Estimation of Ionic Impurities in Poly(propylene Glycol) Diacrylate Monomers/Liquid Crystal E7 Mixtures Using Dielectric Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
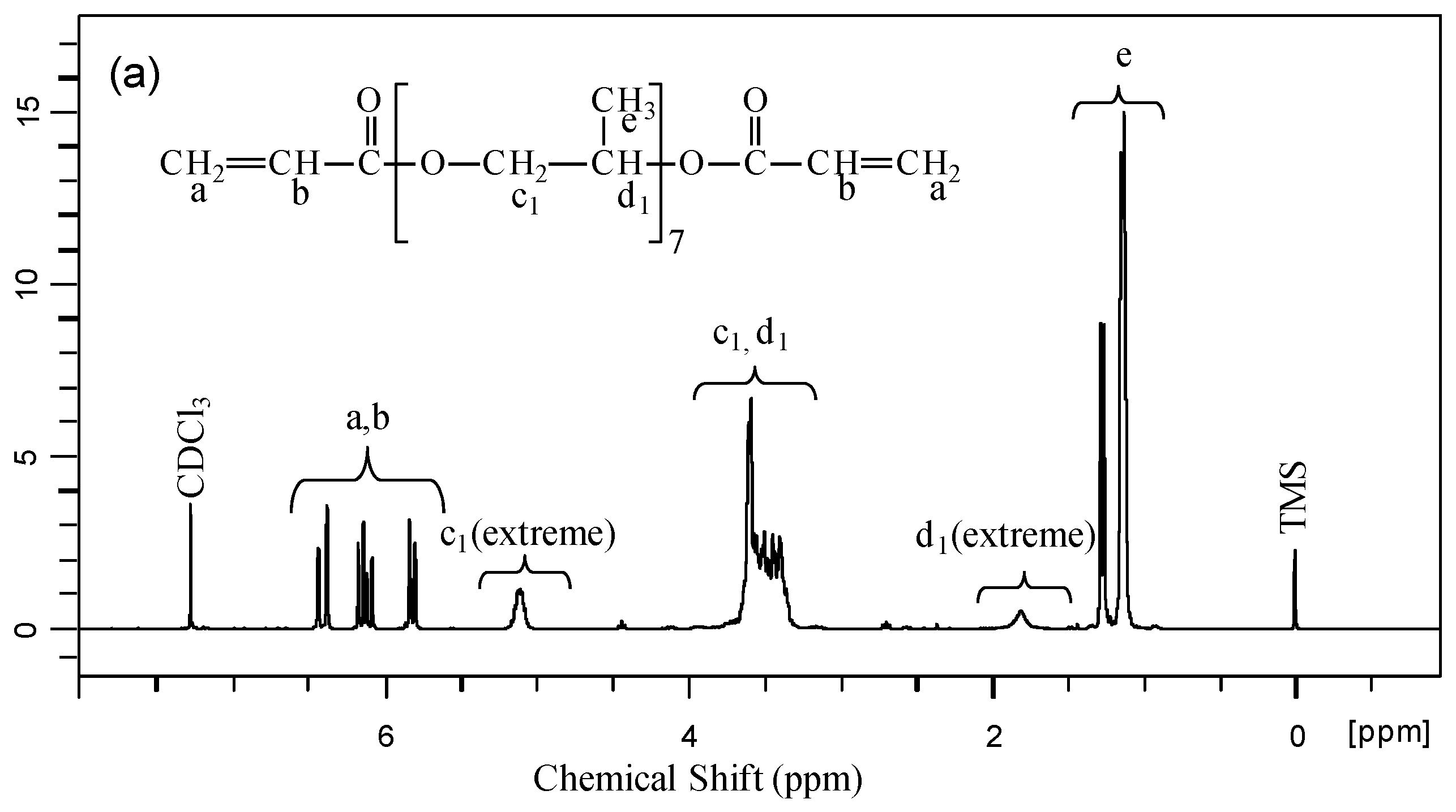
2.2.1. Nuclear Magnetic Resonance Spectroscopy
2.2.2. Gel Permeation Chromatography
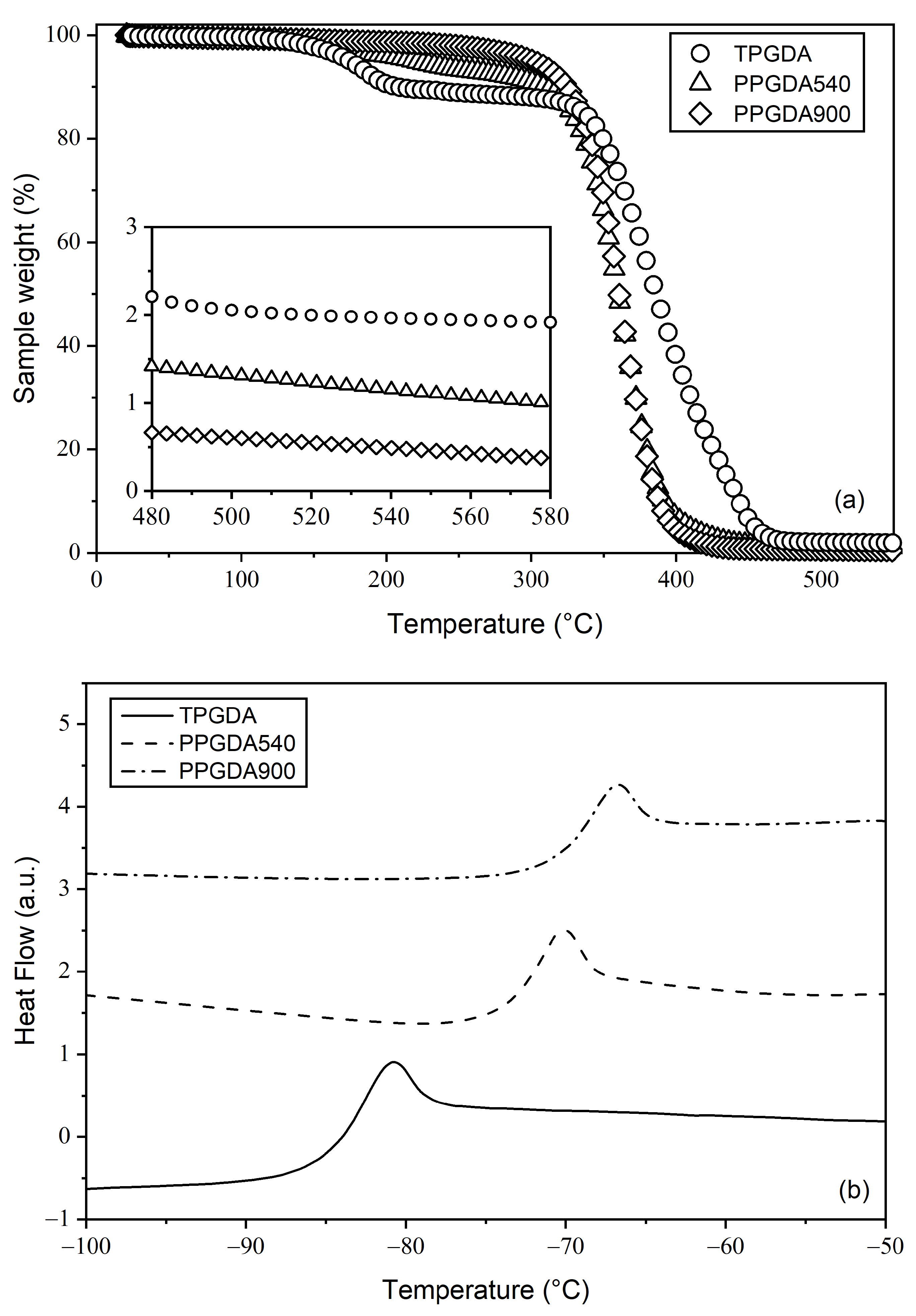
2.2.3. Thermogravimetric Analysis
2.2.4. Differential Scanning Calorimetry
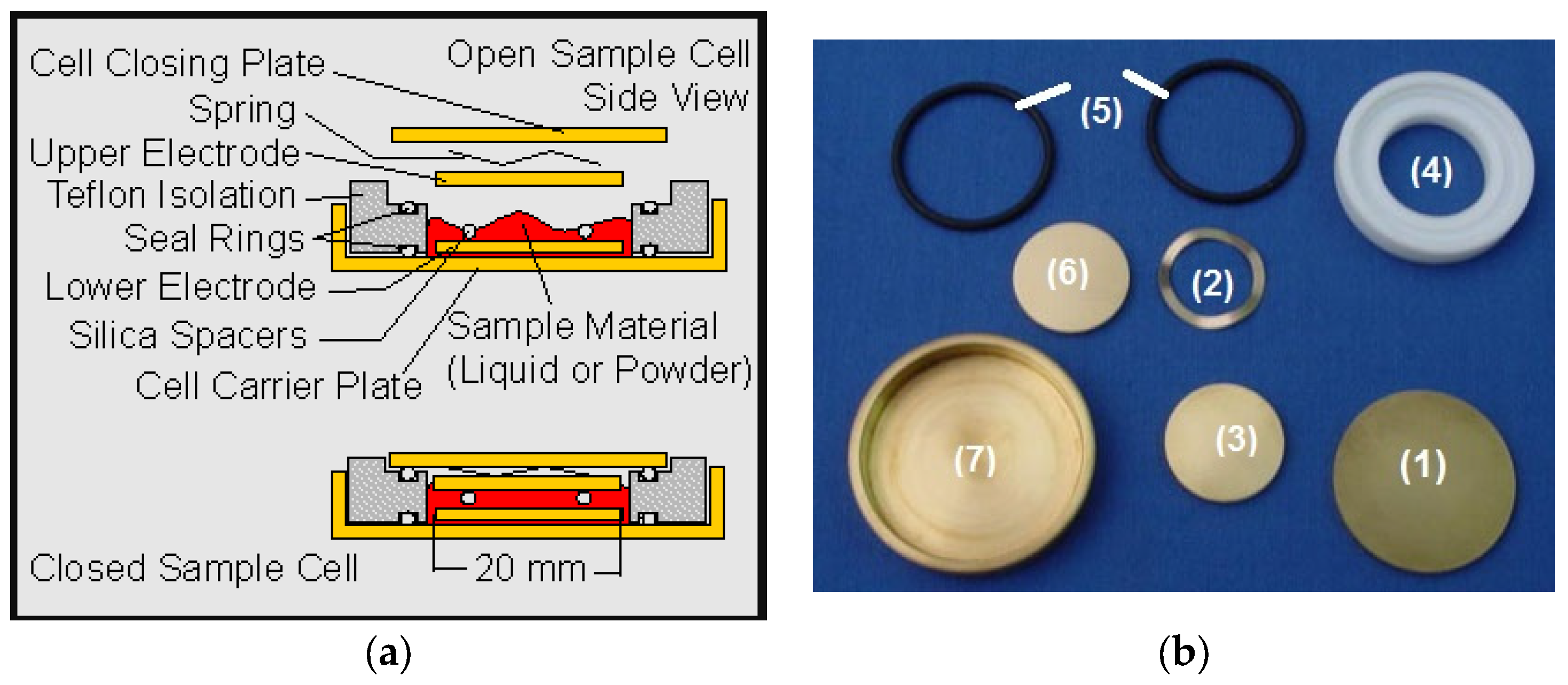
2.2.5. Dielectric Spectroscopy
3. Results and Discussion
3.1. Chemical and Structural Properties of Materials
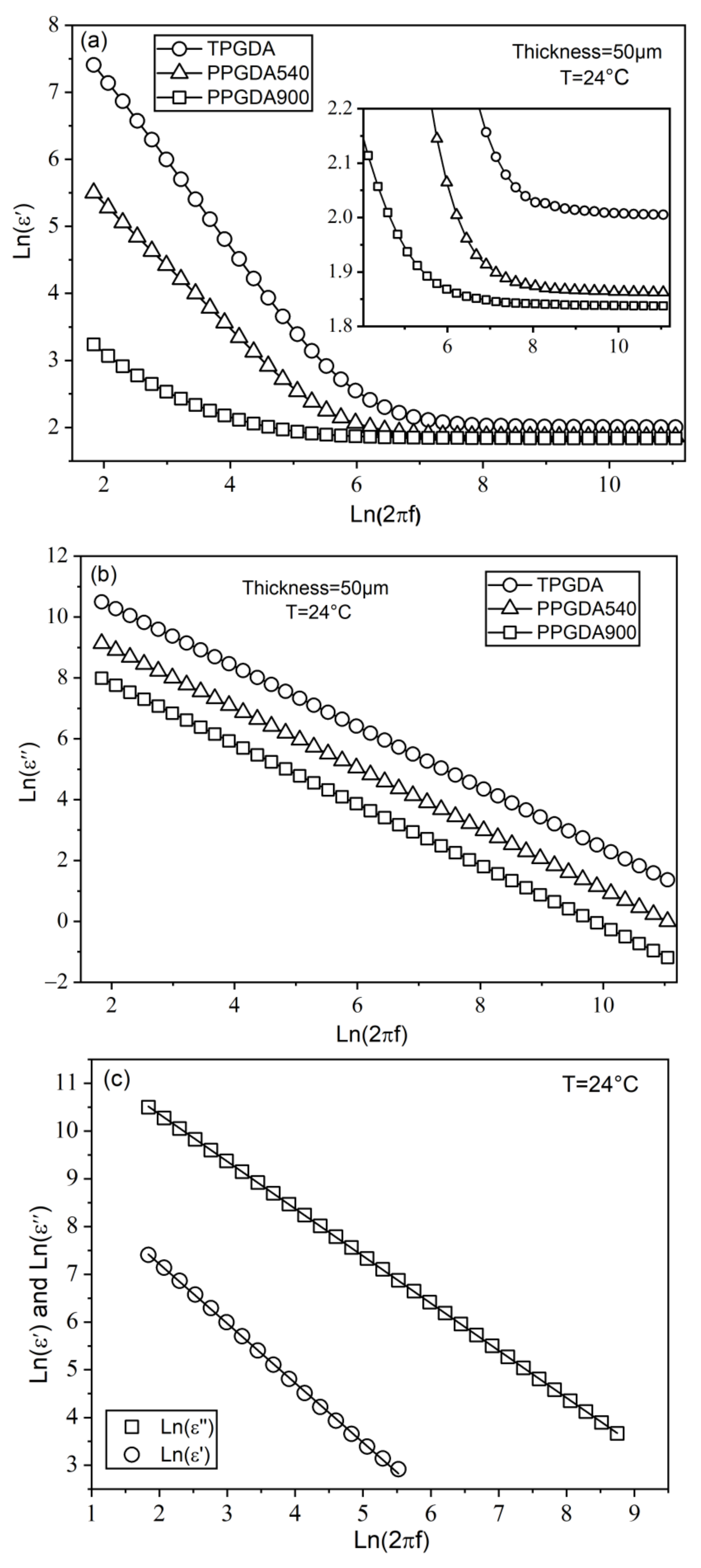
3.2. Dielectric Characterization
3.3. Conductivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Capacitance (F) | |
| Activation energy (eV) | |
| Pseudo-activation energy (eV) | |
| Frequency (Hz) | |
| Conductance () | |
| Boltzmann constant () | |
| Molecular weight (g.mol−1) | |
| q | Electronic charge () |
| Glass transition temperature (K) | |
| Relative permittivity spectrum | |
| Loss factor spectrum | |
| Permittivity of free space () | |
| Conductivity (S.m−1) | |
| Pre-exponential factor () | |
| TPGDA | Tri(propylene glycol) diacrylate (n = 3, CAS Number: 42978-66-5) |
| PPGDA540 | Poly(propylene glycol) diacrylate (n = 7, CAS Number: 52496-08-9) |
| PPGDA900 | Poly(propylene glycol) diacrylate (n = 12, CAS Number: 52496-08-9) |
References
- Bronnikov, S.; Kostromin, S.; Zuev, V. Polymer-Dispersed Liquid Crystals: Progress in Preparation, Investigation, and Application. Soft Mater. 2013, 52, 1718–1735. [Google Scholar] [CrossRef]
- Sasani Ghamsari, M.; Carlescu, I. An overview of polymer-dispersed liquid crystals composite films and their applications. In Liquid Crystal and Display Technology; IntechOpen: London, UK, 2020; Chapter 2; p. 11. [Google Scholar]
- Jeon, Y.-J.; Bingzhu, Y.; Rhee, J.-T.; Cheung, D.-L.; Jamil, M. Application and new developments in polymer-dispersed liquid crystal simulation studies. Macromol. Theory Simul. 2007, 16, 643–659. [Google Scholar] [CrossRef]
- Maschke, U.; Coqueret, X.; Benmouna, M. Electro-Optical Properties of Polymer-Dispersed Liquid Crystals. Macromol. Rapid Commun. 2002, 23, 159–170. [Google Scholar] [CrossRef]
- Saeed, M.H.; Zhang, S.; Cao, Y.; Zhou, L.; Hu, J.; Muhammad, I.; Xiao, J.; Zhang, L.; Yang, H. Recent advances in the Polymer Dispersed Liquid Crystal Composite and its Applications. Molecules 2020, 25, 5510. [Google Scholar] [CrossRef]
- Islam, M.S.; Chan, K.-Y.; Thien, G.S.H.; Low, P.-L.; Lee, C.-L.; Wong, S.K.; Noor, E.E.M.; Au, B.W.-C.; Ng, Z.-N. Performances of Polymer-Dispersed Liquid Crystal Films for Smart Glass Applications. Polymers 2023, 15, 3420. [Google Scholar] [CrossRef] [PubMed]
- Derouiche, Y.; Koynov, K.; Dubois, F.; Douali, R.; Legrand, C.; Maschke, U. Optical, electro-optical, and dielectric properties of acrylic tripropyleneglycol based polymer network systems including LCs. Mol. Cryst. Liq. Cryst. 2012, 561, 124–135. [Google Scholar] [CrossRef]
- Derouiche, Y.; Dubois, F.; Douali, R.; Legrand, C.; Maschke, U. Some properties of nematic liquid crystal E7/acrylic polymer networks. Mol. Cryst. Liq. Cryst. 2011, 541, 201–210. [Google Scholar] [CrossRef]
- Garbovskiy, Y.; Glushchenko, I. Nano-objects and ions in liquid crystals: Ion trapping effect and related phenomena. Crystals. 2015, 5, 501–533. [Google Scholar] [CrossRef]
- Neyts, K.; Beunis, F. Ion transport in liquid crystals. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Tschierske, C., Raynes, P., Gleeson, H., Kato, T., Collings, P.J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2014; Volume 2, Part 1, Chapter 11. [Google Scholar]
- Wojnarowska, Z.; Lange, A.; Taubert, A.; Paluch, M. Ion and proton transport in aqueous/nonaqueous acidic ionic liquids for fuel-cell applications-insight from high-pressure dielectric studies. ACS Appl. Mater. Interfaces 2021, 13, 30614–30624. [Google Scholar] [CrossRef]
- Kumbhakar, K.; Pham, T.-D.; Lee, K.-K.; Kwak, K.; Cho, M. Dielectric relaxation spectroscopy for the characterization of ion transport in solid polymer electrolytes in Li-ion cells. Electrochim. Acta 2023, 462, 142759. [Google Scholar] [CrossRef]
- Dan, L.; Zhang, K.; Huang, Z.; Wang, F.; Wang, Q.; Li, J. Molecular-level evaluation of ionic transport under external electric fields in biological dielectric liquids. J. Mol. Liq. 2021, 340, 116883. [Google Scholar] [CrossRef]
- Cîrcu, V.; Ganea, C.P.; Secu, M.; Manaila-Maximean, D.; Marinescu, G.C.; Popescu, R.G.; Pasuk, I. Columnar Liquid Crystals of Copper (I) Complexes with Ionic Conductivity and Solid State Emission. Molecules 2023, 28, 4196. [Google Scholar] [CrossRef] [PubMed]
- Raymond, M.H.; Ronald, G.O.; St-Onge, H. The Dielectric Properties of Silicone Fluids. IEEE Trans. Dielectr. Electr. Insul. 1977, EI-12, 360–370. [Google Scholar]
- Mazuki, N.F.; Kufian, M.Z.; Saari, M.M.; Samsudin, A.S. Influencing of [EDIMP]TFSI in PMMA-PLA doped LiTFSI based hybrid gel polymer electrolyte on the variation in crystallinity phase and ionic conduction properties. J. Non-Cryst. Solids 2023, 621, 122634. [Google Scholar] [CrossRef]
- Tominaga, Y.; Tsunesada, N.; Miura, S.; Kodama, H.; Furukawa, T. Dielectric relaxation and ionic conduction in solid polymer electrolyte based on a random copolymer of ethylene carbonate and ethylene oxide. Electrochim. Acta 2023, 465, 142995. [Google Scholar] [CrossRef]
- Tian, L.; Wang, M.; Liu, Y.; Su, Z.; Niu, B.; Zhang, Y.; Dong, P.; Long, D. Multiple ionic conduction highways and good interfacial stability of ionic liquid-encapsulated cross-linked polymer electrolytes for lithium metal batteries. J. Power Sources 2022, 543, 231848. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D. Simultaneous establishment of high conductivity and mechanical stability via pore-filling of porous PTFE substrate with poly(ethylene glycol) and ionic liquid for lithium secondary battery. J. Membr. Sci. 2021, 624, 119029. [Google Scholar] [CrossRef]
- Lufrano, E.; Coppol, L.; Nicotera, I.; Simari, C. UV-cured gel polymer electrolytes based on poly(ethylene glycol) and organo-modified nanoclays for lithium ions batteries. Appl. Clay Sci. 2023, 246, 107163. [Google Scholar] [CrossRef]
- Hamrahjoo, M.; Hadad, S.; Dehghani, E.; Salami-Kalajahi, M.; Roghani-Mamaqani, M. Poly(poly(ethylene glycol) methyl ether methacrylate-co-acrylonitrile) gel polymer electrolytes for high performance lithium ion batteries: Comparing controlled and conventional radical polymerization. Eur. Polym. J. 2022, 173, 111276. [Google Scholar] [CrossRef]
- Costa, M.-R.; Altafim, R.A.C.; Mammana, A.-P. Ionic impurities in nematic liquid crystal displays. Liq. Cryst. 2001, 28, 1779–1783. [Google Scholar] [CrossRef]
- Son, J.-H.; Park, S.B.; Zin, W.-C.; Song, J.-K. Ionic impurity control by a photopolymerisation process of reactive mesogen. Liq. Cryst. 2013, 40, 458–467. [Google Scholar] [CrossRef]
- Shcherbinin, D.P.; Konshina, E.A. Ionic impurities in nematic liquid crystal doped with quantum dots CdSe/ZnS. Liq. Cryst. 2017, 44, 648–655. [Google Scholar] [CrossRef]
- Iwamoto, M. The dielectric dispersion of insulating films with long-range movements of charge carriers. J. Appl. Phys. 1995, 77, 5314–5321. [Google Scholar] [CrossRef]
- Bouchakour, M.; Derouiche, Y.; Bouberka, Z.; Beyens, C.; Mechernène, L.; Riahi, F.; Maschke, U. Optical properties of electron beam- and UV-cured polypropyleneglycoldiacrylate/liquid crystal E7 systems. Liq. Cryst. 2015, 42, 1527–1536. [Google Scholar]
- Bouchakour, M.; Derouiche, Y.; Bouberka, Z.; Beyens, C.; Supiot, P.; Dubois, F.; Riahi, F.; Maschke, U. Chapter 4—Electron beam curing of monomer/liquid crystal blends. In Polymer-Modified Liquid Crystals; Dierking, I., Ed.; Soft Matter Series No. 8; RSC: London, UK, 2019. [Google Scholar]
- Zair, L.; Berrayah, A.; Arabeche, K.; Bouberka, Z.; Best, A.; Koynov, K.; Maschke, U. Temperature Controlled Mechanical Reinforcement of Polyacrylate Films Containing Nematic Liquid Crystals. Polymers 2022, 14, 5024. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, R.; Dunmur, D. Dielectric properties of liquid crystals. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Tschierske, C., Raynes, P., Gleeson, H., Kato, T., Collings, P.J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA.: Weinheim, Germany, 2014; Volume 2, Part 1, Chapter 4. [Google Scholar]
- Kremer, F.; Schönhals, A. Broadband Dielectric Spectroscopy, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2003. [Google Scholar]
- Vogel, H. Das Temperaturabhaengigkeitsgesetz der Viskositaet von Fluessigkeiten. Phys. Zeitschr. 1921, 22, 645. [Google Scholar]
- Fulcher, G.-S. Analysis of recent measurements of the viscosity of glasses. J. Am. Chem. Soc. 1925, 8, 339–355. [Google Scholar] [CrossRef]
- Tamman, G.; Hesse, W.; Anorg, Z. Die Abhaengigkeit der Viskositat von der Temperatur bei unterkiihlten Fliissigkeiten. Allg. Chem. 1926, 156, 245–257. [Google Scholar] [CrossRef]
- Maschke, U.; Roussel, F.; Buisine, J.-M.; Coqueret, X. Liquid crystal-polymer composite materials: A thermophysical and electro-optical study. J. Therm. Anal. 1998, 51, 737–746. [Google Scholar] [CrossRef]
- Kozanecki, M.; Pastorczak, M.; Okrasa, L.; Ulanski, J.; Yoon, J.-A.; Kowalewski, T.; Matyjaszewski, K.; Koynov, K. Evolution of high-temperature molecular relaxations in poly(2-(2-methoxyethoxy)ethyl methacrylate) upon network formation. Colloid Polym. Sci. 2015, 293, 1357–1367. [Google Scholar] [CrossRef] [PubMed]
- Liquid parallel plate sample cell BDS1308. Available online: https://www.novocontrol.de/php/sa_cell_liquid.php (accessed on 6 March 2024).
- Chakraborty, S.; Bandyopadhyay, S.; Dasgupta, S.; Mukhopadhyay, R.; Deuri, A.-S. Application of GPC in characterization of MP resin through correlation of softening point and methylol content with weight average molecular weight. Polym. Test. 2006, 25, 12–15. [Google Scholar] [CrossRef]
- Fox, T.G.; Flory, P.J. The glass temperature and related properties of polystyrene. Influence of molecular weight. J. Polym. Sci. 1954, 14, 315–319. [Google Scholar] [CrossRef]
- Cherfi, A.; Santos, A.F.; Pinto, J.C.; Seytre, G.; Boiteux, G.; McKenna, T.F.; Fevotte, G. Application of dielectric analysis to the measurement of conversion during batch solution copolymerizations. Chem. Eng. Process. 2003, 42, 121–128. [Google Scholar] [CrossRef]
- Coelho, R.; Aladenize, B. Les Diélectriques; Editions Hermes Science: Paris, France, 1993. [Google Scholar]
- Jonscher, A.K. Dielectric Relaxation in Solids; Chelsea Dielectrics Press: London, UK, 1983. [Google Scholar]
- Williams, M.L.; Landell, R.F.; Ferry, J.D. The Temperature Dependence of Relaxation Mechanisms in Amorphous Polymers and Other Glass-forming Liquids. J. Am. Chem. Soc. 1955, 77, 3701–3707. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, J.Y.; Kim, H.S.; Chao, H.N. Ionic conductivity of polymer electrolytes based on phosphate and polyether copolymers. Solid State Ion. 1999, 116, 63–71. [Google Scholar] [CrossRef]
- Kishikawa, Y.; Shinohara, H.; Maeda, K.; Nakamura, Y.; Weigand, S.; Kita, R. Temperature dependence of thermal diffusion for aqueous solutions of monosaccharides, oligosaccharides, and polysaccharides. Phys. Chem. Chem. Phys. 2012, 14, 10147–10153. [Google Scholar] [CrossRef] [PubMed]


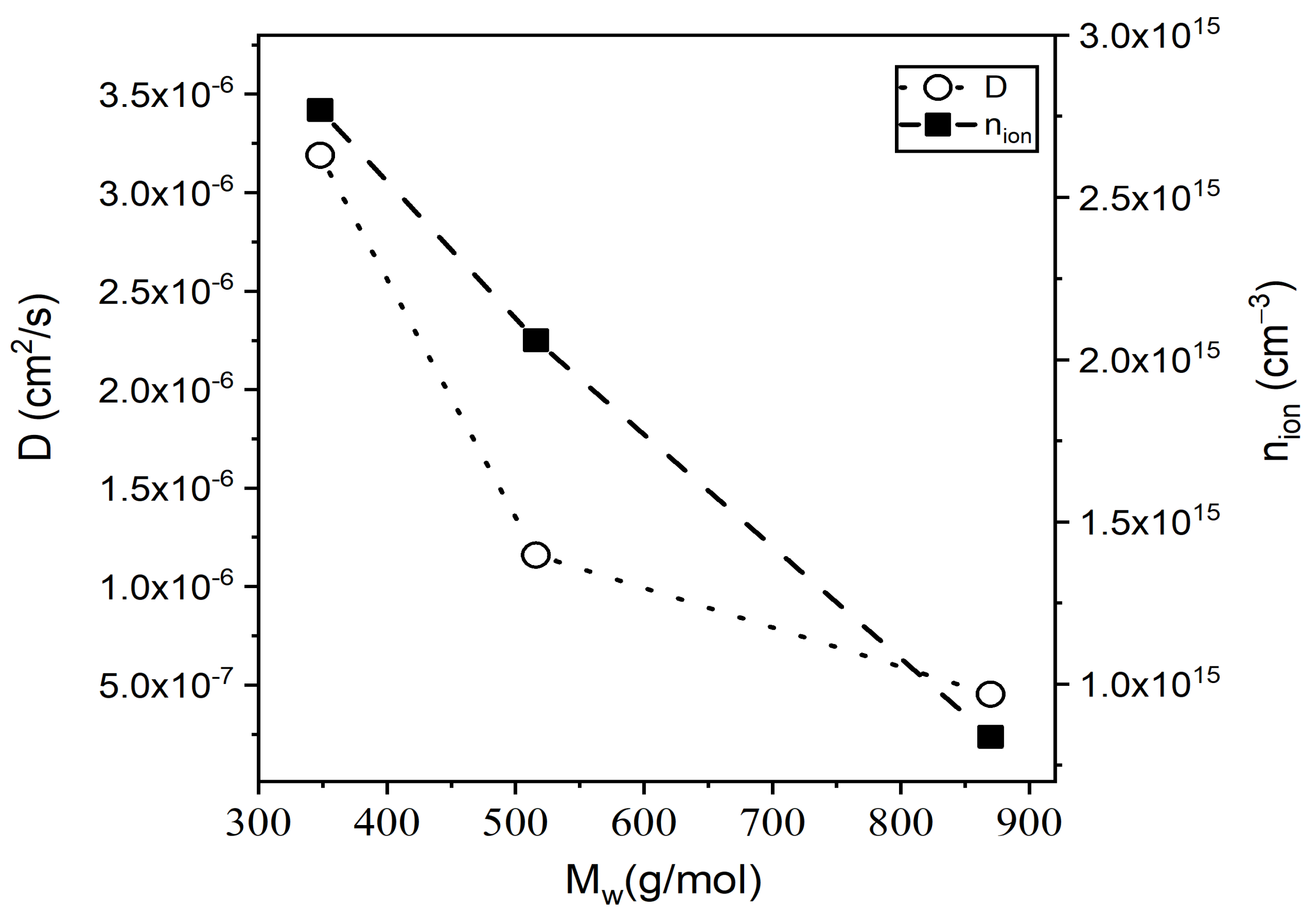
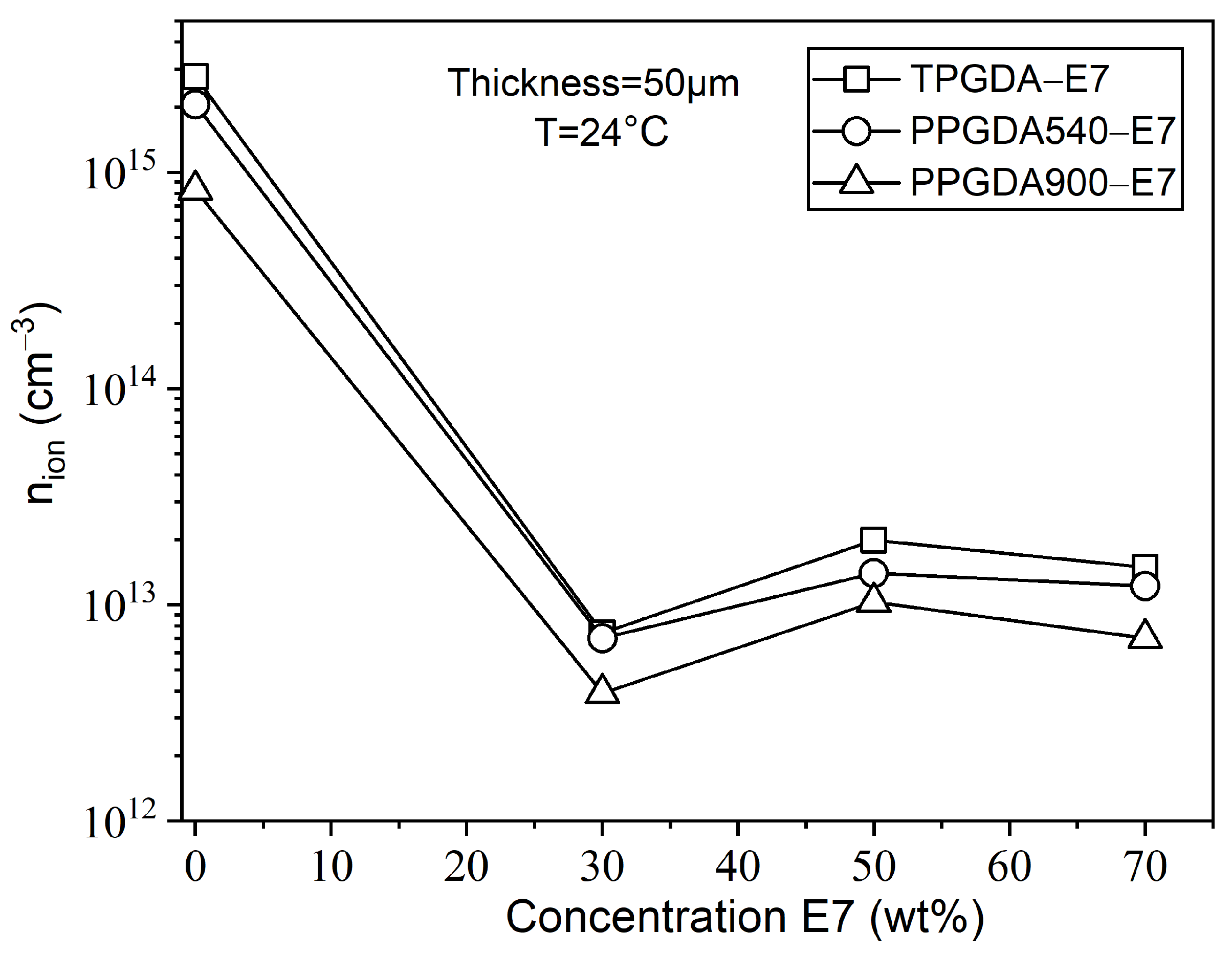
| Monomer | |||||
|---|---|---|---|---|---|
| TPGDA | 0.230 | 0.095 | 148 | ||
| PPGDA540 | 0.342 | 0.094 | 159 | ||
| PPGDA900 | 0.348 | 0.090 | 163 |
| Monomer/E7 Mixture | |||||
|---|---|---|---|---|---|
| 70wt%TPGDA–30wt%E7 | 0.291 | 0.104 | 157 | ||
| 50wt%TPGDA–50wt%E7 | 0.304 | 0.098 | 157 | ||
| 30wt%TPGDA–70wt%E7 | 0.311 | 0.109 | 157 | ||
| 70wt%PPGDA540–30wt%E7 | 0.319 | 0.101 | 162 | ||
| 50wt%PPGDA540–50wt%E7 | 0.291 | 0.094 | 163 | ||
| 30wt%PPGDA540–70wt%E7 | 0.262 | 0.113 | 163 | ||
| 70wt%PPGDA900–30wt%E7 | 0.355 | 0.107 | 162 | ||
| 50wt%PPGDA900–50wt%E7 | 0.350 | 0.118 | 166 | ||
| 30wt%PPGDA900–70wt%E7 | 0.334 | 0.116 | 165 |
| Monomer | εs | ||
|---|---|---|---|
| TPGDA | 7.41 | 2.77 | 3.19 |
| PPGDA540 | 6.47 | 2.06 | 1.16 |
| PPGDA900 | 6.25 | 0.83 | 0.45 |
| Monomer/E7 Mixture | |||
|---|---|---|---|
| 70wt%TPGDA–30wt%E7 | 9.10 | ||
| 50wt%TPGDA–50wt%E7 | 10.30 | ||
| 30wt%TPGDA–70wt%E7 | 10.00 | ||
| 70wt%PPGDA540–30wt%E7 | 8.60 | ||
| 50wt%PPGDA540–50wt%E7 | 9.00 | ||
| 30wt%PPGDA540–70wt%E7 | 10.20 | 1.22 | |
| 70wt%PPGDA900–30wt%E7 | 8.43 | ||
| 50wt%PPGDA900–50wt%E7 | 9.56 | ||
| 30wt%PPGDA900–70wt%E7 | 11.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkouider, T.; Derouiche, Y.; Souli, L.; Dubois, F.; Barrera, A.; Bouberka, Z.; Maschke, U. Estimation of Ionic Impurities in Poly(propylene Glycol) Diacrylate Monomers/Liquid Crystal E7 Mixtures Using Dielectric Spectroscopy. Crystals 2024, 14, 286. https://doi.org/10.3390/cryst14030286
Benkouider T, Derouiche Y, Souli L, Dubois F, Barrera A, Bouberka Z, Maschke U. Estimation of Ionic Impurities in Poly(propylene Glycol) Diacrylate Monomers/Liquid Crystal E7 Mixtures Using Dielectric Spectroscopy. Crystals. 2024; 14(3):286. https://doi.org/10.3390/cryst14030286
Chicago/Turabian StyleBenkouider, Tayeb, Yazid Derouiche, Lahcene Souli, Frédéric Dubois, Ana Barrera, Zohra Bouberka, and Ulrich Maschke. 2024. "Estimation of Ionic Impurities in Poly(propylene Glycol) Diacrylate Monomers/Liquid Crystal E7 Mixtures Using Dielectric Spectroscopy" Crystals 14, no. 3: 286. https://doi.org/10.3390/cryst14030286






