Figure 2.
Examples of homonuclear 3d complexes available in the CSD database [12,13,14,15,16,17,18,19,20,23].
Figure 2.
Examples of homonuclear 3d complexes available in the CSD database [12,13,14,15,16,17,18,19,20,23].

In the reported 3d–LnIII complexes there are examples of hetero- di- [24,25,26,27], tri- [28,29,30], tetra- [27,31,32], and octanuclear [33] compounds (Figure 3). The 3d and 4f metal centers are linked by two phenoxo oxygen atoms of the Schiff base ligands. Moreover, very often the auxiliary ligands additionally connect 3d and LnIII ions [27,29,30,31,32]. The heterotetranuclear carbonato/nitrato-bridged compounds [3d2LnIII2] consist of double phenoxo-bridged 3dLnIII binuclear units linked by two carbonato/nitrate anions [27,31,32]. Similarly, the heterooctanuclear complexes [3d4LnIII4] (Ln = SmIII, GdIII) are built of 3dLnIII binuclear units that are connected by one nitrate ion (bridging only LnIII ions) and form tetranuclear species. The 3d2LnIII2 subunits are then bridged by azide ions [33]. According to the literature [34,35,36,37,38], significant efforts have been made in recent years to construct 3d-4f compounds to obtain novel single-molecule magnets (SMMs). Lanthanide ions, especially TbIII, DyIII, HoIII, and ErIII, are widely used in the synthesis of 3d-4f SMMs due to their large magnetic anisotropies, which are induced by strong spin-orbit coupling. It is worth noting that among the 3d-4f complexes, those containing Cu(II) metal ions are particularly interesting because they exhibit favorable magnetic properties, including ferromagnetic Cu(II)-Ln(III) interactions.
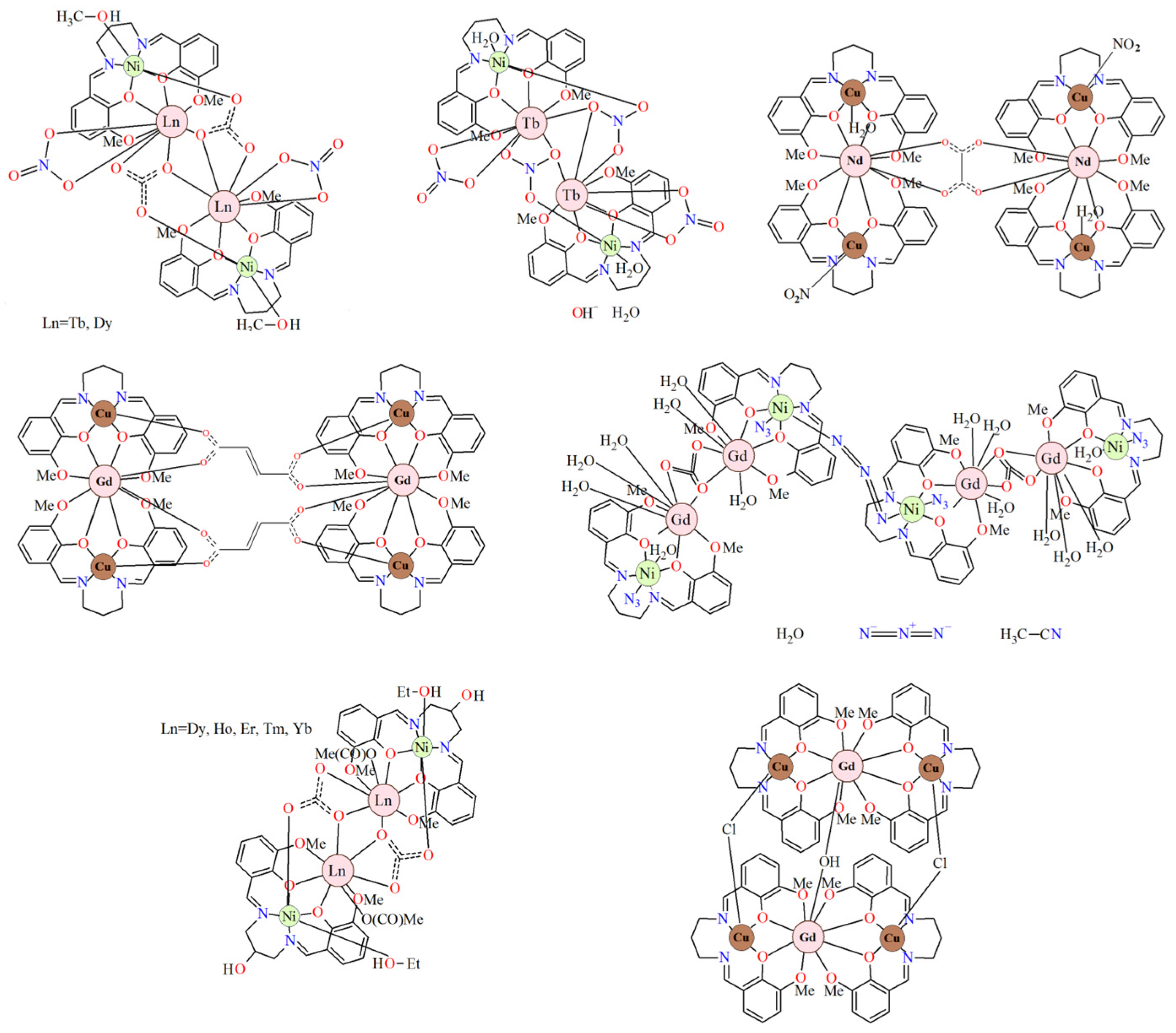

Figure 3.
Chemical diagrams of selected heteronuclear complexes 3d-LnIII, including salen-type Schiff base ligands [27,33].
Figure 3.
Chemical diagrams of selected heteronuclear complexes 3d-LnIII, including salen-type Schiff base ligands [27,33].

In this article, we report on the synthesis and the crystal characterization of new heteronuclear complexes, as well as their thermal and magnetic properties. These complexes are [Cu4Tb2(H2L)4(NO3)4(H2O)3](NO3)2·5.5H2O·2MeOH (1), [Cu4Ho2(H2L)4(NO3)4(H2O)3](NO3)2·7.5H2O (2), and [Cu4Er2(H2 L)4(NO3)4(H2O)3](NO3)2·7H2O·3MeOH (3) and include the hexadentate N2O4-donor Schiff base. The compounds were synthesized in a step-wise manner without the isolation of the mononuclear complex.
2. Materials and Methods
2.1. Materials
The chemicals used, i.e., 1,3-diaminopropane, 2,3-dihydroxybenzaldehyde, Cu(CH3COO)2·H2O, Tb(NO3)3·6H2O, Ho(NO3)3·5H2O, Er(NO3)3·5H2O, and MeOH, were of analytical grade and were purchased from commercial sources.
2.1.1. Synthesis of the N,N′-bis(2,3-dihydroxybenzylidene)-1,3-diaminopropane
The Schiff base H4L was synthesized straightforwardly by condensation of 2-hydroxy-3-methoxybenzaldehyde and propane-1,3-diamine according to the reported procedure [39].
2.1.2. Synthesis of Heterometallic Complexes [Cu4Ln2] (Ln = Tb (1), Ho (2), Er (3))
Complexes 1–3 were synthesized using a similar procedure, with the only difference being the lanthanide (III) ions that were used (Figure 4). First, the hexadentate Schiff base H4L (0.4 mmol, 0.1248 g) was dissolved in 30 mL of hot MeOH and stirred for a few minutes. Next, 10 mL of methanol solution of Cu(OAc)2·H2O (0.4 mmol, 0.0799 g) was added, resulting in a green mixture. Then, Tb(NO3)3·6H2O (0.2 mmol, 0.0906 g), Ho(NO3)3·5H2O (0.2 mmol, 0.0882 g), or Er(NO3)3·5H2O (0.2 mmol, 0.0887 g) dissolved in 5 mL of methanol was added to a suspension, and the reaction mixture was stirred for 30 min. As a result, a green solution was achieved. The crystals of the desired complexes were obtained through the slow evaporation of the solvent at 4°C over several days.
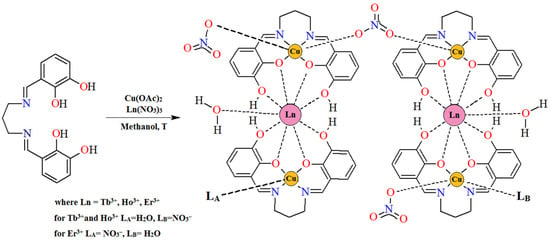
Figure 4.
The scheme of the synthetic route of complexes 1–3.
- Yield 20% 1. Anal. C70H89Cu4N14O44.5Tb2 2410.55 (%): C, 34.88; H, 3.72; N, 8.14; Cu, 10.55; Tb, 13.19. Found: C, 35.50; H, 3.70; N, 8.10; Cu, 10.55; Tb, 13.20.
- Yield 23% 2. Anal. C68H83Cu4Ho2N14O44.5, 2392.50 (%): C, 34.14; H, 3.50; N, 8.20; Cu, 10.62; Ho, 13.79. Found: C, 34.20; H, 3.35; N, 8.00; Cu, 10.30; Ho, 13.60.
- Yield 22% 3. Anal. C71H96Cu4Er2N14O47, 2486.29 (%): C, 34.30; H, 3.90; N, 7.89; Cu, 10.22; Er, 13.46. Found: C, 34.80; H, 3.45; N, 8.10; Cu, 10.40; Er, 13.50.
2.2. Methods
The C, H, and N contents in 1–3 were determined using a CHN 2400 Perkin Elmer analyzer, whereas the copper and lanthanide amounts were established using an ED XRF spectrophotometer (Canberra Packard). The measurements were performed with a Canberra XRF System 100 with an Si(Li) detector (an active area of 30 mm2, a thickness of Be window 0.025 mm, and an FWHM resolution) using 109Cd and 24lAm as excitation sources. Quantitative analysis was conducted using the AXIL software (CanberraPackard, Belgium) and calibration curves. The samples were prepared by the addition of an internal standard suitable for the element being measured. The weighed composite was ground in an alumina mortar for homogenization; then, a tablet was pressed for measurement. The thermogravimetric investigation (TG/DTG/DSC) was performed on a Setaram Setsys 16/18 thermal analyzer in an open Al2O3 crucible in a flowing air atmosphere. Samples of 7.23 mg (1), 7.94 mg (2), and 7.49 mg (3) were heated (10 °C min−1) in the temperature range of 30–1000 °C. The dc magnetic susceptibility 1–3 investigated using a Quantum Design MPMS SQUID-VSM magnetometer in the temperature range of 1.8–300 K. The magnetization of samples was studied at 2K in an applied field up to 5 T. Diamagnetic corrections were estimated using Pascal’s constants [40].
X-ray Crystal Structure Determination
The selected crystals of 1–3 that were suitable for X-ray diffraction data collection were mounted on the tip of a glass fiber. The X-ray diffraction intensities for 1 and 3 were collected at 100 K on the Oxford Diffraction Xcalibur CCD diffractometer with graphite-monochromatized MoKα radiation (λ = 0.71073 Å). The data for 2 were registered at 120 K on the SuperNova diffractometer using CuKα radiation (λ = 1.54184 Å). All the data were collected using the ω scan technique, with an angular scan width of 1.0°. The structures were solved with the olex2.solve structure solution program using charge flipping and refined with the olex2.refine refinement package using Gauss–Newton minimization. The O-bonded H atoms were found where possible in the difference Fourier maps and refined isotropically. The C-bonded H atoms were positioned geometrically and allowed to ride on their parent atoms, with Uiso(H) = 1.5 Ueq(O) and 1.2 Ueq(C) [41,42]. Because of a high content of disordered solvent molecules in the crystal, the SQUEEZE procedure was applied to calculate the solvent masks and to refine the structures [43]. The details are given in Table S1.
3. Results and Discussion
The reaction of a multidentate Schiff base (that may selectively coordinate MII and LnIII ions) with the copper(II) acetate and the terbium(III)/holmium(III)/erbium(III)/nitrate, respectively, results in the hexanuclear cationic complexes [Cu4Tb2(H2L)4(NO3)4(H2O)3](NO3)2·5.5H2O·2MeOH (1), [Cu4Ho2(H2L)4(NO3)4(H2O)3](NO3)2·7.5H2O (2), and [Cu4Er2(H2 L)4(NO3)4(H2O)3](NO3)2·7H2O·3MeOH (3) that crystallize in the triclinic system, space group P-1. The coordination sphere of 1 and 3 is the same as that in the complexes of [CuII4GdIII2], [CuII4EuIII2] [44,45], whereas in 2 it is identical to that in [CuII4SmIII2] [45]. In comparison to the compounds reported earlier, the complexes discussed here differ in terms of the kind and amount of solvents (water and methanol) and the lack of an acetic acid molecule in the outer sphere; they also differ in terms of the magnetic and thermal properties. The magnetic exchange interaction between the paramagnetic centers CuII and TbIII/HoIII is ferromagnetic.
3.1. Crystal and Molecular Structure
The results of the single-crystal X-ray studies show that [Cu4Tb2(H2L)4(NO3)4(H2O)3](NO3)2·5.5H2O·2MeOH (1), [Cu4Ho2(H2L)4(NO3)4(H2O)3](NO3)2·7.5H2O (2), and [Cu4Er2(H2 L)4(NO3)4(H2O)3](NO3)2·7H2O·3MeOH (3) crystallize in the triclinic space group P-1.
Table 1 summarizes the details of the structure solution and refinement, while Table 2 and Table 3 list selected bond distances and angles. The structure analysis revealed that 1–3 differ in terms of the type and amount of solvent molecules (water and methanol).

Table 1.
Summary of crystallographic data for 1, 2, and 3.

Table 2.
Bond lengths and distances for 1, 2, and 3 [Å].

Table 3.
Selected interatomic bond angles for 1, 2, and 3 [°].
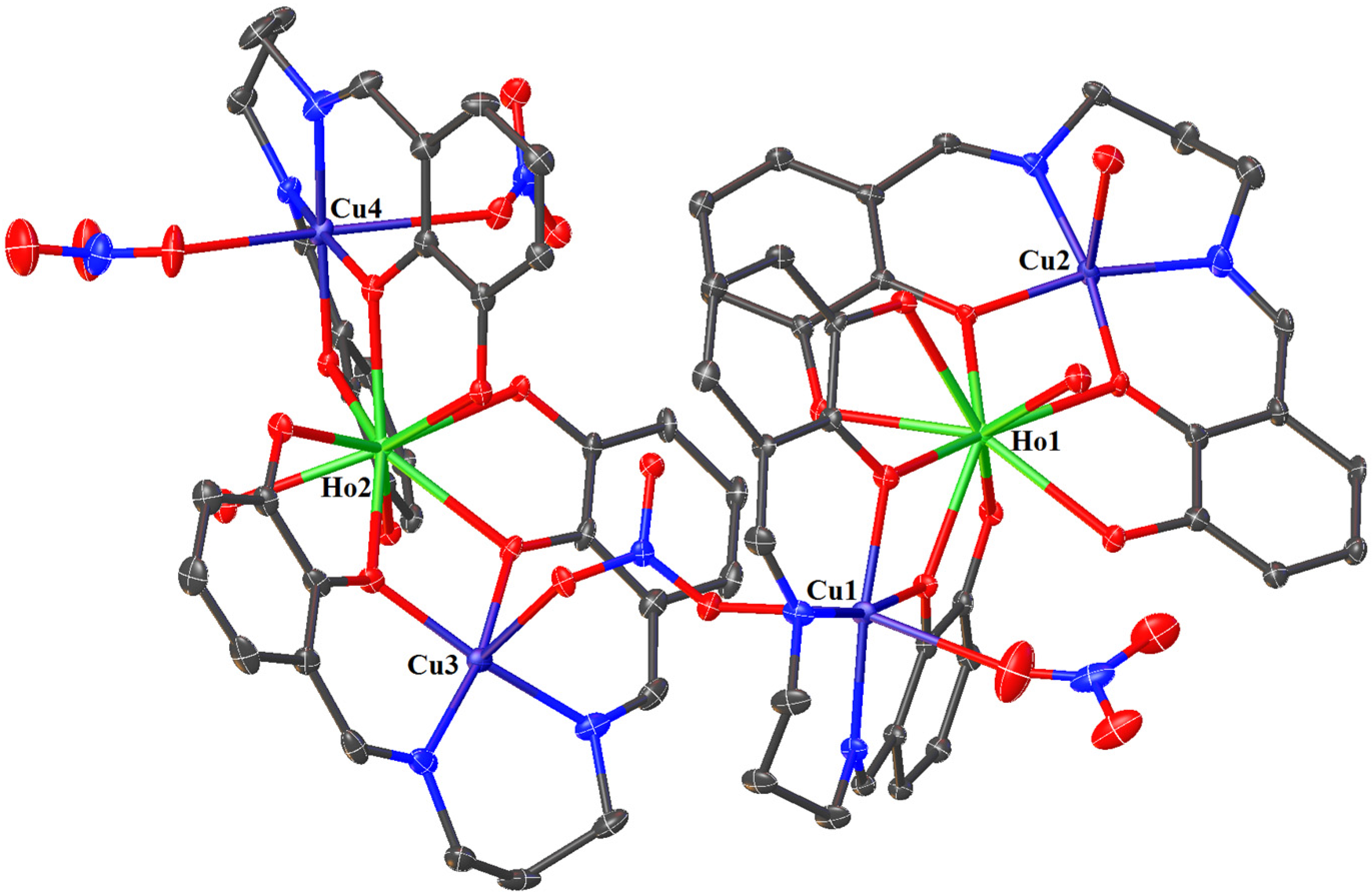
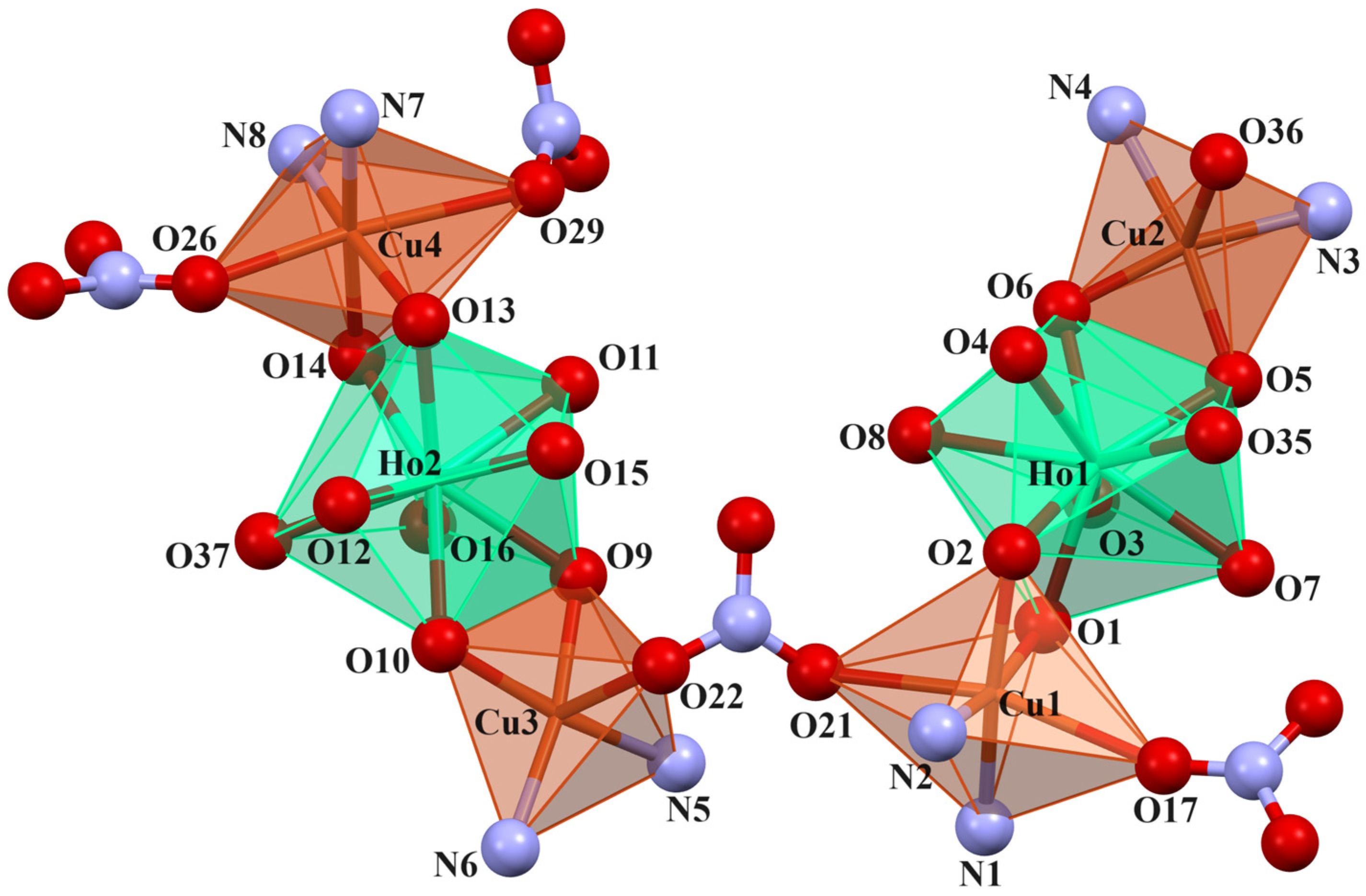
In crystals 1–3, the nitrate ions act as counter ions, as well as monodentate and bidentate (bridging) ligands. The 1–3 heterohexanuclear cationic complexes consist of double phenoxy-bridged CuIILnIII trinuclear units linked by the nitrate ion. In contrast to the nitrate/carbonate-bridged 3d-4f polynuclear compounds in which these ions mainly connect LnIII ions in 1–3, the nitrate ion joins the CuII centers. The distance between these copper(II) ions is approximately 6.13–6.21 Å. The CuII ions exhibit two types of coordination environment, while the LnIII centers display one. Cu1 and Cu4 are located in the six-coordinate environments with the four O atoms from the two bridging phenoxyl groups, two nitrate ions (monodentate or bidentate bridging), and two imine N atoms. Compared with Cu1 and Cu4, Cu2 and Cu3 are penta-coordinated by two imine N atoms, two bridging phenoxyl O atoms, and one O atom of a water molecule or a monodentate nitrate; thus, the distances range from 1.955(10) to 2.015(10) Å and 1.927(6) to 1.985(7) Å, respectively (Table 2). The two LnIII centers exhibit the same coordination environments (tricapped trigonal prismatic geometry), and they are nine-coordinated with the O atoms of the bridging phenoxyl groups, hydroxy groups, and a water molecule (Figure 5, Figure 6 and Figures S1–S4 in the ESI†). The range of distances between Ln-O is between 2.296(8)–2.485(6)Å, with the shortest being Ln-Ophenoxo and the longest being Ln-Ohydroxyl bonds (Table 2).
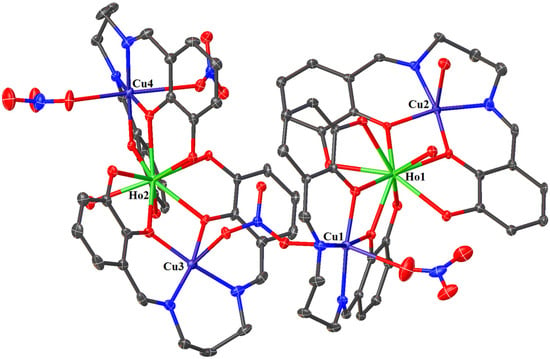
Figure 5.
The molecular structure of 2. Displacement ellipsoids are drawn at the 30% probability level. For clarity, the H atoms, counter ions, and the non-coordinated solvent molecules are omitted.
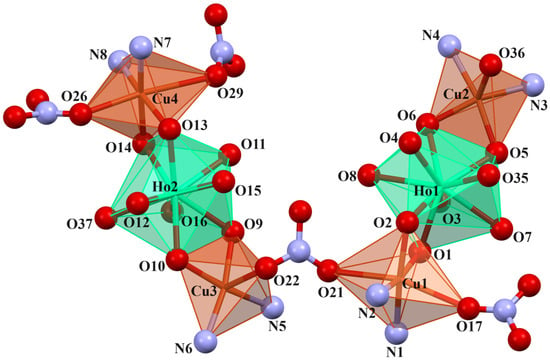
Figure 6.
Coordination environment of Cu(II) and Ho(III) ions in compound 2.
In crystals 1–3, the nitrate ions act as counter ions, as well as monodentate and bidentate (bridging) ligands. The 1–3 heterohexanuclear cationic complexes consist of double phenoxy-bridged CuIILnIII trinuclear units linked by the nitrate ion. In contrast to the nitrate/carbonate-bridged 3d-4f polynuclear compounds in which these ions mainly connect LnIII ions in 1–3, the nitrate ion joins the CuII centers. The distance between these copper(II) ions is approximately 6.13–6.21 Å. The CuII ions exhibit two types of coordination environment, while the LnIII centers display one. Cu1 and Cu4 are located in the six-coordinate environments with the four O atoms from the two bridging phenoxyl groups, two nitrate ions (monodentate or bidentate bridging), and two imine N atoms. Compared with Cu1 and Cu4, Cu2 and Cu3 are penta-coordinated by two imine N atoms, two bridging phenoxyl O atoms, and one O atom of a water molecule or a monodentate nitrate; thus, the distances range from 1.955(10) to 2.015(10) Å and 1.927(6) to 1.985(7) Å, respectively (Table 2). The two LnIII centers exhibit the same coordination environments (tricapped trigonal prismatic geometry), and they are nine-coordinated with the O atoms of the bridging phenoxyl groups, hydroxy groups, and a water molecule (Figure 5 and Figure 6, and Figures S1–S4 in the ESI†). The range of distances between Ln-O is between 2.296(8)–2.485(6)Å, with the shortest being Ln-Ophenoxo and the longest being Ln-Ohydroxyl bonds (Table 2).
In crystals 1–3, the three metallic centers of a trinuclear subunit [Cu2Ln] are almost collinear, with the values of the Cu-Ln-Cu angle: 171,10(2)° (for Cu1Tb1Cu2), 168,69(3)° (for Cu3Tb2Cu4); 171,43(4)° (for Cu1Ho1Cu2), 170,11(4)° (for Cu3Ho2Cu4); 171,56(3)° (for Cu1Er1Cu2), and 168,93(3)° (for Cu3Er2Cu4), respectively. It is important to mention that the two [CuH2L] fragments that are coordinated to the TbIII/HoIII/ErIII ion are not on the same plane. The magnetic properties of the compounds are affected by the planarity of the bridging CuO2Ln moiety. The planarity can be estimated by measuring the dihedral angle between the CuO(phenoxo)2 and LnO(phenoxo)2 planes. The dihedral angle between the Cu1O1O2 and Tb1/Ho1/Er1/O1O2 planes is equal to 10.81° in 1, 9.43° in 2, and 10.81° in 3; between the Cu2O5O6 and Tb1/Ho1/Er1/O5O6 planes, it is equal to 9.71° in 1, 8.58° in 2, and 8.27° in 3; between the Cu3O9O10 and Tb2/Ho2/Er2/O9O10 planes, it is equal to 9.27° in 1, 8.25° in 2, and 8.74° in 3; between the Cu4O13O14 and Tb2/Ho2/Er2/O13O14 planes, it is equal to 4.29° in 1, 2.74° in 2, and 2.96° in 3, respectively. The intramolecular distances between Cu...Ln and Cu...Cu are approximately 3.5 and 6.9 Å, respectively. There are intra- and intermolecular hydrogen bonds in 1–3. The adjacent molecules are linked by hydrogen bonds through undeprotonated hydroxyl groups, nitrate ions, water, and methanol molecules.
3.2. Thermal Properties
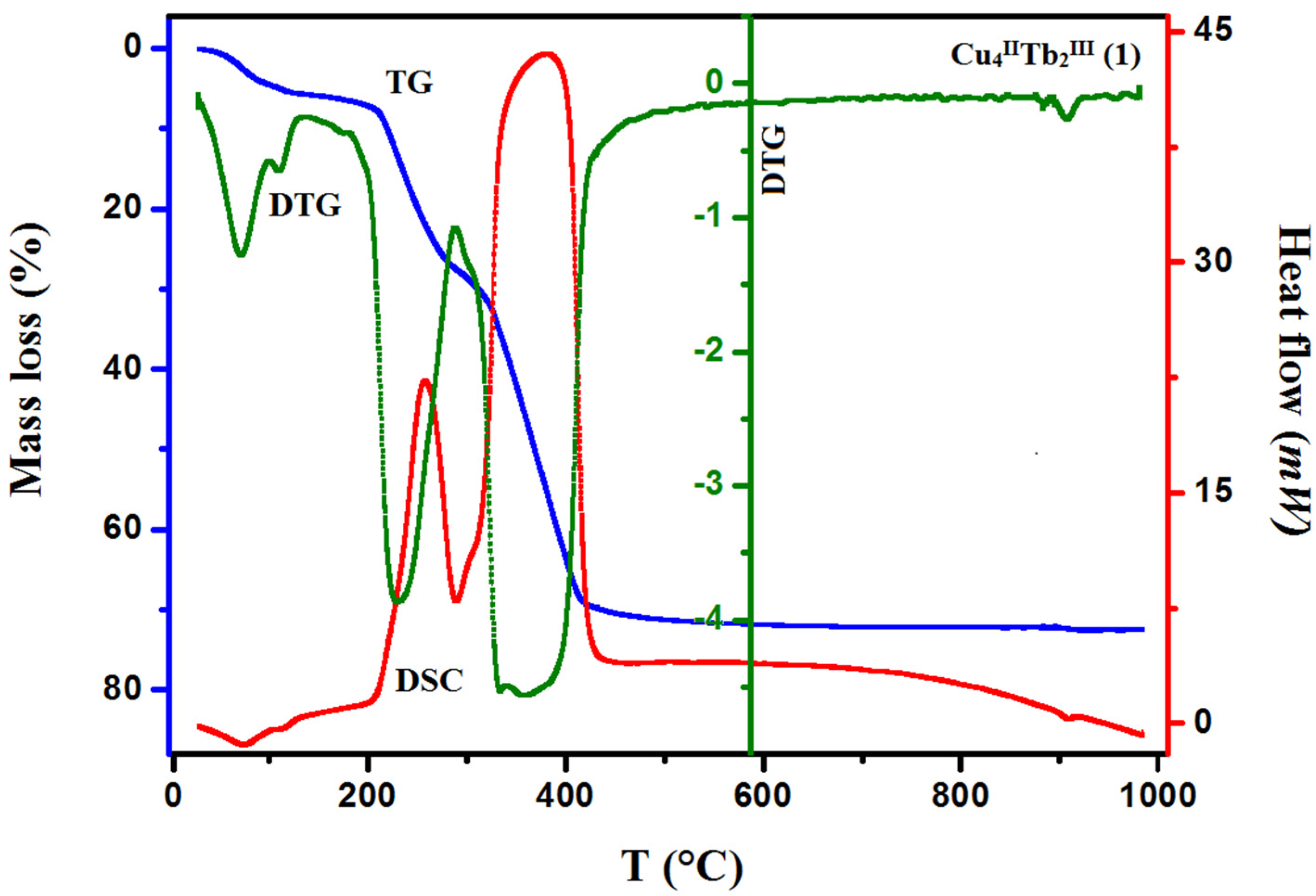
The TG/DTG/DSC techniques were used to study the thermal properties of complexes 1–3 in the air atmosphere. The results are presented in Figure 7 and Figure 8 (see also Figures S5 and S6 in the ESI†). The desolvation process occurs in two steps for 1 and 3 and one step for 2.
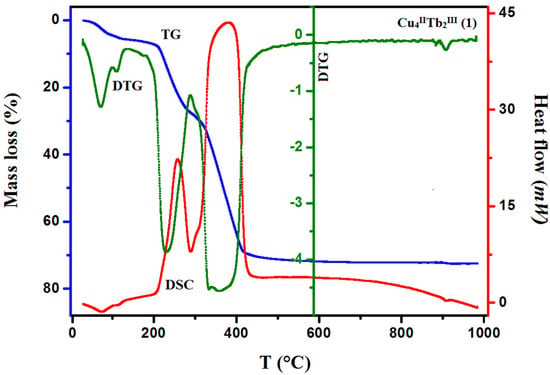
Figure 7.
TG, DTG, and DCS curves of thermal decomposition of complex 1 in air.
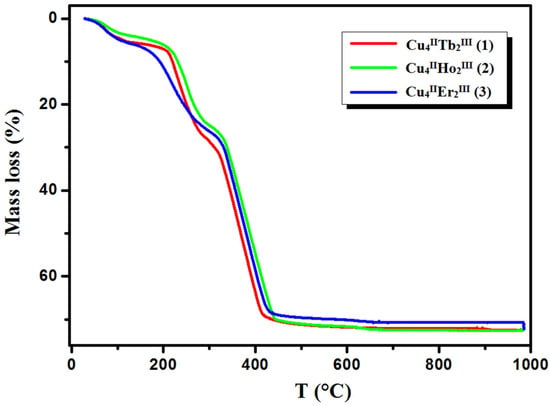
Figure 8.
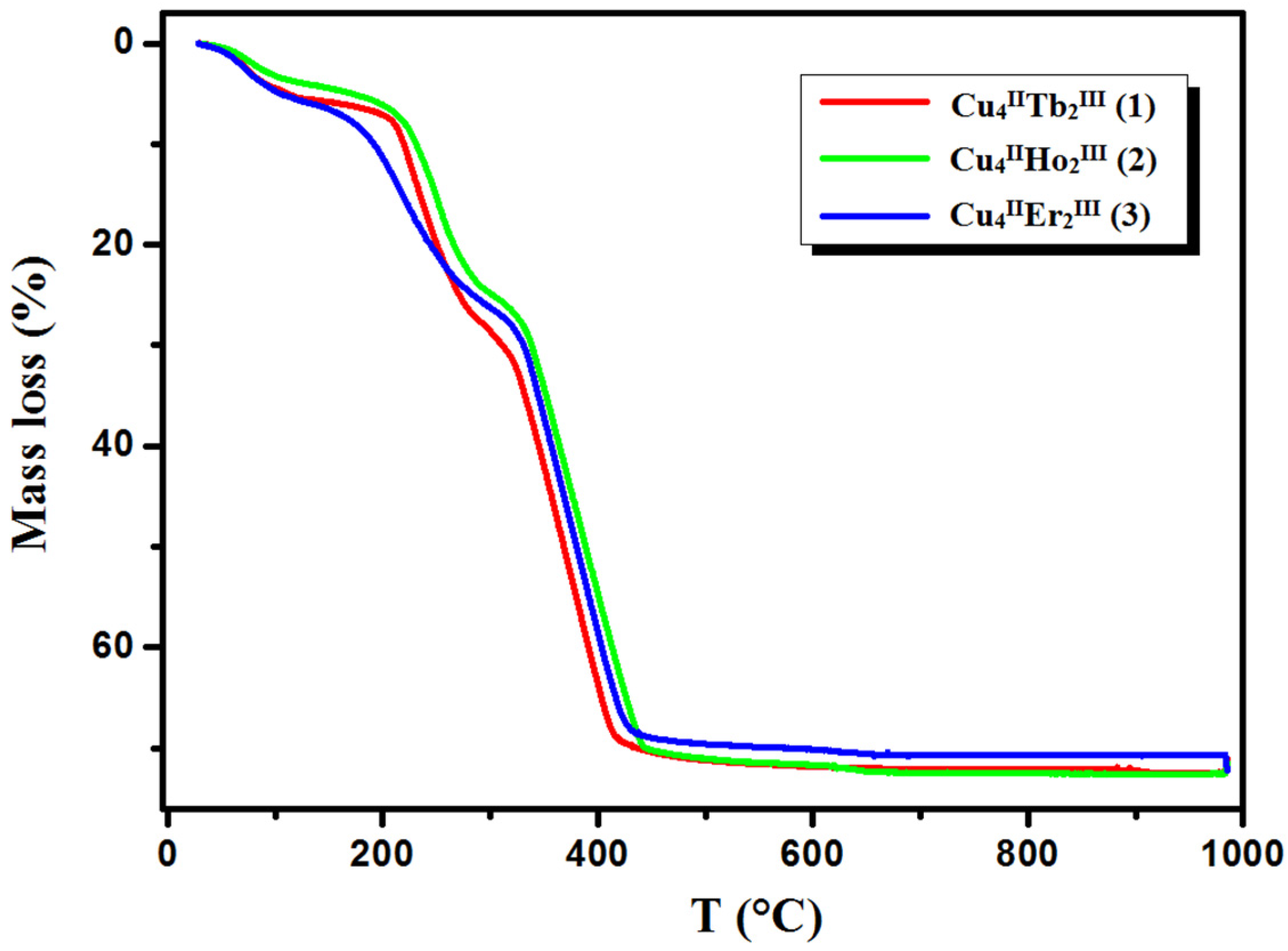
TG curves of thermal decomposition of complexes 1–3 in air.
In compound 1, the first step in the range of 44–100 °C concerns the release of two methanol and two water molecules with a mass loss of 4.40% (calc. 4.14%). In the second step, one and a half molecules of water are lost, resulting in a mass loss of 1.10% (cald. 1.12%). During the heating of 2, seven and a half water molecules are lost in the temperature range of 49–110 °C, resulting in a 5.10% mass loss (calc. 5.64%). As with 1, compound 3 loses the solvent molecules in two steps in the temperature range of 42–100 °C, but the second step is very small, and it is tough to separate. The mass loss at about 5.90% (calc. 6.40%) recorded on the TG curve corresponds to the loss of three methanol and three and a half water molecules. The DSC curves of complexes 1, 2, and 3 are characterized by two 1 or one 2, 3 broad low-intensity endothermic peaks at 75 °C and 112 °C for 1, 76 °C for 2, and 77 °C for 3, respectively. During further heating of the samples, the initial decomposition step begins at about 200 °C for 1, 195 °C for 2, and 190 °C for 3. The mass losses of 18.90% (calc. 18.91%) 1, 19.00% (calc. 17.55%) 2, and 18.80% (calc. 19.24%) 3, respectively, are probably associated with the loss of nitrate ions and coordinated (1, 2, 3) and solvate (1, 3) water molecules The differences in the calculated and found mass loss values may result from the fact that at a higher temperature, the decomposition process of the organic ligand also begins. At a higher temperature, the products decompose with no evident intermediate steps in the TG analysis until stabilization at the temperature of 630 °C 1, 680 °C 2, and 695 °C 3. The combustion of the organic ligand is accompanied by a significant exothermic effect, as seen in the DSC curves.
The final products of the heteronuclear complex decomposition are presumably a mixture of respective oxides: CuO and Tb4O7 1; CuO and Ho2O3 2; and CuO and Er2O3 3. The mass residue coincides with the theoretical values 28.40% (calc. 28.70%) 1, 28.00% (calc. 29.09%) 2, and 28.50% (calc. 28.18%) 3, respectively.
3.3. Magnetic Properties
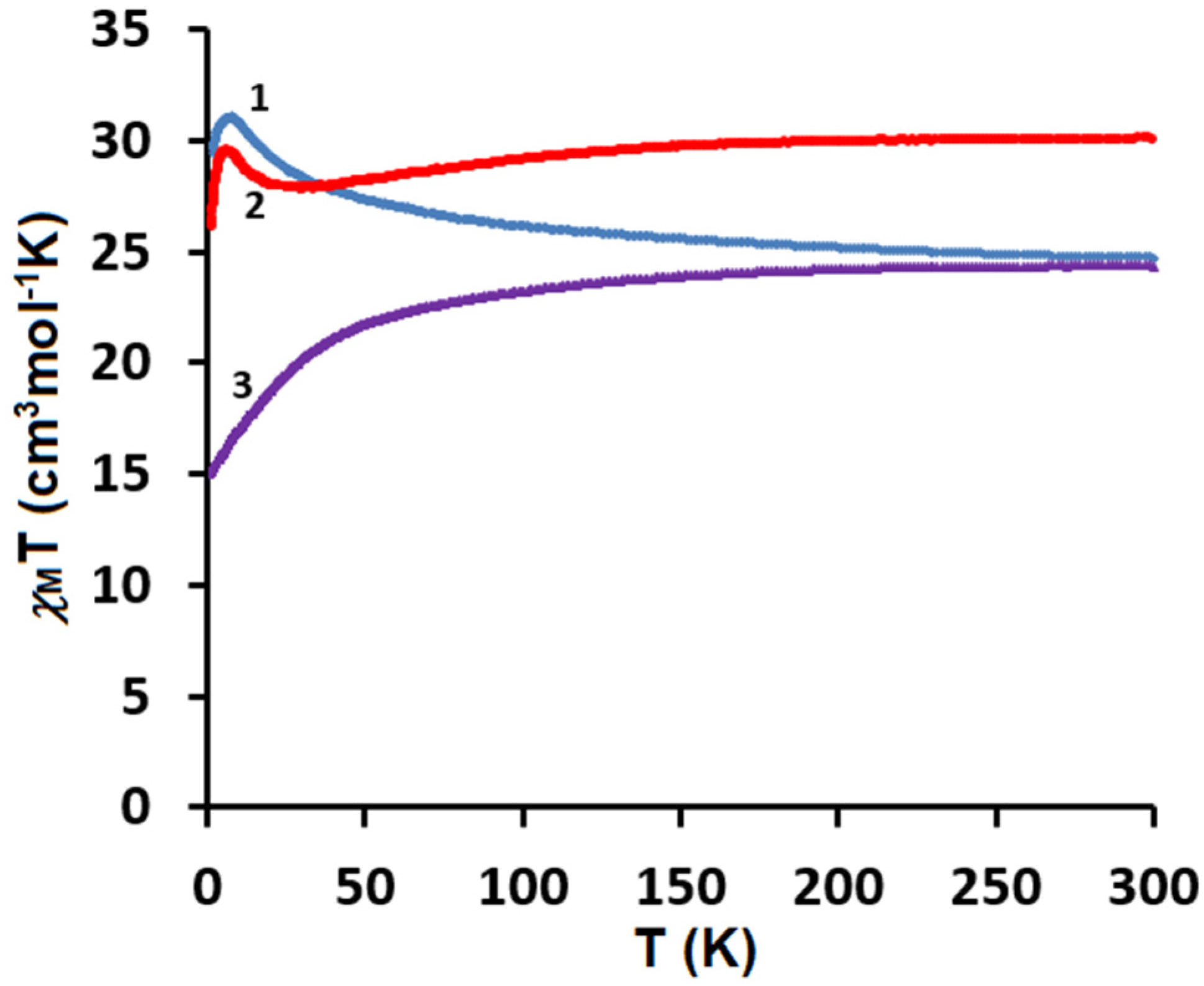
The direct current (dc) magnetic susceptibilities of the polycrystalline samples of CuII4–TbIII2 (1), CuII4–HoIII2 (2), and CuII4–ErIII2 (3) were measured using a SQUID-VSM magnetometer at the temperatures of 1.8–300 K under the applied magnetic field of 0.1 T. The plots of the temperature dependence of χMT versus T, where χM is the molar magnetic susceptibility and T is the absolute temperature, are presented in Figure 9. As shown in this figure, at 300 K the experimental χMT values are 24.72, 30.01, and 24.38 cm3Kmol−1, respectively, which are almost in agreement with the expected values of 25.13, 29.63, and 24.45 cm3Kmol−1 for four CuII (S = 1/2, g = 2) and two LnIII noninteracting ions: two TbIII (7F6, S = 3, L = 3, J = 6, g = 3/2) for 1, two HoIII (5I8, J = 8, L = 6, S = 2, g = 5/4) for 2, and two ErIII (4I15/2, S = 3/2, L = 6, J = 15/2, g = 6/5) for 3. For CuII4–TbIII2 1, with the lowering of the temperature, the χMT values gradually increase and reach 31.06 cm3Kmol−1 at 7.9 K, which suggests the existence of the weak ferromagnetic interaction between the CuII and TbIII paramagnetic centers. For CuII4–HoIII2 2, as the temperature decreases, the χMT values stay almost constant until 155 K and then start to decrease, reaching the value of 27.90 cm3Kmol−1 at 21 K. Afterwards, the χMT product values slowly increase, reaching 29.48 cm3Kmol−1 at 6.6 K. Finally, the χMT versus T curve shows a rapid decrease, indicating 26.05 cm3Kmol−1 at 1.8 K. For CuII4–ErIII2 3, the χMT values decrease with the lowering of the temperature to 15.90 cm3Kmol−1 at 1.8 K. The decrease in χMT values in the low-temperature range may be attributed to the presence of the intramolecular antiferromagnetic coupling interactions and the large magnetic anisotropy of TbIII/HoIII/ErIII ions [35,46,47,48,49]. However, the decrease in χMT in the high temperature range mainly results from the thermal depopulation of the mJ states of the Ln(III) ions, which are responsible for the increased value of χMT due to general ferromagnetic coupling interactions [35,47,50]. The obtained results 1, 2 are consistent with the other empirical studies on 3d–4f complexes. According to research, the 3d-LnIII exchange interaction is usually ferromagnetic in the case of heavy lanthanides [34,35,36,37,38,46,47,48,49]. The results are also in a good agreement with Kahn et al.’s theoretical model, which suggested that for the light lanthanide ions(III) with a 4f1 –4f6 configuration, the angular and spin moments were antiparallel in the 2S+1LJ free-ion ground state (J = L − S). A parallel alignment of the CuII and LnIII spin moment would lead to an antiparallel alignment of the angular moment, that is, to an overall antiferromagnetic interaction, whereas for the heavy lanthanide ions(III) with a 4f8–4f13 configurations (J = L + S), a parallel alignment of the CuII and LnIII spin moment would result in an overall ferromagnetic interaction [51]. To confirm the nature of the ground state of 1, 2, and 3, we investigated the variation in the magnetization, M, with respect to the field, at 2 K. The result is shown in Figure 10, where the molar magnetization M is expressed in µB units.
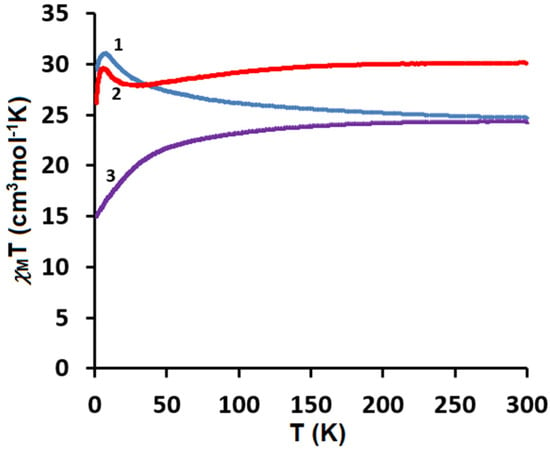
Figure 9.
Temperature dependence of experimental χMT versus T for 1–3.
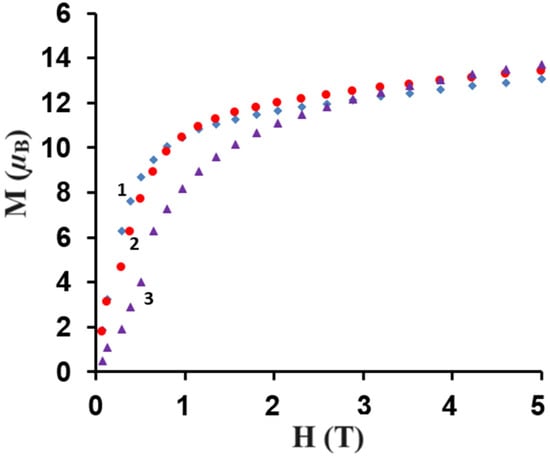
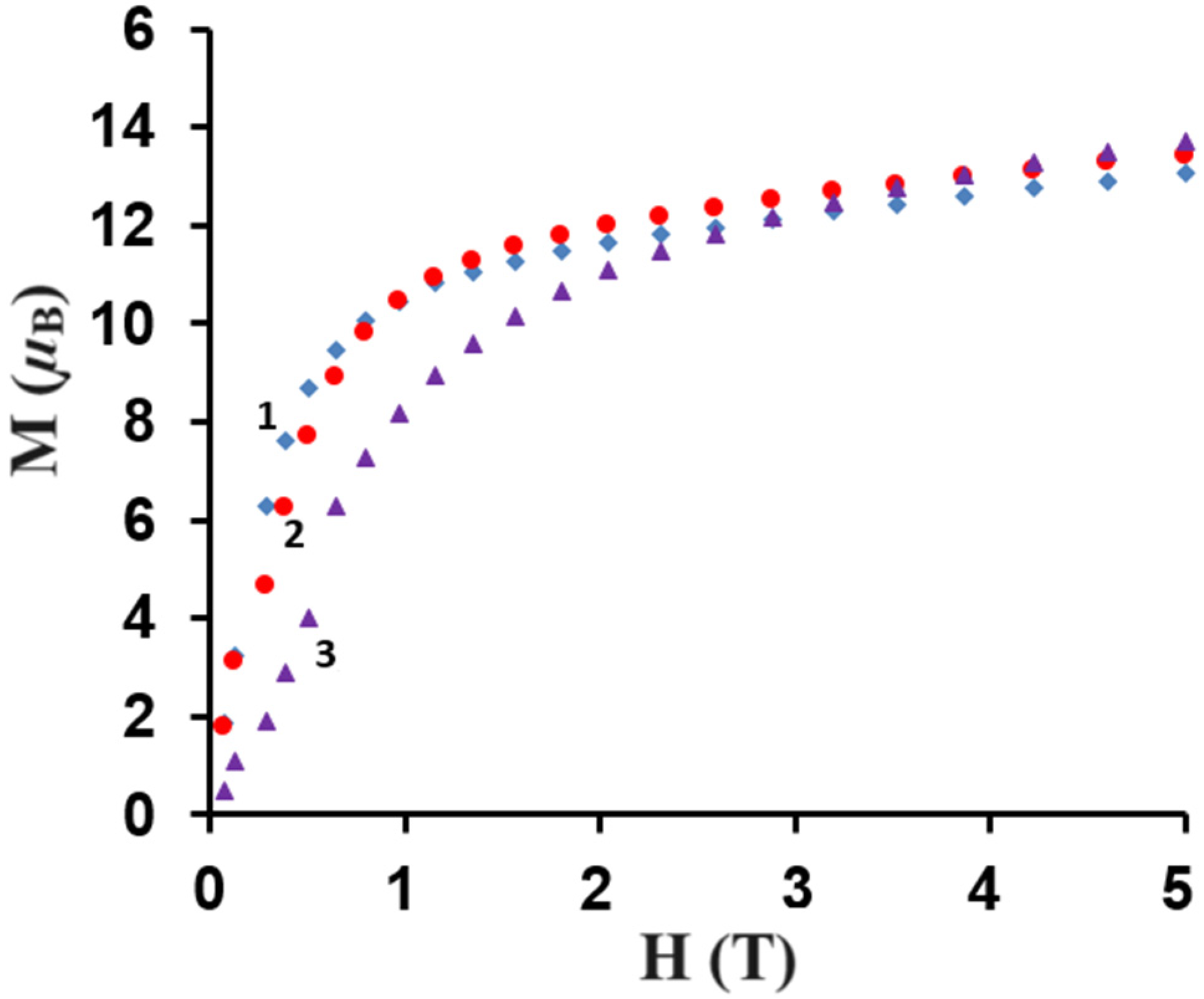
Figure 10.
Field dependence of the magnetization for complexes 1–3 at 2 K.
The M = f(H) curves show a slow increase in the magnetization up to 13.1 µB for 1, 13.6 µB for 2, and 13.7 µB for 3, respectively, at 5 T. These results are far from the theoretical saturated values of 22 µB for 1, 24 µB for 2, and 22 µB for 3, respectively, as anticipated for two uncoupled LnIII and four CuII ions. This could be due to the presence of the magnetic anisotropy of the LnIII ions and/or the low-lying excited states [35,47,49].
4. Conclusions
The hexanuclear solvates 1−3 are stable at room temperature. The structural determination revealed that 1−3 are composed of two diphenoxo-bridged [CuII2LnIII] trinuclear clusters with slight structural differences. Interestingly, the nitrate groups exhibit not the chelating but the bridging mode. In each heterotrinuclear unit, the CuII and LnIII ions are situated in the smaller N2O2 and the larger O2O2 pockets, respectively, both of which are formed by the N2O4-donor ligands. During heating in air, 1–3 decompose similarly. In the first step, they lose solvent molecules; this is connected with the endothermic process. The decomposition of ligands is associated with an exothermic process. The mixture of the corresponding oxides is finally formed. The weak ferromagnetic interaction between the paramagnetic centers CuII and TbIII/HoIII of 1 and 2 is indicated by the temperature dependence of the magnetic susceptibility. According to research, the 3d-LnIII exchange interaction is usually ferromagnetic in the case of heavy lanthanides.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14020189/s1. Crystallographic data for 1, 2, and 3 were deposited in the Cambridge Crystallographic Data Centre: CCDC 2314649, 2314651, 2314650. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 31 December 2023), or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033. Table S1: Results of SQUEEZE procedure for crystals of 1-3, Figure S1: The molecular structure of 1. Displacement ellipsoids are drawn at the 30% probability level. For clarity, the H atoms, counter ions and the non-coordinated solvent molecules are omitted, Figure S2: Coordination environment of Cu(II) and Tb(III) ions in compound 1, Figure S3: The molecular structure of 3. Displacement ellipsoids are drawn at the 30% probability level. For clarity, the H atoms, counter ions and the non-coordinated solvent molecules are omitted, Figure S4: Coordination environment of Cu(II) and Er(III) ions in compound 3, Figure S5: TG, DTG, and DCS curves of thermal decomposition of complex 2 in air, Figure S6: TG, DTG, and DCS curves of thermal decomposition of complex 2 in air.
Author Contributions
Conceptualization, B.C. and D.O.; methodology, B.C., D.O. and B.M.; software, B.M., D.O. and B.C.; formal analysis, D.O., B.M., and B.C.; investigation, B.C., D.O. and B.M; writing—original draft preparation, B.C. and D.O.; writing—review and editing, B.C. and D.O.; visualization, D.O. and B.C; supervision, B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors. The CIF files were deposited in the Cambridge Crystallographic Data Center (CCDC; No. 2314649, 2314651, 2314650). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif (accessed on 31 December 2023) (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shukla, P.; Das, S.; Bag, P.; Dey, A. Magnetic materials based on heterometallic CrII/III–LnIII complexes. Inorg. Chem. Front. 2023, 10, 4322. [Google Scholar] [CrossRef]
- Amirkhanov, O.V.; Moroz, O.V.; Znovjyak, K.O.; Sliva, T.Y.; Penkova, L.V.; Yushchenko, T.; Szyrwiel, L.; Konovalova, I.S.; Dyakonenko, V.; Shishkin, O.V. Heterobinuclear Zn–Ln and Ni–Ln complexes with Schiff-base and carbacylamidophosphate ligands: Synthesis, crystal structures, and catalytic activity. Eur. J. Inorg. Chem. 2014, 23, 3720–3730. [Google Scholar] [CrossRef]
- Kajiwara, T.; Nakano, M.; Takahashi, K.; Takaishi, S.; Yamashita, M. Structural design of easy-axis magnetic anisotropy and determination of anisotropic parameters of LnIII-CuII single-molecule magnets. Chem. Eur. J. 2011, 17, 196–205. [Google Scholar] [CrossRef]
- Novitchi, G.; Costes, J.P.; Donnadieu, B. Synthesis and structure of 1-D heterometallic thiocyanato-bridged CuIIGdIII polymers with ferromagnetic properties. Eur. J. Inorg. Chem. 2004, 1808–1812. [Google Scholar] [CrossRef]
- Okazawa, A.; Watanabe, R.; Nezu, M.; Shimada, T.; Yoshii, S.; Nojiri, H.; Ishida, T. Ferromagnetic Gd Cu, Tb Cu, and Ho Cu couplings in isomorphous [Ln2Cu] complexes. Chem. Lett. 2010, 39, 1331 1332. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, D.-Y.; Suo, J.-J.; Gu, W.; Tian, J.-L.; Liu, X.; Yan, S.-P. Synthesis, magnetism and spectral studies of six defective dicubane tetranuclear {M4O6} (M = NiII, CoII, ZnII) and three trinuclear CdII complexes with polydentate Schiff base ligands. Dalton Trans. 2016, 45, 10233–10248. [Google Scholar] [CrossRef]
- Liua, K.; Shia, W.; Chenga, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 290, 74–122. [Google Scholar] [CrossRef]
- Dong, Y.-J.; Ma, J.-C.; Zhu, L.-C.; Dong, W.-K.; Zhang, Y. Four 3d–4f heteromultinuclear zinc(II)–lanthanide(III) complexes constructed from a distinct hexadentate N2O2-type ligand: Syntheses, structures and luminescence properties. J. Coord. Chem. 2017, 70, 103–115. [Google Scholar] [CrossRef]
- Beata Cristóvão, B.; Miroslaw, B.; Kłak, J.; Rams, M. Carbonato-bridged heteronuclear NiII2LnIII2 (Ln = Tb, Dy, Ho, Er, Tm, Yb, Lu) complexes synthesized by fixation of atmospheric CO2—Structural and magnetic studies. Polyhedron 2015, 85, 697–704. [Google Scholar] [CrossRef]
- Kori, D.; Dote, Y.; Koikawa, M.; Yamada, Y. Syntheses, crystal structures, and solid-state photoluminescence properties of heterotrinuclear Zn2Ln (Ln: La, Sm, Eu, Tb) complexes derived from 1,4-diaminobutane-based N2O4 compartmental ligand. Polyhedron 2019, 170, 612–621. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, R.; Fujiwara, K.; Okazawa, A.; Kojima, N.; Tanaka, G.; Yoshiic, S.; Nojiri, H. Exchange coupling in TbCu and DyCu single-molecule magnets and related lanthanide and vanadium analogs. Dalton Trans. 2012, 41, 13609–13619. [Google Scholar] [CrossRef]
- Das, S.; Sahu, A.; Joshi, M.; Paul, S.; Shit, M.; Choudhury, A.R.; Biswas, B. Ligand-centered radical activity by a zinc-Schiff-base complex towards catechol oxidation. Chem. Sel. 2018, 3, 10774–11078. [Google Scholar] [CrossRef]
- Pala, C.K.; Mahatoa, S.; Joshib, M.; Paulc, S.; Choudhuryb, A.R.; Biswas, B. Transesterification activity by a zinc(II)-Schiff base complex with theoretical interpretation. Inorg. Chim. Acta 2020, 506, 119541. [Google Scholar]
- Mukherjee, A.; Saha, M.K.; Rudra, I.; Ramasesha, S.; Nethaji, M.; Chakravarty, A.R. Synthesis, crystal structure and magnetic properties of quasi-linear tetranuclear copper(II) Schiff base complexes formed by covalent linkage of asymmetrically dibridged dicopper(II) units. Inorg. Chim. Acta 2004, 357, 1077–1082. [Google Scholar] [CrossRef]
- Elmali, A.; Zeyrek, C.T.; Elerman, Y. Crystal structure, magnetic properties and molecular orbital calculations of a binuclear copper(II) complex bridged by an alkoxo-oxygen atom and an acetate ion. J. Mol. Struct. 2004, 693, 225–234. [Google Scholar] [CrossRef]
- Sanatkar, T.H.; Khorshidi, A.; Janczak, J. Dinuclear Zn(II) and tetranuclear Co(II) complexes of a tetradentate N2O2 Schiff base ligand: Synthesis, crystal structure, characterization, DFT studies, cytotoxicity evaluation, and catalytic activity toward benzyl alcohol oxidation. Appl. Organomet. Chem. 2020, 34, e5493. [Google Scholar] [CrossRef]
- Yasuda, S.; Takase, M.; Sano, A.; Akitsu, T. Schiff base dinuclear Zn(II) complexes as assisting fluorescent probe for CD19 antibody protein. Int. J. Chem. Eng. Appl. 2017, 8, 290–293. [Google Scholar] [CrossRef][Green Version]
- Kargar, H.; Fallah-Mehrjardi, M.; Behjatmanesh-Ardakani, R.; Rudbari, H.; Ardakani, A.; Sedighi-Khavidak, S.; Munawarf, K.; Ashfaq, M.; Tahir, M. Binuclear Zn(II) Schiff base complexes: Synthesis, spectral characterization, theoretical studies and antimicrobial investigations. Inorg. Chim. Acta 2022, 530, 120677. [Google Scholar] [CrossRef]
- Dey, D.; Kaur, G.; Patra, M.; Choudhury, A.; Kole, N.; Biswas, B. A perfectly linear trinuclear zinc–Schiff base complex: Synthesis, luminescence property and photocatalytic activity of zinc oxide nanoparticle. Inorg. Chim. Acta 2014, 421, 335–341. [Google Scholar] [CrossRef]
- Mal, S.K.; Mitra, M.; Purohit, C.S.; Ghosh, R. A trimetallic zinc(II) complex and its catecholase activity. Polyhedron 2015, 101, 191–195. [Google Scholar] [CrossRef]
- Song, Y.; Massera, C.; Roubeau, O.; Gamez, P.; Manotti Lanfredi, A.M.; Reedijk, J. An unusual open cubane structure in 1,1-azido- and alkoxo-bridged tetranuclear copper(II) complex, [Cu4L2(1,1-N3)2]‚5H2O(H3L) N,N′-(2-Hydroxylpropane-1,3-diyl)bis-salicylideneimine). Inorg. Chem. 2004, 43, 6842–6847. [Google Scholar] [CrossRef]
- Yang, F.-L.; Shao, F.; Zhu, G.-Z.; Shi, Y.-H.; Gao, F.; Li, X.-L. Structures and magnetostructural correlation analyses for two novel hexanuclear complexes based on a pentadentate Schiff base ligand. ChemistrySelect 2017, 2, 110–117. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Liu, Y.-Q.; Deng, X.-W.; Zhu, Z.-X.; Yao, M.-X.; Jing, S. Synthesis, structures and magnetism of heterodinuclear Ni–Ln complexes: Field-induced single-molecule magnet behavior in the dysprosium analogue. New J. Chem. 2015, 39, 3467–3473. [Google Scholar] [CrossRef]
- Pasatoiu, T.D.; Etienne, M.; Madalan, A.M.; Sessoli, R.; Andruh, M. Crystal structures and magnetic properties of two new heterodinuclear [NiIILnIII] complexes obtained using a side-off compartmental ligand and 2,6-pirydin-dicarboxylato coligand. Rev. Roum. Chim. 2012, 57, 507–512. [Google Scholar]
- Wang, J.-H.; Yan, P.-F.; Li, G.-M.; Zhang, J.-W.; Chen, P.; Suda, M.; Einag, Y. N,N-bis(2-hydroxy-3-methoxybenzylidene)-1,3-diaminopropane dimeric 4f and 3d–4f heterodinuclear complexes: Syntheses, crystal structures and magnetic properties. Inorg. Chim. Acta 2010, 363, 3706–3713. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Liu, X.; Tian, J.; Yan, S. Three series of heterometallic NiII–LnIII Schiff base complexes: Synthesis, crystal structures and magnetic characterization. Dalton Trans. 2017, 46, 12558–12573. [Google Scholar] [CrossRef]
- Costes, J.-P.; Donnadieu, B.; Gheorghe, R.; Novitchi, G.; Tuchagues, J.-P.; Vendier, L. Di- or trinuclear 3d–4f Schiff base complexes: The role of anions. Eur. J. Inorg. Chem. 2008, 5235–5244. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Pełka, R.; Miroslaw, B.; Hnatejko, Z. Heterometallic trinuclear 3d–4f–3d compounds based on a hexadentate Schiff base ligand. Polyhedron 2014, 68, 180–190. [Google Scholar] [CrossRef]
- Cristóvão, B.; Kłak, J.; Miroslaw, B. Synthesis, crystal structures and magnetic behavior of NiII–4f–NiII compounds. Polyhedron 2012, 43, 47–54. [Google Scholar] [CrossRef]
- Sakamoto, S.; Fujinami, T.; Nishi, K.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Re, N. Carbonato-bridged NiII2LnIII2 (LnIII = GdIII, TbIII, DyIII) complexes generated by atmospheric CO2 fixation and their single-molecule magnet behavior: [(μ4-CO3)2{NiII(3-MeOsaltn)(MeOH or H2O)LnIII(NO3)}2]·solvent [3-MeOsaltn = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato]. Inorg. Chem. 2013, 52, 7218–7229. [Google Scholar] [PubMed]
- Im, S.Y.; Park, S.J.; Im, H.J.; Lee, S.W. Conversion of Ni–Nd and Ni–Tb compartment compounds into one-dimensional coordination polymers or tetranuclear dimers. Polyhedron 2016, 117, 231–243. [Google Scholar] [CrossRef]
- Pasatoiu, T.D.; Ghirri, A.; Madalan, A.M.; Affronte, M.; Andruh, M. Octanuclear [NiII4LnIII4] complexes. Synthesis, crystal structures and magnetocaloric properties. Dalton Trans. 2014, 43, 9136–9142. [Google Scholar] [CrossRef]
- Wang, H.-S.; Zhang, Z.; Song, Y.; Pan, Z.-Q. Recent advances in 3d-4f magnetic complexes with several types of non-carboxylate organic ligands. Inorg. Chim. Acta 2021, 521, 120318. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Q.-Q.; Hu, Z.-B.; Wang, H.-S.; Huang, Z.-Y.; Chen, W.; Song, Y.; Zhang, Z.-C.; Yang, F.-J. Synthesis, crystal structures and magnetic properties of a series of pentanuclear heterometallic [CuII3LnIII2 ] (Ln = Ho, Dy, and Gd) complexes containing mixed organic ligands. New J. Chem. 2019, 43, 8101–8108. [Google Scholar] [CrossRef]
- Sun, J.; Sun, Z.; Wang, K.; Xi, L.; Ma, Y.; Li, L. Slow relaxation of magnetization in unprecedented Cu–Ln-Rad hetero-tri-spin chains constructed from multidentate nitronyl nitroxide. J. Mater. Chem. C 2019, 7, 9057. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, F.; Zheng, J. Syntheses, Structures, and Magnetic Properties of a Series of Heterotri-, Tetra- and Pentanuclear LnIII–CoII Compounds. Polymers 2019, 11, 196. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, Y.; Li, M.-X.; Nfor, E.; Wang, Z.-X. Controllable fabrication of one-dimensional and two-dimensional compounds with heterometallic units: Syntheses, crystal structures, and magnetic properties. Cryst. Growth Des. 2020, 20, 5760–5766. [Google Scholar] [CrossRef]
- Zeyrek, C.T.; Elmali, A.; Elerman, Y. Magnetic characterization, synthesis and crystal structure of a heterodinuclear CuIIGdIII Schiff base complex bridged by the two phenolic oxygen atoms. J. Mol. Struct. 2005, 740, 47–52. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH Publishers Inc.: New York, NY, USA, 1993. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Cryst. 2015, A71, 59–75. [Google Scholar]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Cryst. 2015, C71, 9–18. [Google Scholar]
- Cristóvão, B.; Pełka, R.; Miroslaw, B. A novel hexanuclear CuII4–GdIII2 cluster obtained from heterotrinuclear building blocks. Inorg. Chem. Commun. 2015, 54, 81–84. [Google Scholar] [CrossRef]
- Cristóvão, B.; Miroslaw, B.; Bartyzel, A. Hexanuclear [Cu4IILn2III] compounds incorporating N,O-donor ligands –Synthesis, crystal structures and physicochemical properties. Inorg. Chim. Acta 2017, 466, 160–165. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, M.-X.; Sui, Y.; Nfor, E.N.; Wang, Z.-X. Two 1D homochiral heterometallic chains: Crystal structures, spectra, ferroelectricity and ferromagnetic properties. RSC Adv. 2020, 10, 7004–7010. [Google Scholar] [CrossRef]
- Wang, H.-S.; Yang, F.-J.; Long, Q.-Q.; Huang, Z.-Y.; Chen, W.; Pan, Z.-Q. Syntheses, crystal structures, and magnetic properties of a family of heterometallic octanuclear [Cu6Ln2] (Ln = Dy(III), Tb(III), Ho(III), Er(III), and Gd(III)) complexes. New J. Chem. 2017, 41, 5884–5892. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Li, H.; Che, Y.-X.; Zheng, J.-M.; Vieru, V.; Chibotaru, L.F.; Grandjean, F.; Long, G.J. Synthesis, structure, and magnetic properties of Dy2Co2L10(bipy)2 and Ln2Ni2L10 (bipy)2, Ln= La, Gd, Tb, Dy, and Ho: Slow magnetic relaxation in Dy2Co2L10(bipy)2. Inorg. Chem. 2014, 53, 9785–9799. [Google Scholar] [CrossRef]
- Wen, H.-R.; Bao, J.; Liu, S.-J.; Liu, C.-M.; Zhang, C.-W.; Tang, Y.-Z. Temperature-controlled polymorphism of chiral CuII–LnIII dinuclear complexes exhibiting slow magnetic relaxation. Dalton Trans. 2015, 44, 11191–11201. [Google Scholar] [CrossRef]
- Mahapatra, P.; Koizumi, N.; Kanetomo, T.; Ishida, T.; Ghosh, A. A Series of CuII−LnIII Complexes of an N2O3 donor asymmetric ligand and a possible CuII−TbIII SMM candidate in no bias field. New J. Chem. 2019, 43, 634–643. [Google Scholar] [CrossRef]
- Andruh, M.; Ramade, I.; Codjovi, E.; Guillou, O.; Kahn, O.; Trombe, J.C. Crystal structure and magnetic properties of [Ln2Cu4] hexanuclear clusters (where Ln = trivalent lanthanide). Mechanism of the gadolinium(III)-copper(II) magnetic interaction. J. Am. Chem. Soc. 1993, 115, 1822–1829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).