High-Performance Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol over Pt Nanoparticles Supported on Co-Al LDH Nanosheets
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Preparation of Co-Al LDH
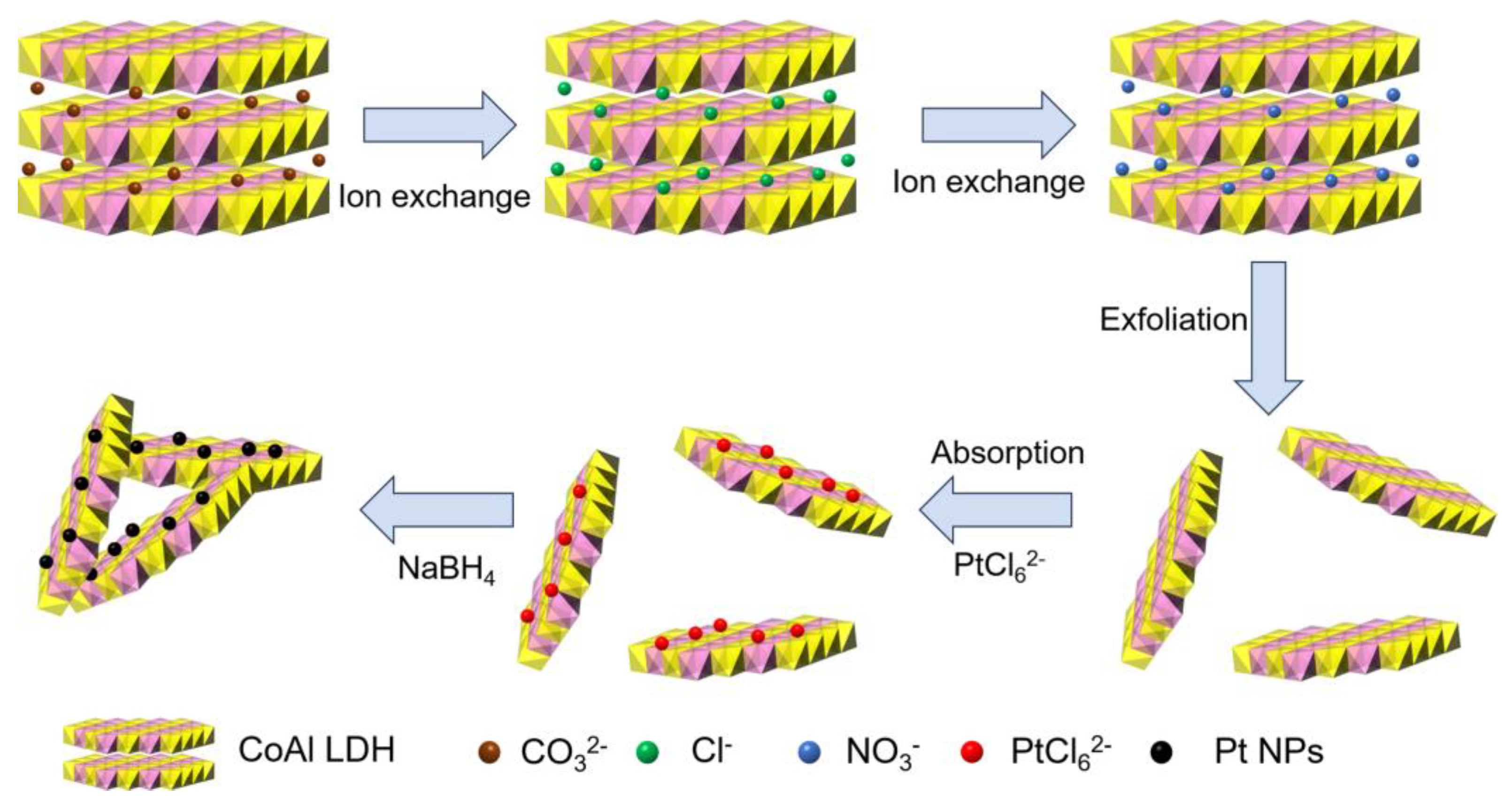
2.3. Anion Exchange and Exfoliation of Co-Al LDH
2.4. Preparation of Pt@Co-Al LDH Hybrid
2.5. Catalyst Characterization
2.6. Evaluation of Catalytic Performance
3. Results and Discussion
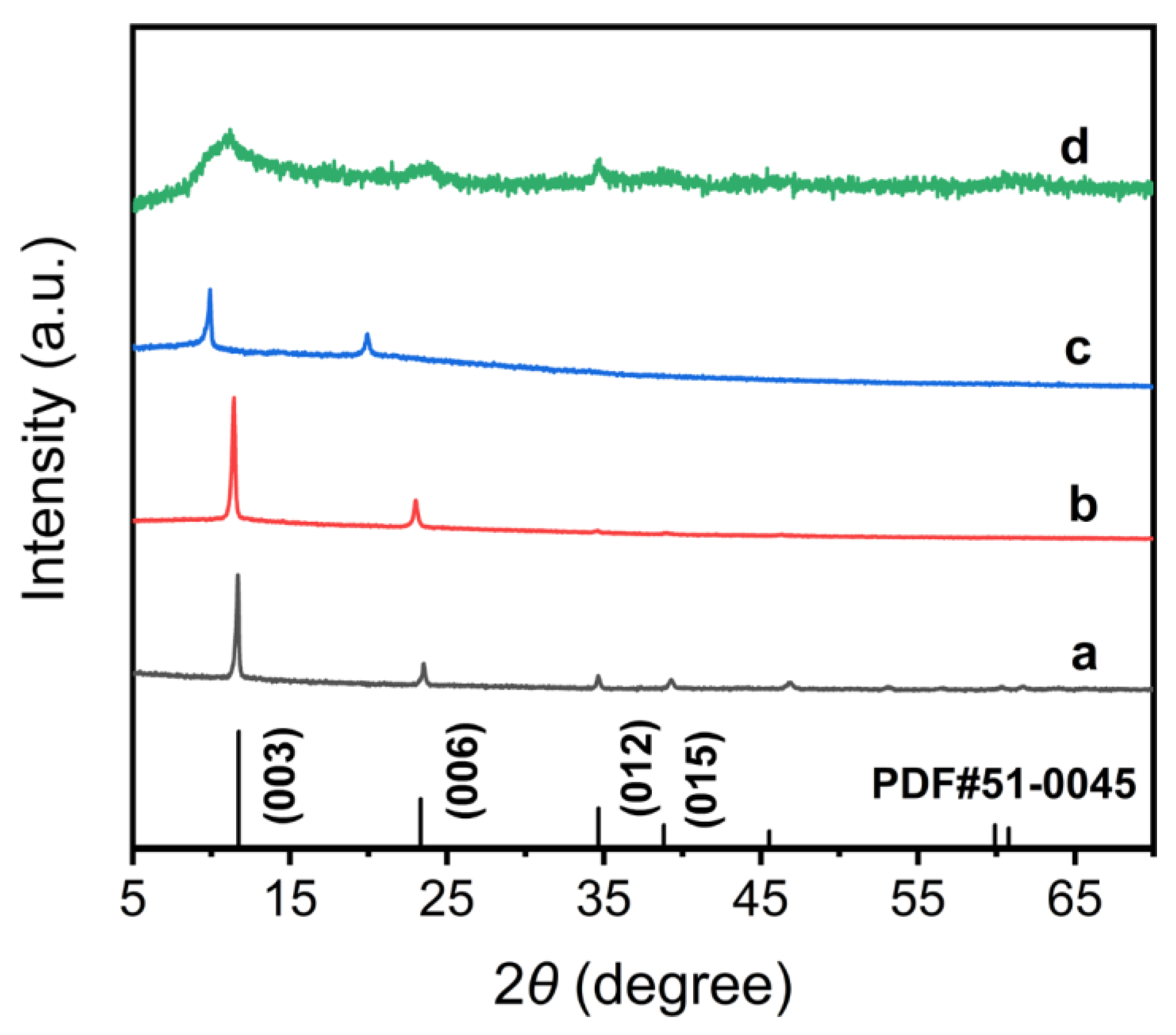
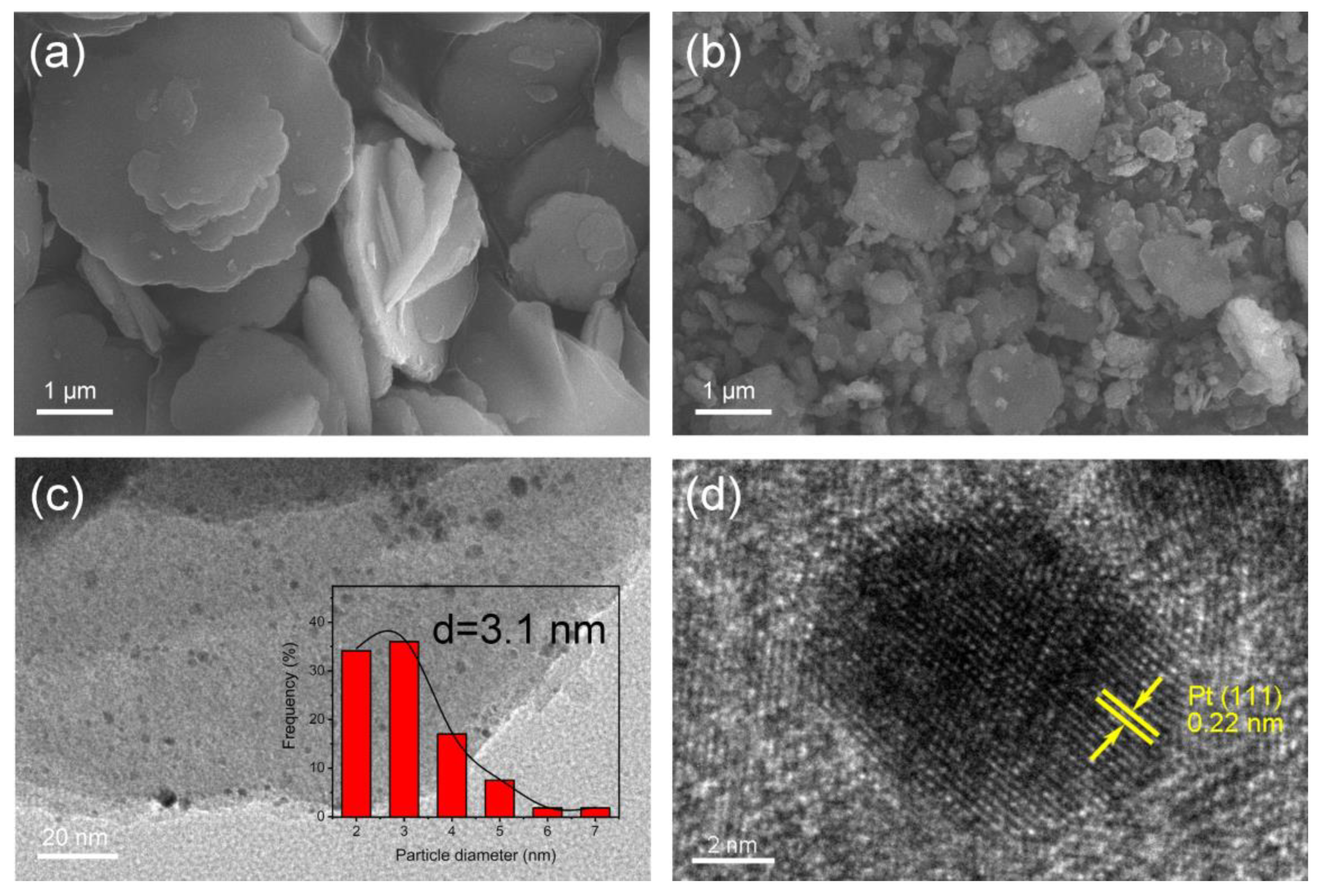
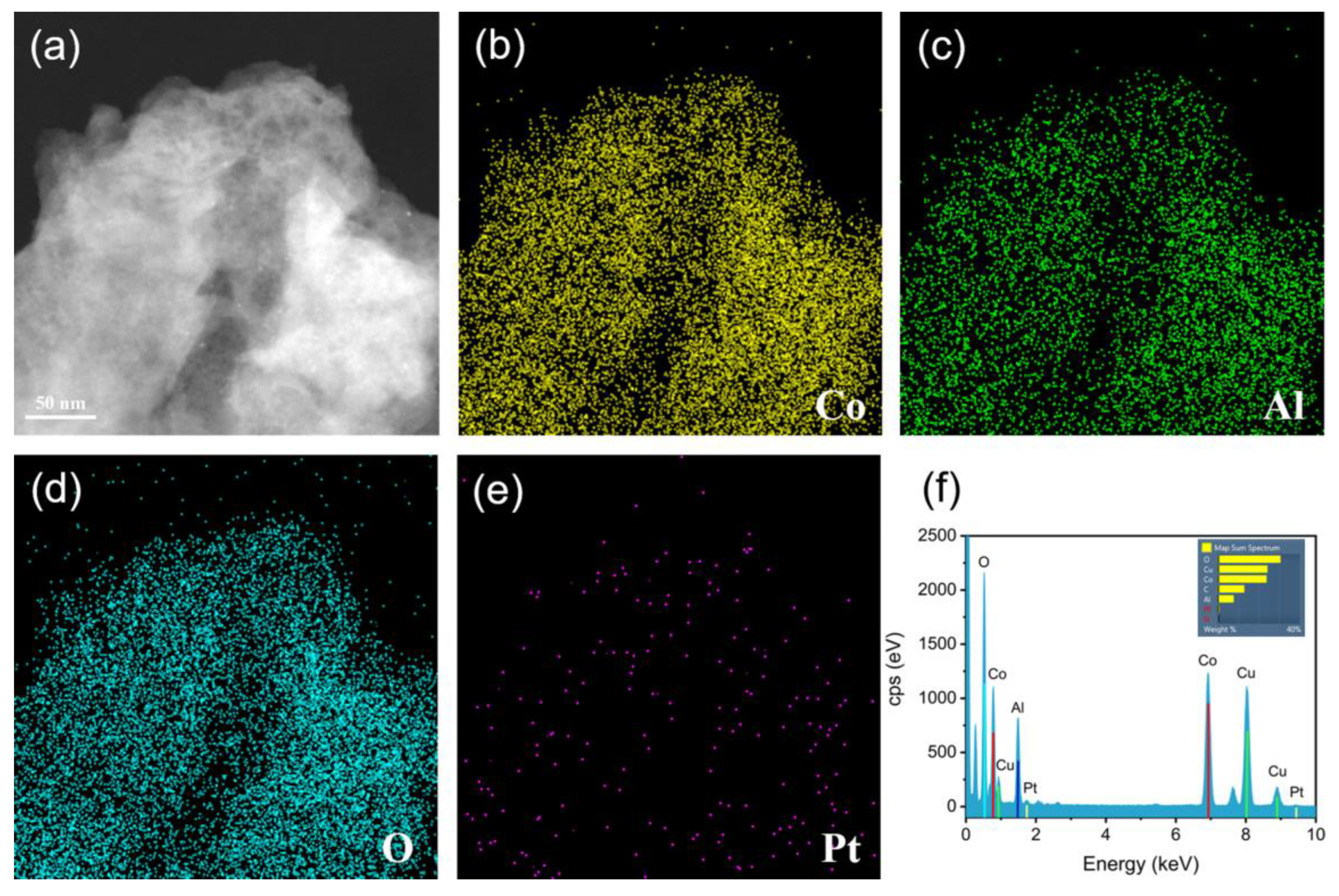
3.1. Characterization of Pt@Co-Al LDH Hybrid
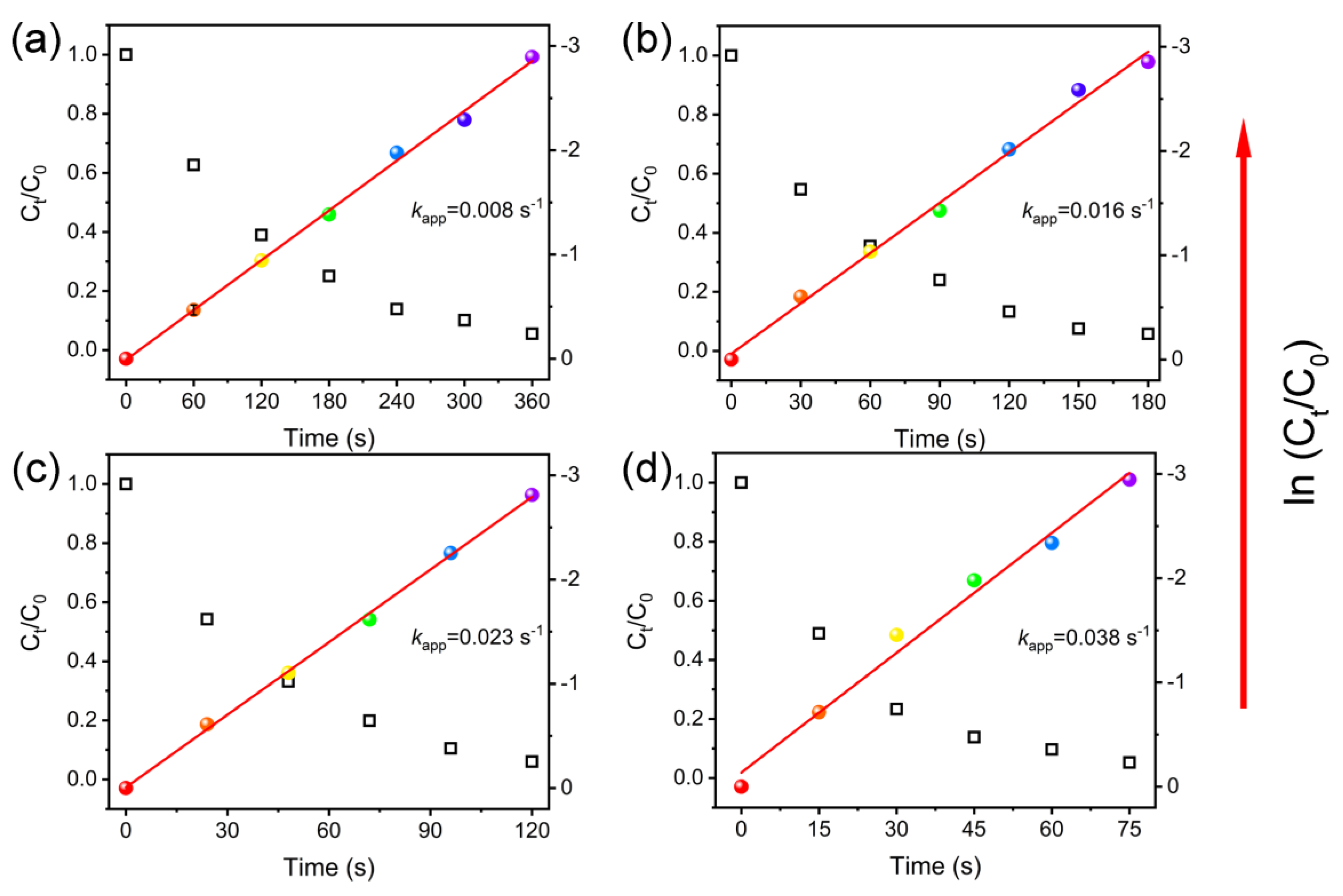
3.2. Catalytic Performance of Pt@Co-Al LDH Hybrid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, H.; Zhang, A.; Jin, F.; Guo, H.; Huang, W.; Cheng, W.; Liu, J. Origami and layered-shaped ZnNiFe-LDH synthesized on Cu(OH)2 nanorods array to enhance the energy storage capability. J. Colloid Interface Sci. 2022, 607, 1269–1279. [Google Scholar] [CrossRef] [PubMed]
- El-Bahy, Z.M. Preparation and characterization of Pt-promoted NiY and CoY catalysts employed for 4-nitrophenol reduction. Appl. Catal. A 2013, 468, 175–183. [Google Scholar] [CrossRef]
- Neal, R.D.; Inoue, Y.; Hughes, R.A.; Neretina, S. Catalytic reduction of 4-nitrophenol by gold catalysts: The influence of borohydride concentration on the induction time. J. Phys. Chem. C 2019, 123, 12894–12901. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Heterogeneous catalytic reduction of anthropogenic pollutant, 4-nitrophenol by silver-bionanocomposite using Cylindrocladium floridanum. Bioresour. Technol. 2011, 102, 10737–10740. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of TiO2 nanoparticles synthesized by sol-gel method for effectual photodegradation, oxidation and reduction reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Wi-Afedzi, T.; Yeoh, F.-Y.; Yang, M.-T.; Yip, A.C.K.; Lin, K.-Y.A. A comparative study of hexacyanoferrate-based Prussian blue analogue nanocrystals for catalytic reduction of 4-nitrophenol to 4-aminophenol. Sep. Purif. Technol. 2019, 218, 138–145. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, B.; Wang, X.; Zhang, L.; Yang, X.; Li, C. Sandwich-like MIL-100(Fe)@Pt@MIL-100(Fe) nanoparticles for catalytic hydrogenation of 4-nitrophenol. Catal. Commun. 2017, 102, 17–20. [Google Scholar] [CrossRef]
- Liu, B.; Yan, S.; Zhang, A.; Song, Z.; Sun, Q.; Huo, B.; Yang, W.; Barrow, C.J.; Liu, J. Insight into catalytic mechanisms for the reduction of nitrophenol via heterojunctions of gold nanoclusters on 2D boron nitride nanosheets. ChemNanoMat 2019, 5, 784–791. [Google Scholar] [CrossRef]
- Karki, H.P.; Ojha, D.P.; Joshi, M.K.; Kim, H.J. Effective reduction of p-nitrophenol by silver nanoparticle loaded on magnetic Fe3O4/ATO nano-composite. Appl. Surf. Sci. 2018, 435, 599–608. [Google Scholar] [CrossRef]
- Lee, S.J.; Yu, Y.; Jung, H.J.; Naik, S.S.; Yeon, S.; Choi, M.Y. Efficient recovery of palladium nanoparticles from industrial wastewater and their catalytic activity toward reduction of 4-nitrophenol. Chemosphere 2021, 262, 128358. [Google Scholar] [CrossRef]
- Mejía, C.H.; van Deelen, T.W.; de Jong, K.P. Activity enhancement of cobalt catalysts by tuning metal-support interactions. Nat. Commun. 2018, 9, 4459–4466. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Saenz-Arana, R.; Wang, H.; Bernal, R.; Noveron, J.C. Green synthesis of gold, silver, platinum, and palladium nanoparticles reduced and stabilized by sodium rhodizonate and their catalytic reduction of 4-nitrophenol and methyl orange. New J. Chem. 2018, 42, 6472–6478. [Google Scholar] [CrossRef]
- Menumerov, E.; Hughes, R.A.; Neretina, S. Catalytic reduction of 4-nitrophenol: A quantitative assessment of the role of dissolved oxygen in determining the induction time. Nano Lett. 2016, 16, 7791–7797. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, S.B. Catalytic reduction of p-nitrophenol by using platinum nanoparticles stabilised by guar gum. Carbohydr. Polym. 2014, 113, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Akilandaeaswari, B.; Muthu, K. One-pot green synthesis of Au-Ag bimetallic nanoparticles from Lawsonia inermis seed extract and its catalytic reduction of environmental polluted methyl orange and 4-nitrophenol. J. Taiwan Inst. Chem. Eng. 2021, 127, 292–301. [Google Scholar] [CrossRef]
- Baran, N.Y.; Çalışkan, M.; Baran, T. Pd@MgAl LDH/2-ThCA: A sustainable, active and recyclable nanocatalyst system for the rapid and efficient catalytic reduction of organic pollutants. Inorg. Chem. Commun. 2023, 157, 111333–111346. [Google Scholar] [CrossRef]
- Wang, X.; Tan, F.; Wang, W.; Qiao, X.; Qiu, X.; Chen, J. Anchoring of silver nanoparticles on graphitic carbon nitride sheets for the synergistic catalytic reduction of 4-nitrophenol. Chemosphere 2017, 172, 147–154. [Google Scholar] [CrossRef]
- Tian, X.; Zahid, M.; Li, J.; Sun, W.; Niu, X.; Zhu, Y. Pd/Mo2N-TiO2 as efficient catalysts for promoted selective hydrogenation of 4-nitrophenol: A green bio-reducing preparation method. J. Catal. 2020, 391, 190–201. [Google Scholar] [CrossRef]
- Tian, X.; Zahid, M.; Sun, W.; Zhu, Y. Facile fabrication of TiO2 with 3D hierarchical structure and its supported Pd catalysts for high catalytic hydrogenation performance of 4-nitrophenol to 4-aminophenol. Appl. Surf. Sci. 2021, 566. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, J.; Wen, W.; Zhang, X.; Wang, S. Spherical covalent organic framework supported Cu/Ag bimetallic nanoparticles with highly catalytic activity for reduction of 4-nitrophenol. J. Solid State Chem. 2022, 311, 123116–123123. [Google Scholar] [CrossRef]
- Malik, M.A.; Alshehri, A.A.; Patel, R. Facile one-pot green synthesis of Ag–Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021, 12, 455–470. [Google Scholar] [CrossRef]
- Ye, W.; Yu, J.; Zhou, Y.; Gao, D.; Wang, D.; Wang, C.; Xue, D. Green synthesis of Pt–Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4- nitrophenol reduction. Appl. Catal. B 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Xia, B.; He, F.; Li, L. Preparation of bimetallic nanoparticles using a facile green synthesis method and their application. Langmuir 2013, 29, 4901–4907. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhou, Y.; You, Z.; Xia, H.; Tuo, Y.; Wang, S.; Jia, C.; Zhang, J. Bi-functional Fe3O4 /Au/CoFe-LDH sandwich-structured electrocatalyst for asymmetrical electrolyzer with low operation voltage. Small 2021, 17, 2103307–2103320. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Liu, S.; Lai, C.; Zeng, G.; Li, M.; Liu, X.; Li, B.; Huo, X.; Qin, L.; Li, L.; et al. Recent advance of transition-metal-based layered double hydroxide nanosheets: Synthesis, properties, modification, and electrocatalytic applications. Adv. Energy Mater. 2021, 11, 2002863–2002888. [Google Scholar] [CrossRef]
- Wei, C.; Yan, X.; Zhou, Y.; Xu, W.; Gan, Y.; Zhang, Y.; Zhang, N. Morphological control of layered double hydroxides prepared by co-precipitation method. Crystals 2022, 12, 1713. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, B.; Liu, S.; Li, M.; Zheng, W.; Deng, L.; Liu, J. Novel approach to immobilize Au nanoclusters on micro/nanostructured carbonized natural lotus leaf as green catalyst with highly efficient catalytic activity. Chem. Eng. J. 2019, 371, 876–884. [Google Scholar] [CrossRef]
- Murayama, T.; Haruta, M. Preparation of gold nanoparticles supported on Nb2O5 by deposition precipitation and deposition reduction methods and their catalytic activity for CO oxidation. Chin. J. Catal. 2016, 37, 1694–1701. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, B.; Tang, Q.; Liu, L.; Dong, J. Adjusting Pt nanoparticle size on SBA-15 by a sol-immobilisation method towards naphthalene hydrogenation. Catal. Lett. 2022, 152, 3489–3497. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Sasaki, T.; Watanabe, M.; Hashizume, H.; Yamada, H.; Nakazawa, H. Macromolecule-like aspects for a colloidal suspension of an exfoliated titanate. pairwise association of nanosheets and dynamic reassembling process initiated from It. J. Am. Chem. Soc. 1996, 118, 8329–8335. [Google Scholar] [CrossRef]
- Abe, R.; Shinohara, K.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Preparation of porous niobium oxides by soft-chemical process and their photocatalytic activity. Chem. Mater. 1997, 9, 2179–2184. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Zhou, Z.; Deng, L.; Liu, L.; Xu, M. Platinum nanoparticles with low content and high dispersion over exfoliated layered double hydroxide for photocatalytic CO2 reduction. Energy Fuels 2021, 35, 10820–10831. [Google Scholar] [CrossRef]
- Huang, S.; Cen, X.; Peng, H.; Guo, S.; Wang, W.; Liu, T. Heterogeneous ultrathin films of poly(vinyl alcohol)/layered double hydroxide and montmorillonite nanosheets via layer-by-layer assembly. J. Phys. Chem. B 2009, 113, 15225–15230. [Google Scholar] [CrossRef]
- Liu, Z.; Ma, R.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.; Sasaki, T. Synthesis, anion exchange and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, R.; Zhang, Z.; Wu, T.; Yue, C.; Pan, W. Loading metal nanoparticles on the CoAl-LDH/CGCNN S-scheme heterojunction for efficient photocatalytic CO2 reduction under visible light. Sep. Purif. Technol. 2023, 322, 124266–124276. [Google Scholar] [CrossRef]
- Tao, J.; Yu, X.; Liu, Q.; Liu, G.; Tang, H. Internal electric field induced S–scheme heterojunction MoS2/CoAl LDH for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 585, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Liu, Z.; Li, L.; Iyi, N.; Sasaki, T. Exfoliating layered double hydroxides in formamide: A method to obtain positively charged nanosheets. J. Mater. Chem. 2006, 16, 3809–3813. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, C.; Zhang, X.; Jiang, D.; Liu, X.; Dong, B.; Feng, L.; Li, S.; Zhang, Y. Acid-salt treated CoAl layered double hydroxide nanosheets with enhanced adsorption capacity of methyl orange dye. J. Colloid Interface Sci. 2019, 548, 100–109. [Google Scholar] [CrossRef]
- Huang, S.; Peng, H.; Tjiu, W.W.; Yang, Z.; Zhu, H.; Tang, T.; Liu, T. Assembling exfoliated layered double hydroxide (LDH) nanosheet/carbon nanotube (CNT) hybrids via electrostatic force and fabricating nylon nanocomposites. J. Phys. Chem. B 2010, 114, 16766–16772. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Zhang, B.; Lin, Y.; Wang, Q.; Zhang, Q.; Su, D.S. Enhanced chemoselective hydrogenation through tuning the interaction between Pt nanoparticles and carbon supports: Insights from identical location transmission electron microscopy and X-ray photoelectron spectroscopy. ACS Catal. 2016, 6, 7844–7854. [Google Scholar] [CrossRef]
- Mao, N.; Zhou, C.H.; Tong, D.S.; Yu, W.H.; Lin Cynthia, C.X. Exfoliation of layered double hydroxide solids into functional nanosheets. Appl. Clay Sci. 2017, 144, 60–78. [Google Scholar] [CrossRef]
- Chen, C.; Tao, L.; Du, S.; Chen, W.; Wang, Y.; Zou, Y.; Wang, S. Advanced exfoliation strategies for layered double hydroxides and applications in energy conversion and storage. Adv. Funct. Mater. 2020, 30, 1909832–1909849. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, Q.; Li, J.; Rao, P.; Li, R.; Du, Y.; Jia, C.; Luo, J.; Deng, P.; et al. Recent advances in two-dimensional Pt based electrocatalysts for methanol oxidation reaction. Int. J. Hydrogen Energy 2021, 46, 31202–31215. [Google Scholar] [CrossRef]
- Qian, Q.; Yu, G.; Zhao, Q.; Zhang, X. One-step hydrothermal preparation of bilayer films of NiCo LDH/Pt loaded on nickel foam surface for HER catalytic activity. New J. Chem. 2023, 47, 1040–1044. [Google Scholar] [CrossRef]
- Cai, T.; Zhang, P.; Shen, X.; Huang, E.; Shen, X.; Shi, J.; Wang, Z.; Sun, Q. Synthesis of Pt-loaded NiFe-LDH nanosheets on wood veneer for efficient gaseous formaldehyde degradation. ACS Appl. Mater. Interfaces 2020, 12, 37147–37154. [Google Scholar] [CrossRef]
- Jang, S.W.; Dutta, S.; Kumar, A.; Hong, Y.R.; Kang, H.; Lee, S.; Ryu, S.; Choi, W.; Lee, I.S. Holey Pt nanosheets on NiFe-hydroxide laminates: Synergistically enhanced electrocatalytic 2D interface toward hydrogen evolution reaction. ACS Nano 2020, 14, 10578–10588. [Google Scholar] [CrossRef]
- Lin, B.; He, L.; Zhu, B.; Chen, Y.; Gao, B. Visible-light photocatalytic activity of mesoporous nanohybrid assembled by tantalotungstate nanosheets and manganese ions. Catal. Commun. 2012, 29, 166–169. [Google Scholar] [CrossRef]
- Gu, T.H.; Gunjakar, J.L.; Kim, I.Y.; Patil, S.B.; Lee, J.M.; Jin, X.; Lee, N.S.; Hwang, S.J. Porous hybrid network of graphene and metal oxide nanosheets as useful matrix for improving the electrode performance of layered double hydroxides. Small 2015, 11, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, J.; Fjellvag, H.; Bundli, S.; Sjastad, A.O. Efficient exfoliation of layered double hydroxides; effect of cationic ratio, hydration state, anions and their orientations. Materials 2021, 14, 346. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Almashhadani, H.A.; Jawad, S.F.; Khan, M.F.; Ismail, A. Stabilization of Pt nanoparticles within MOFs for selective hydrogenation of hazardous 4-nitrophenol to valuable 4-aminophenol: Confinement and synergistic effect. J. Solid State Chem. 2022, 316, 123565–123574. [Google Scholar] [CrossRef]
- Kiani, Z.; Zhiani, R.; Khosroyar, S.; Motavalizadehkakhky, A.; Hosseiny, M. UiO-66/btb/Pd as a stable catalyst reduction of 4-nitrophenol into 4-aminophenol. Inorg. Chem. Commun. 2021, 124, 108382–108388. [Google Scholar] [CrossRef]
- Kharlamova, T.S.; Salina, M.V.; Svetlichnyi, V.A.; Salaev, M.A.; Stadnichenko, A.I.; Mamontov, G.V. CeO2-supported Pt–Ag bimetallic catalysts for 4-nitrophenol reduction. Catal. Today 2022, 384–386, 12–24. [Google Scholar] [CrossRef]
- Wu, Y.; Hao, W.; Li, X.; Qin, L.; Zhang, T.; Kang, S.-Z. An efficient integrated catalyst for the reduction of 4-nitrophenol in a continuous flow system: Ag nanoparticles loaded graphene nanosheets immobilized on Ti meshes. Diam. Relat. Mater. 2022, 126, 109135–109141. [Google Scholar] [CrossRef]
- Fu, J.; Wang, S.; Wang, X.; Yan, Y.; Wang, K.; Gao, M.; Xu, Q. Facile preparation of highly dispersed Pt nanoparticles supported on heteroatom-containing porous carbon nanospheres and their catalytic properties for the reduction of 4-nitrophenol. J. Porous Mater. 2017, 25, 1081–1089. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Yaseen, M.; Marwat, A.; Ahmad, A. Catalytic reduction of nitrophenol and MB waste water using homogeneous Pt NPs confined in hierarchically porous silica. J. Environ. Chem. Eng. 2021, 9, 105567–105578. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Zhou, Y.; Zhang, Z.; Xue, J.; Xu, Y.; Zhang, C.; Sheng, X.; Kui, N. Nanocasting synthesis of an ordered mesoporous CeO2-supported Pt nanocatalyst with enhanced catalytic performance for the reduction of 4-nitrophenol. RSC Adv. 2016, 6, 730–739. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, J.; Zhang, Y.; Zhang, C.; Zhao, S.; Zhou, Y.; Huang, M.; Sheng, X. Novel synthesis of Fe2O3–Pt ellipsoids coated by double-shelled La2O3 as a catalyst for the reduction of 4-nitrophenol. Appl. Organomet. Chem. 2018, 32, 4208–4219. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Bahrami, F.; Gholami, M.R. Au and Pt nanoparticles supported on Ni promoted MoS2 as efficient catalysts for p-nitrophenol reduction. J. Water Process Eng. 2020, 34, 101142–101150. [Google Scholar] [CrossRef]
- Sharma, A.; Dutta, R.K.; Roychowdhury, A.; Das, D.; Goyal, A.; Kapoor, A. Cobalt doped CuO nanoparticles as a highly efficient heterogeneous catalyst for reduction of 4-nitrophenol to 4-aminophenol. Appl. Catal. A 2017, 543, 257–265. [Google Scholar] [CrossRef]
- Kassem, A.A.; Abdelhamid, H.N.; Fouad, D.M.; Ibrahim, S.A. Catalytic reduction of 4-nitrophenol using copper terephthalate frameworks and CuO@C composite. J. Environ. Chem. Eng. 2021, 9, 104401–104410. [Google Scholar] [CrossRef]
- Aykut, E.; Sert, M.; Sert, E. Catalytic activity of MOF derived CuFe@C catalysts for catalytic reduction of 4-nitrophenol. J. Water Process Eng. 2023, 54, 103970–103983. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Pal, U.; Kumar, M.K.; Domínguez, J.M.; Gomez, L.M.; Agarwal, V. Green fabrication of 2D platinum superstructures and their high catalytic activity for mitigation of organic pollutants. Catal. Today 2021, 360, 185–193. [Google Scholar] [CrossRef]
- Mejia, Y.R.; Reddy, N.K. Bogireddy Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Sahare, P.; Pal, U.; Méndez, S.F.O.; Gomez, L.M.; Agarwal, V. Platinum nanoparticle-assembled porous biogenic silica 3D hybrid structures with outstanding 4-nitrophenol degradation performance. Chem. Eng. J. 2020, 388, 124237–124250. [Google Scholar] [CrossRef]
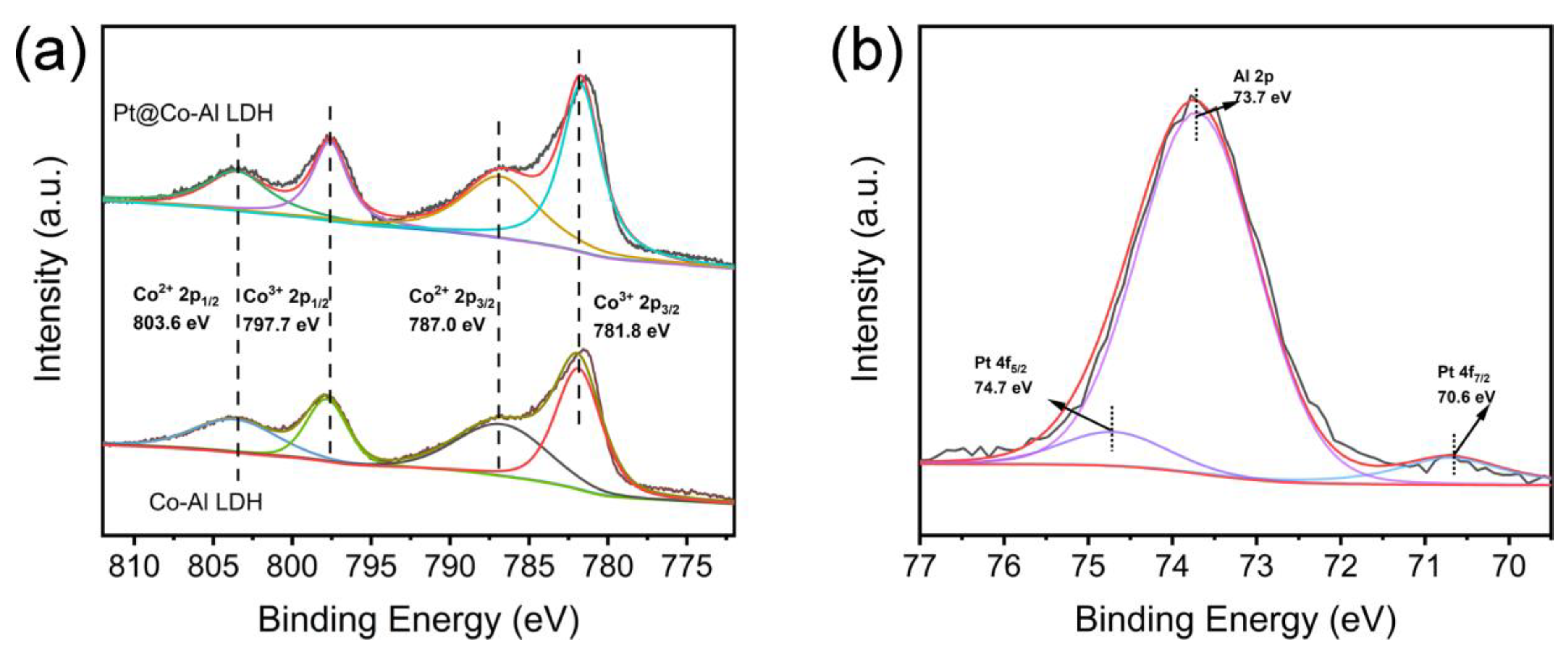
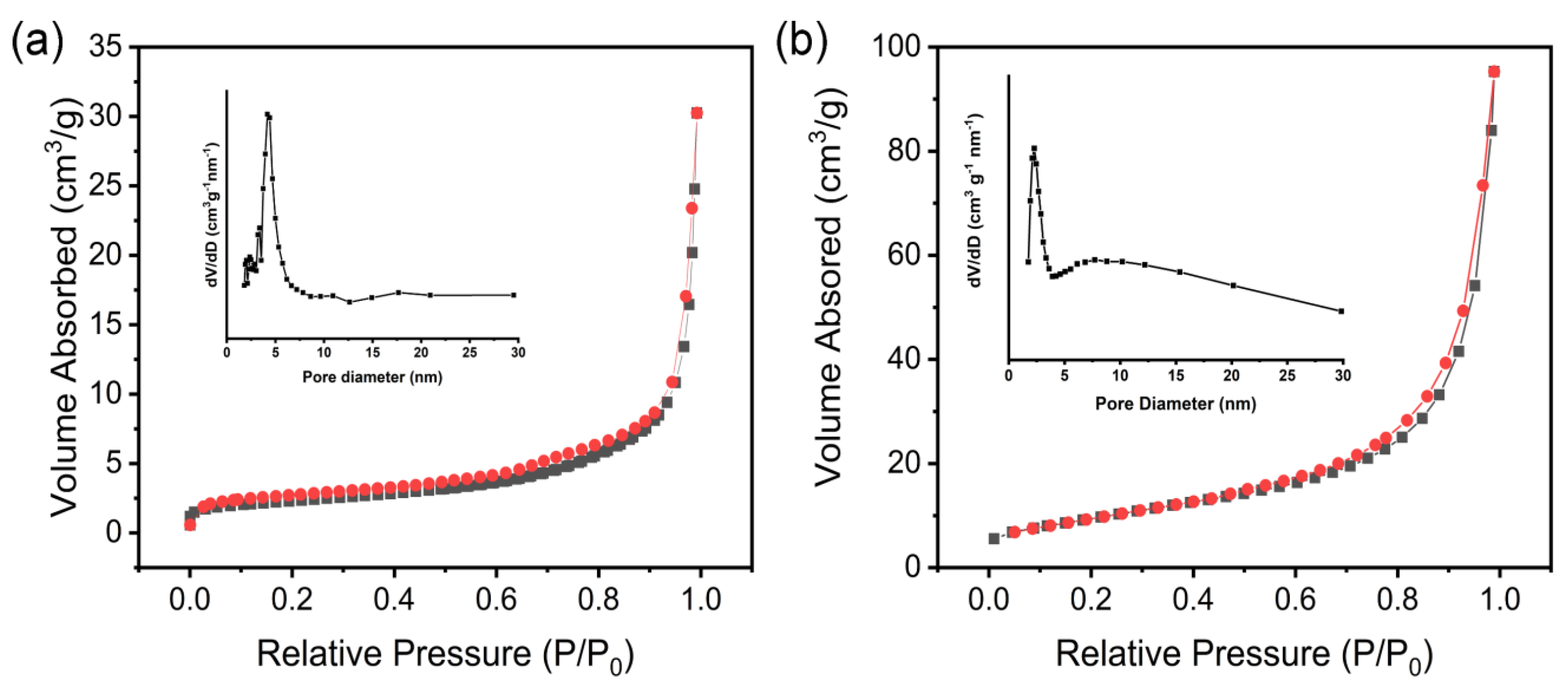
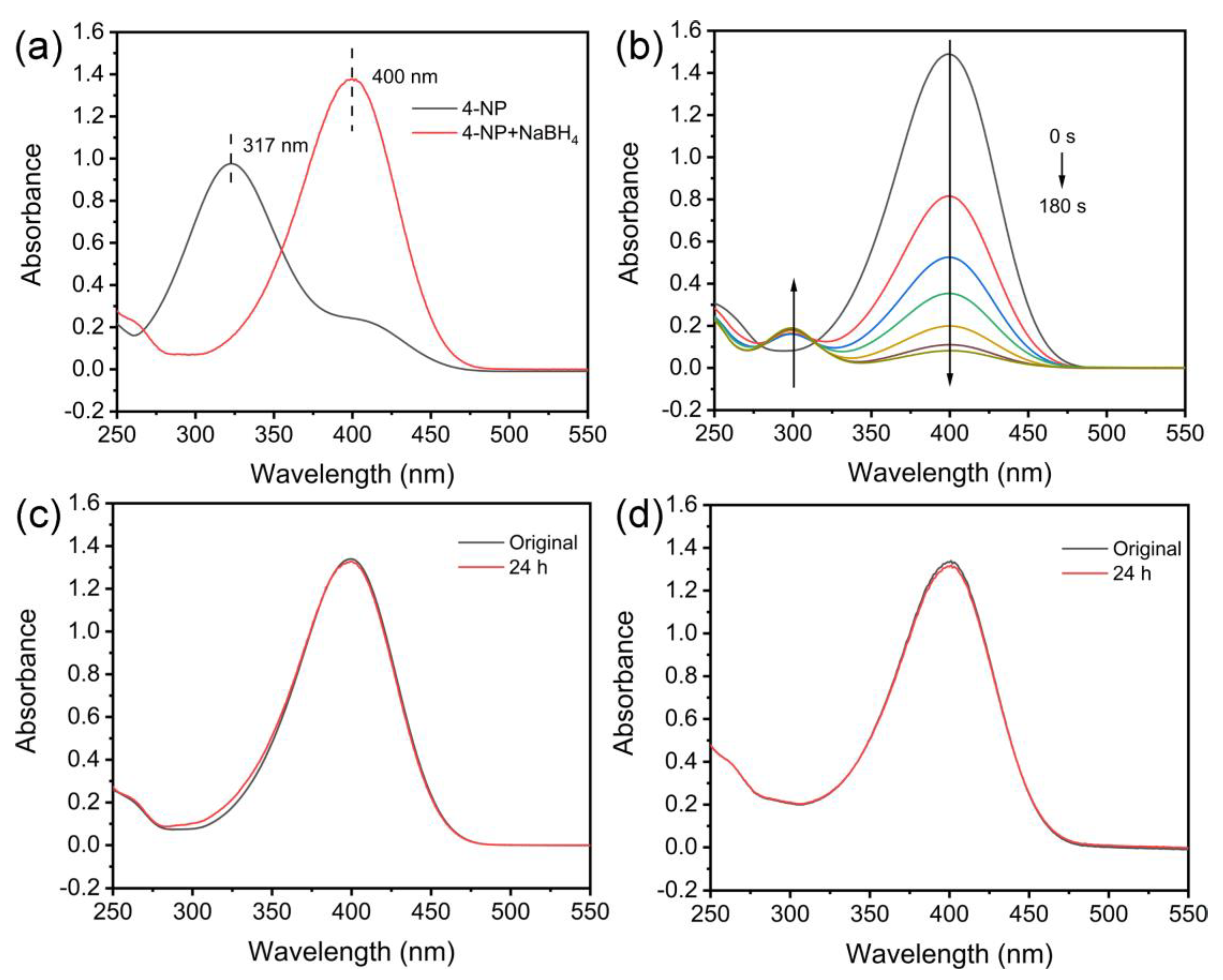
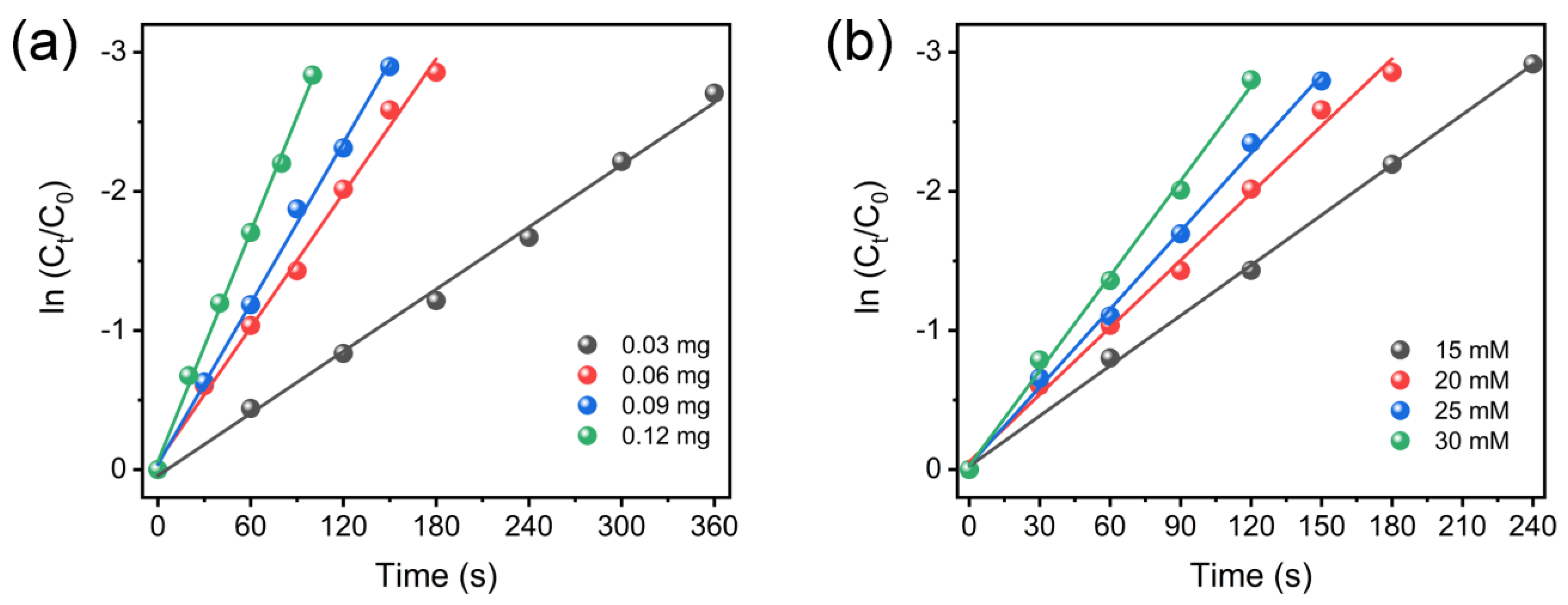


| Material | S/m2 g−1 | A/nm | P/cm3 g−1 |
|---|---|---|---|
| Co-Al LDH | 8 | 6.1 | 0.04 |
| Pt@Co-Al LDH | 35 | 4.5 | 0.15 |
| Catalyst | Reaction Conditions (4-NP, NaBH4, Cat.) | kapp × 103 (s−1) | Time (min) | Ref. |
|---|---|---|---|---|
| Pt NPs@C-PZS | 0.1 mM, 5 mM, 0.2 mg | 4.6 | 16 | [55] |
| Pt/MMZ | 0.03 mM, 0.016 mM, 2 mg | 5.35 | 14 | [56] |
| Pt/meso-CeO2 | 0.1 mM, 5 mM, 0.3 mg | 6.0 | 8 | [57] |
| Fe2O3-Pt@DSL-Pt | 0.1 mM, 41 mM, 0.5 mg | 6.32 | 10 | [58] |
| PtNi/MoS2 | 0.1 mM, 80 mM, 1 mg | 9.2 | 5 | [59] |
| Co-doped CuO NPs | 0.06 mM, 4 mM, 1 mg | 43.8 | 3.5 | [60] |
| Pt@Co-Al LDH | 0.1 mM, 20 mM, 0.06 mg | 16.1 | 3 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Qiu, Y.; Yang, X.; Gao, Z.; Wang, Z.; Liu, C.; Sun, Y.; Ma, J.; Liu, L. High-Performance Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol over Pt Nanoparticles Supported on Co-Al LDH Nanosheets. Crystals 2024, 14, 284. https://doi.org/10.3390/cryst14030284
Xu C, Qiu Y, Yang X, Gao Z, Wang Z, Liu C, Sun Y, Ma J, Liu L. High-Performance Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol over Pt Nanoparticles Supported on Co-Al LDH Nanosheets. Crystals. 2024; 14(3):284. https://doi.org/10.3390/cryst14030284
Chicago/Turabian StyleXu, Chenzhe, Yue Qiu, Xiaoting Yang, Zifei Gao, Zheng Wang, Cai Liu, Yanran Sun, Juanjuan Ma, and Lin Liu. 2024. "High-Performance Catalytic Reduction of 4-Nitrophenol to 4-Aminophenol over Pt Nanoparticles Supported on Co-Al LDH Nanosheets" Crystals 14, no. 3: 284. https://doi.org/10.3390/cryst14030284





