Abstract
For the purpose of improving the wear properties of Ni composite coatings, diamond particles were co-electrodeposited into Ni–diamond composite coatings. The effect of diamond particle concentration in the electrolyte on the surface morphology, microstructure, and wear properties of Ni–diamond composite coatings was investigated. The electrodeposition behaviors of the composite coatings were simulated by COMSOL5.6. The results showed that the content of diamond particles in the coating was elevated by increasing the particle concentration in the electrolyte. The formation of [200] fiber texture was blocked and concurrently brought about crystallite refinement of the Ni deposits by the embedded particles. The COMSOL simulation findings indicated that embedded particles influenced the microstructure of the Ni deposits through processes such as heterogeneous nucleation, rearrangement, and concentration of local current density. The synergistic effect of the tailored microstructure and embedded particles substantially enhanced the wear resistance of the coating. By increasing the particle concentration in the electrolyte, the wear resistance of the coating was gradually enhanced, and the coating electrodeposited at 16 g/L possessed the lowest friction coefficient and the smallest profile of wear scratch owning to the strengthened synergistic effect.
1. Introduction
The electrodeposition technique has been extensively applied to prepare Ni composite coatings reinforced by oxides, carbides, nitrides, and so on, which endows the coatings with the desirable microstructure and properties [1,2,3,4,5,6,7,8,9,10]. Among the reinforcements, the diamond particle is considered as a prospective candidate, due to its excellent hardness, wear resistance, strength, chemical inertness, corrosion resistance, and so on [11,12,13,14]. In recent years, lots of research attention have been directed to electrodeposited Ni–diamond composite coatings, especially the investigation on the preparation parameters and properties of the coatings [15,16,17,18,19,20]. Representatively, Hong et al. [21] adopted the electrodeposition to assemble a kind of Ni–diamond composite coating with high micro-hardness, and a low coefficient of friction and high wear resistance, which also suggested a strong bonding strength between the nickel deposits and the diamond particles. Wang et al. [22] prepared Ni–diamond composite coatings using the double-pulse electrodeposition method, and they reported that the addition of diamond particles enhanced the cathodic polarization effect during electrodeposition. By increasing the concentration of the diamond particles in the coatings, the enhanced tailoring effect of the particles brought in an enhanced grain refinement, consequently improving the wear and corrosion resistance of the coatings. Zhang et al. [23] investigated the effect of mechanical agitation and ultrasonic treatment on the microstructures and properties of the Ni–diamond coatings. The results showed that the uniform distribution of the diamond particles in the Ni deposits could be derived with the assistance of mechanical agitation and ultrasonic treatment, subsequently resulting in an enhanced corrosion resistance of the coatings. Wang et al. [24] electrodeposited the nickel–diamond composite coating and found that increasing the applied current density could lead to an increase and then a decrease in the content of diamond particles in the coatings. The increase in the diamond content and the diamond size led to an intensified wear resistance of the composite coatings. Although the existing literature has extensively explored the relationship of the preparation parameters and the properties of nickel–diamond coatings, it has not yet revealed the deposition behaviors of the diamond particles in nickel deposits and the subsequent tailoring effect of deposition behaviors on the microstructure and the wear resistance of the coatings, which severely limits the further optimization of electrodeposition and widening of the application of the Ni–diamond coatings.
Therefore, the Ni–diamond composite coatings in this work were prepared at various diamond particle concentrations in the electrolyte, and the tailoring effect of diamond particles on the surface morphology and microstructures of the coatings were deeply investigated. The COMSOL5.6 was utilized to reveal the electrodeposition behaviors of the diamond particles into the Ni deposits, so that the tailoring mechanism of the particles was established. Finally, the impact of particle concentration in the electrolyte on coating wear resistance was assessed to demonstrate the role of particles in customization.
2. Experimental Preparation
2.1. Preparation of the Coatings
The substrate was a 304 stainless steel plate with a surface area of 20 mm × 10 mm. Before electrodeposition, it was necessary to remove the oxide layer and rust layer on the substrates, which were abraded with 2000 mesh sandpapers, rinsed with deionized water, then put into an alkaline solution consisting of 40 g/L sodium hydroxide to degrease, then activated in hydrochloric acid at a concentration of 10% for 5 min, and finally cleaned with deionized water.
The typical Watts electrolyte was employed to fabricate the Ni–diamond coatings, comprising 240 g per liter of nickel sulfate, 40 g per liter of nickel chloride, 30 g per liter of boric acid, and 0.2 g per liter of sodium dodecyl sulfate as the primary constituents. Based on previous experimental experience and reference to the experimental parameters of relevant academic papers, the following experimental parameters were used in this experiment. The suspensions consisted of the addition of the diamond particles (1, 2, 4, 8, 16 g/L, particle size of about 1~2 μm) to the Watts solution. Following 30 min of ultrasonic treatment, the suspension underwent magnetic stirring for 3 h at a rotational speed of 400 rpm and a temperature of 50 °C to achieve uniform dispersion of the diamond particles in the electrolyte.
During the electrodeposition process, the nickel–diamond composite coatings were prepared by keeping the rotational speed of 400 rpm and the temperature of 50 °C, using the pure nickel plate as the anode and the 304 stainless steel plate as the cathode, and electrodeposition was conducted for 45 min at a current density of 1 A/dm2. After the electrodeposition, the specimens were successively placed in deionized water and anhydrous ethanol for ultrasonic cleaning treatment for 5 min.
2.2. Characterization of the Coatings
The surface morphologies of the nickel–diamond composite coatings were characterized by scanning electron microscopy (SEM, Hitachi TM3030, Ibaraki, Japan), while the composition of the coatings was characterized by the energy dispersive spectrometer (EDS, Oxford Swift 3000, Abingdon, UK). XRD patterns of the nickel–diamond composite coatings were obtained by X-ray diffractometer (XRD, Ultima IV, Tokyo, Japan), and the microstructures of the coating were analyzed by Rietveld refinement method. The applied voltage and current of XRD were set at 40 kV and 20 mA, and a range of 10° to 90° was scanned at a constant rate of 5°/min.
The wear resistance of the Ni–diamond coating was evaluated using a friction wear instrument (Bruker-Contour GT-K 3D, Karlsruhe, Germany) at room temperature. GCr15 steel balls with a diameter of 6 mm were used as the wear parts, and the load, frequency, total sliding distance and friction time were 5 N, 5 Hz, 60 m, and 10 min, respectively. The surface morphologies of the coatings after the wear experiments were characterized using SEM and white light interferometer (Bruker, Karlsruhe, Germany). Finally, the electrodeposition process of the Ni–diamond coating was simulated using COMSOL5.6, so that the relationship between the electrodeposition behavior of the coating and the wear resistance was revealed.
3. Results and Discussion
3.1. Surface Morphology and Composition of the Nickel–Diamond Coatings
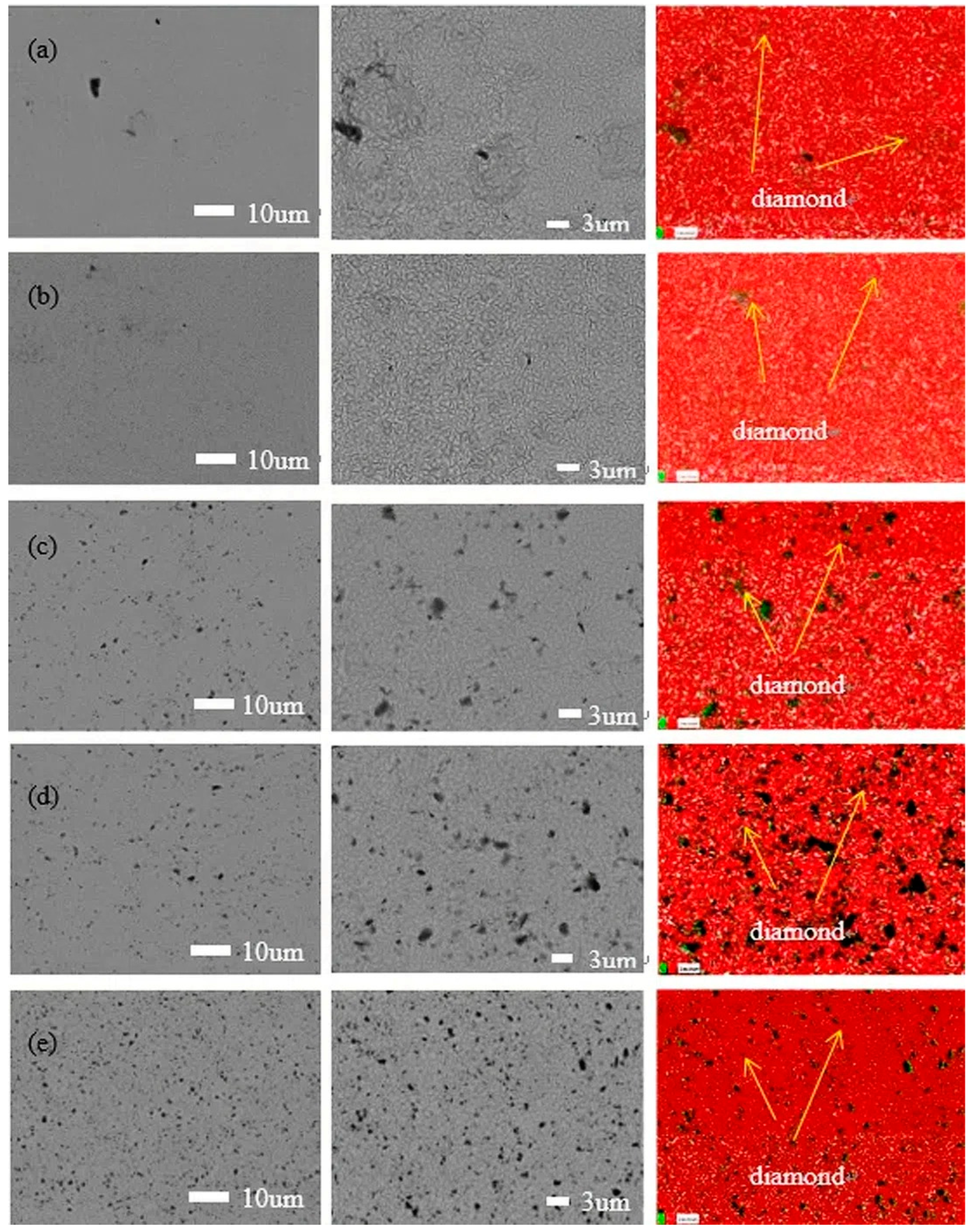
Figure 1 exhibits the surface morphologies of various magnification and elemental mapping of the nickel–diamond coatings prepared at various particle concentrations of 1, 2, 4, 8, and 16 g/L, respectively. It can be observed that the surface morphologies of the coatings are similar to each other, exhibiting typical poly-pyramid structures. However, there were still some differences in the surface structures of the coatings, which were derived from the effect of the co-deposited diamond particles. It can be observed that the poly-pyramid structures are apparent in the coatings prepared at 1 and 2 g/L, while the poly-pyramid structures are reduced, and the black points are gradually increased, with an increase in the diamond particles. Based on the element mapping images, the black points were denoted as diamond particles. Consequently, it was rationally concluded that the increased co-deposition of diamond particles could disturb the poly-pyramid structures of the coatings to some extent, with an increase in the particle concentration. Additionally, the element mapping of Figure 1c,d exhibits some large black sites, which could be caused by the agglomeration of the diamond particles or unremoved contaminants. This could be confirmed by the SEM images of the coatings, in which the diamond particles were reflected by the black points rather than the grey ones. According to the electrodeposition mechanism [25], the diamond particles were adsorbed on the surface of the Ni deposits, which disturbed the growth of the Ni grains and the formation of poly-pyramid surface structures. The increased content of diamond particles enhanced the disturbance effect on the formation of the Ni deposits, which is discussed later.
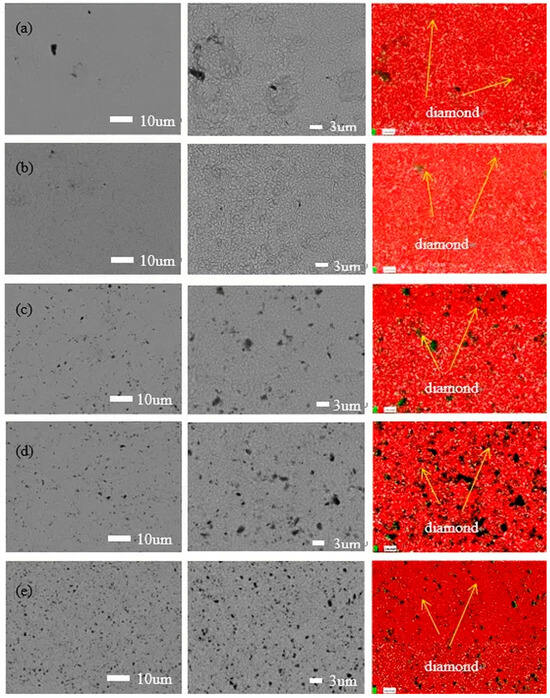
Figure 1.
SEM images and EDS analysis of the nickel–diamond coatings prepared at the particle concentrations of (a) 1, (b) 2, (c) 4, (d) 8, and (e) 16 g/L.
3.2. Microstructure of the Nickel–Diamond Coatings
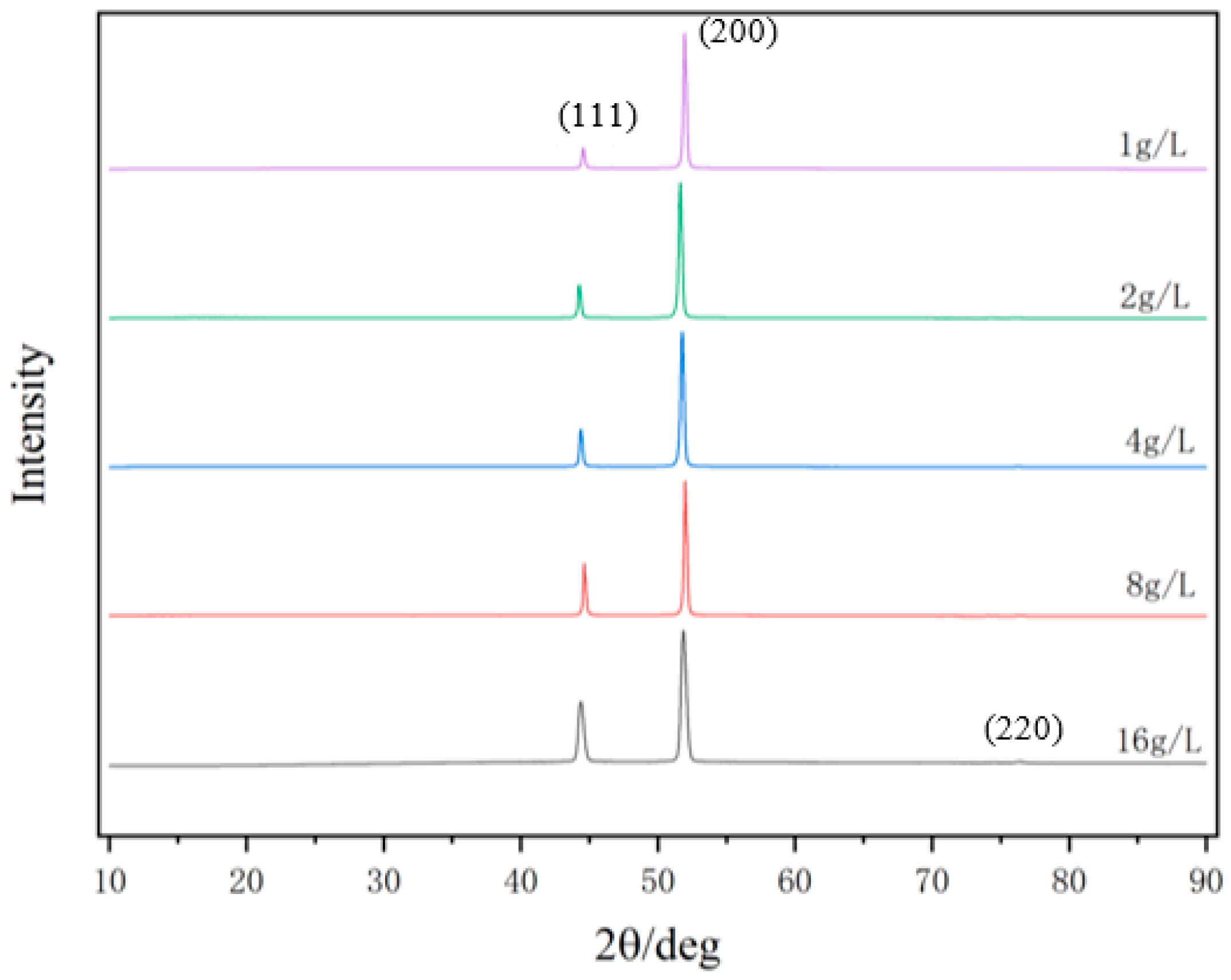
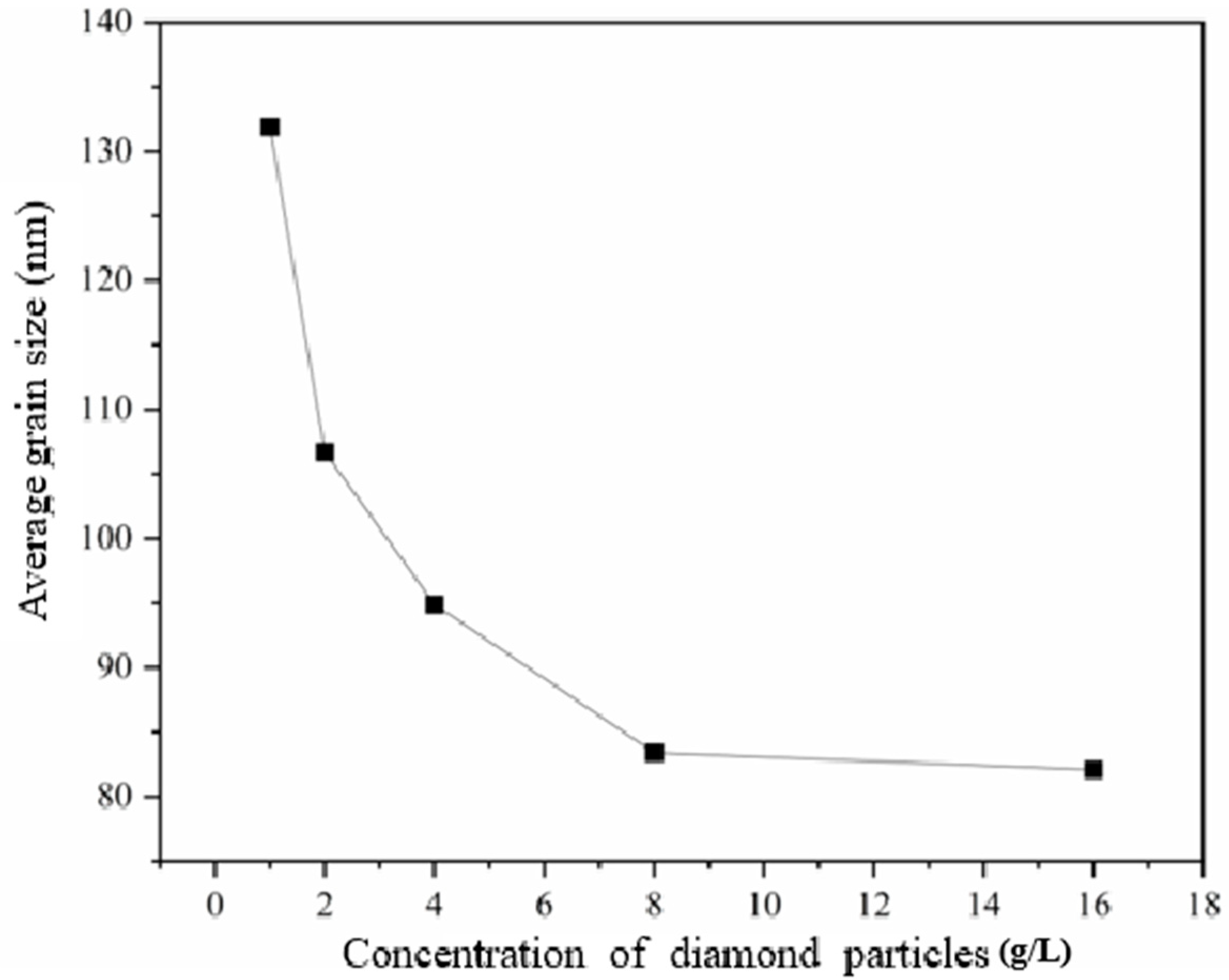
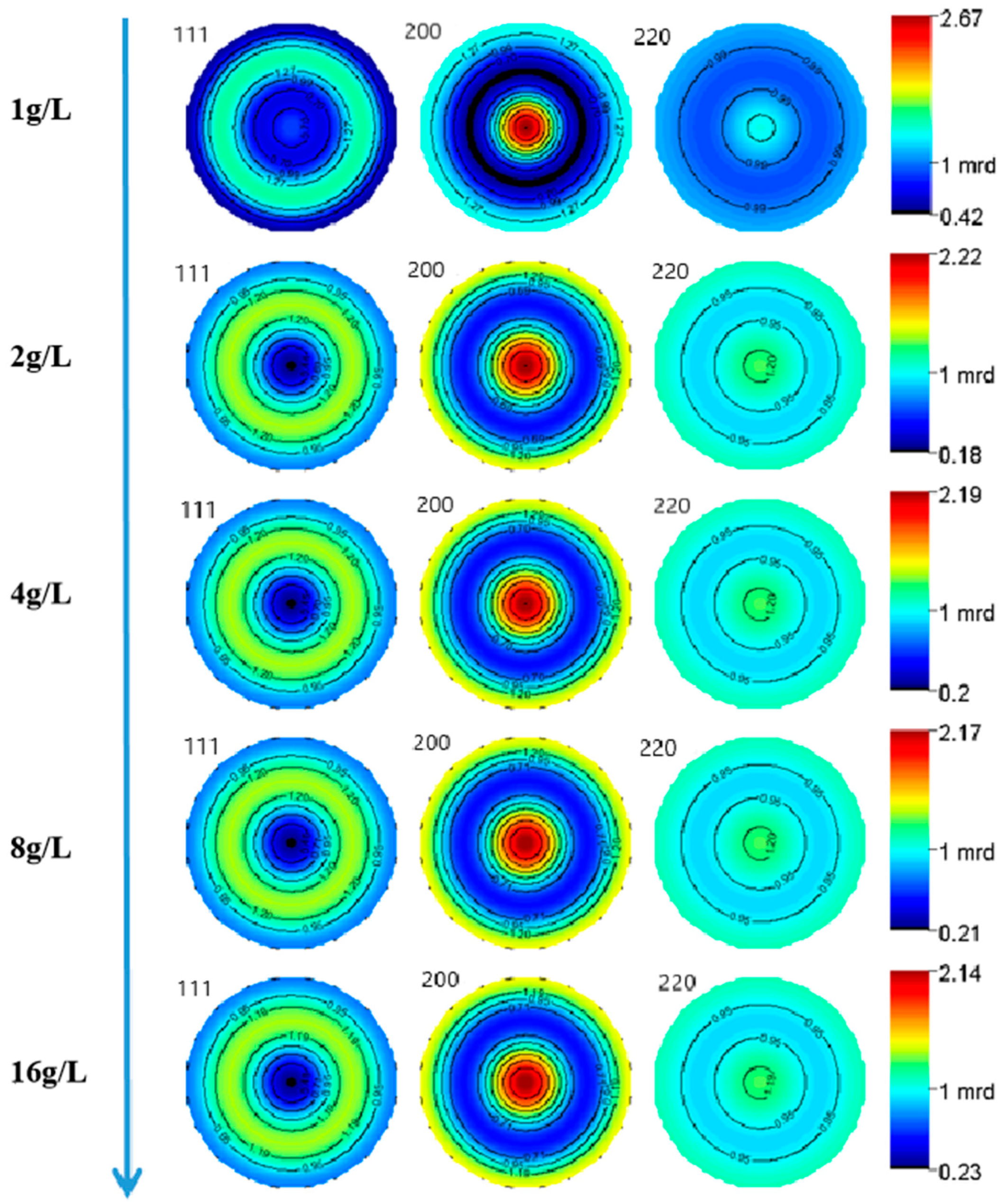
Figure 2 exhibits the XRD patterns of the nickel–diamond coatings. It is visible that the typical Ni phase diffraction peaks including (111), (200), and (220) appear for all the Ni–diamond coatings. The strongest diffraction peak is the (200) peak located at 51.5°, and the weakest one is the (220) peak located at 76.5°, which is almost invisible. With the increase in diamond particle concentration, the diffraction profile of the (111) peak was gradually elevated, and the changes in profiles of the other two peaks were not as obvious, suggesting the microstructure evolution induced by the co-deposition of the particles. Figure 3 depicts the grain size variation of the Ni deposits in the coatings, which is evaluated using the Rietveld refinement method [26,27]. With the increase in the concentration of the diamond particles in the electrolyte, the average grain size of the Ni deposits gradually decreased from 131.9 nm at 1 g/L to 82.1 nm at 16 g/L. Figure 4 exhibits the pole figures of the (111), (200) and (220) crystallographic planes of the Ni–diamond coatings obtained by the Rietveld refinement method. It is observed that all the coatings exhibit a strong [200] fiber texture in the Ni deposits, while the [200] fiber texture gradually decreases as the concentration of the diamond particles in the electrolyte increases, until it reaches a minimum at 16 g/L. The above microstructure evolution of the Ni deposits illustrated that the decreases in the grain size and [200] fiber texture of the Ni deposits were attributed to the strengthened tailoring effect of the diamond on the microstructure of the Ni–diamond coatings, which, in turn, came with the increase in the concentration of diamond particles in the electrolyte.
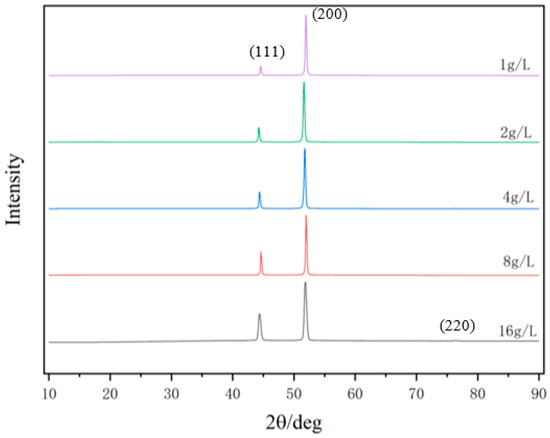
Figure 2.
XRD patterns of the nickel–diamond coatings prepared at various concentrations of diamond particles.
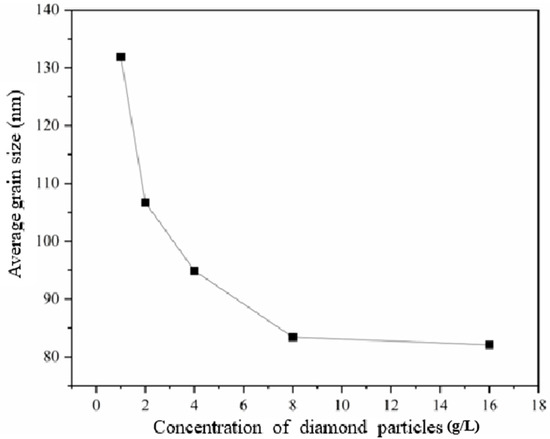
Figure 3.
Grain size variation of the nickel–diamond coatings prepared at various concentrations of diamond particles.
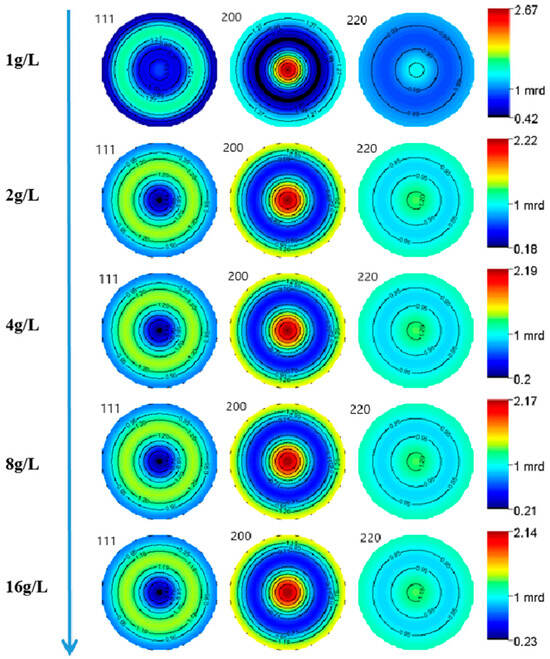
Figure 4.
Pole figures of the Ni deposits in nickel–diamond coatings prepared at various concentrations of diamond particles.
3.3. Simulation of Electrodeposition Process
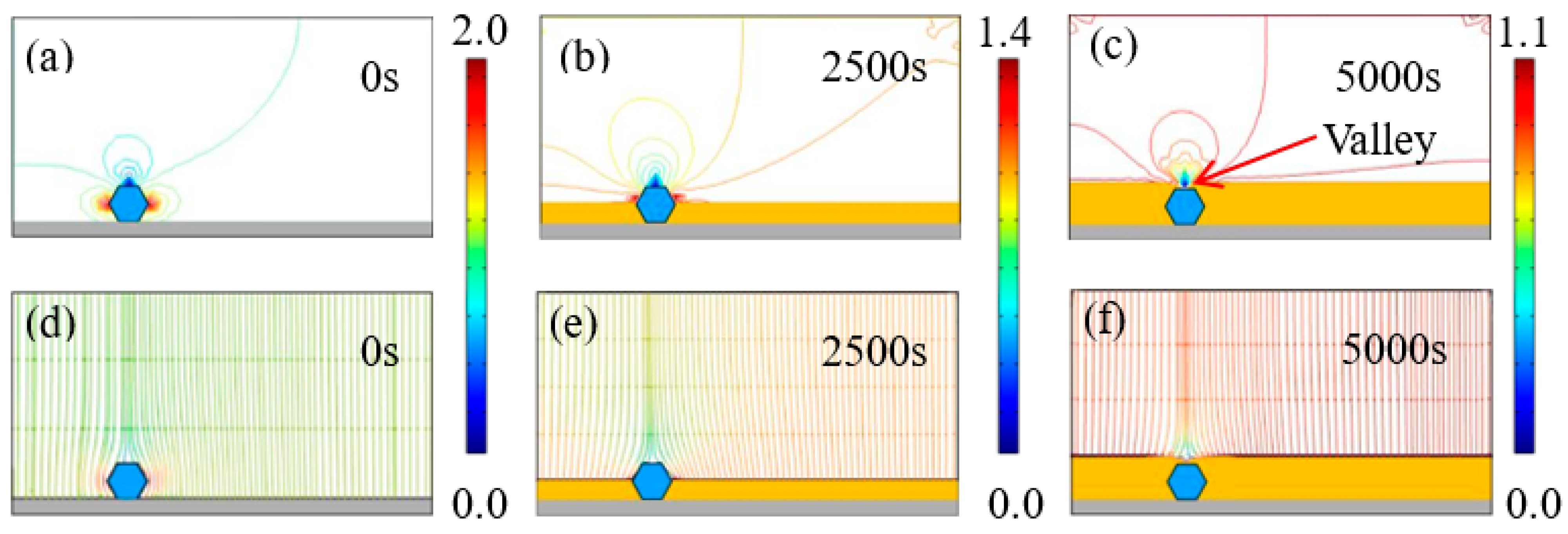
The tailoring effect of diamond particles was examined by analyzing the time-dependent distribution of current density and the electric field on the cathode using COMSOL5.6. In the simulation, it is important to note that different particle numbers (1, 2, 3, and 4) were employed to model the growth of the Ni–diamond composite coating, corresponding to the diamond particle concentrations of 2, 4, 8, and 16 g/L, respectively. The distribution of equipotential current density lines and electric field lines near diamond particles was investigated under an applied current density of 1 A/dm². The instance when particles were captured by the cathode surface (either the steel substrate or the growing Ni deposits) was defined as time 0 s. The Ni deposits were represented by the yellow color, while the diamond particles were depicted as magnified hexagon shapes, marked in blue.
Figure 5 illustrates the time-dependent electrodeposition conditions, depicting current density lines and electric field lines near the diamond particles with a concentration of 2 g/L in the electrolyte. Notably, electric field lines can be categorized into those perpendicular to the cathode surface, and those circumventing diamond particles before penetrating the surface, attributed to the non-conductive nature of diamonds. At time 0 s, a diamond particle adheres to the cathode surface, with current density concentrated on both of its ends, reaching a maximum of 2.05 A/dm². By 2500 s, the diamond particles are strongly wrapped by the growing Ni deposits. The current density is still concentrated at the two ends of the diamond particles, while the highest value was reduced to 1.41 A/dm2. When the deposition time increases to 5000 s, the diamond particles are completely wrapped by the Ni deposits, and the highest current density is continuously reduced to 1.1 A/dm2. However, the highest current density was still larger than the set average current density of 1 A/dm2. Moreover, when the diamond particles were completely wrapped, a concave valley appeared above the diamond, which was caused by the non-conductivity of particles.
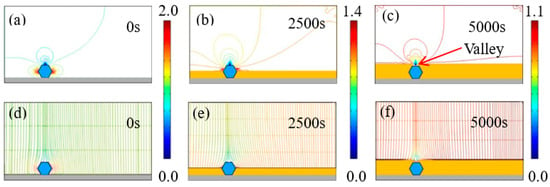
Figure 5.
(a–c) Contours of current density versus time at a diamond concentration of 2 g/L, and (d–f) corresponding electric field lines near the diamond particles at 0, 2500, and 5000 s.
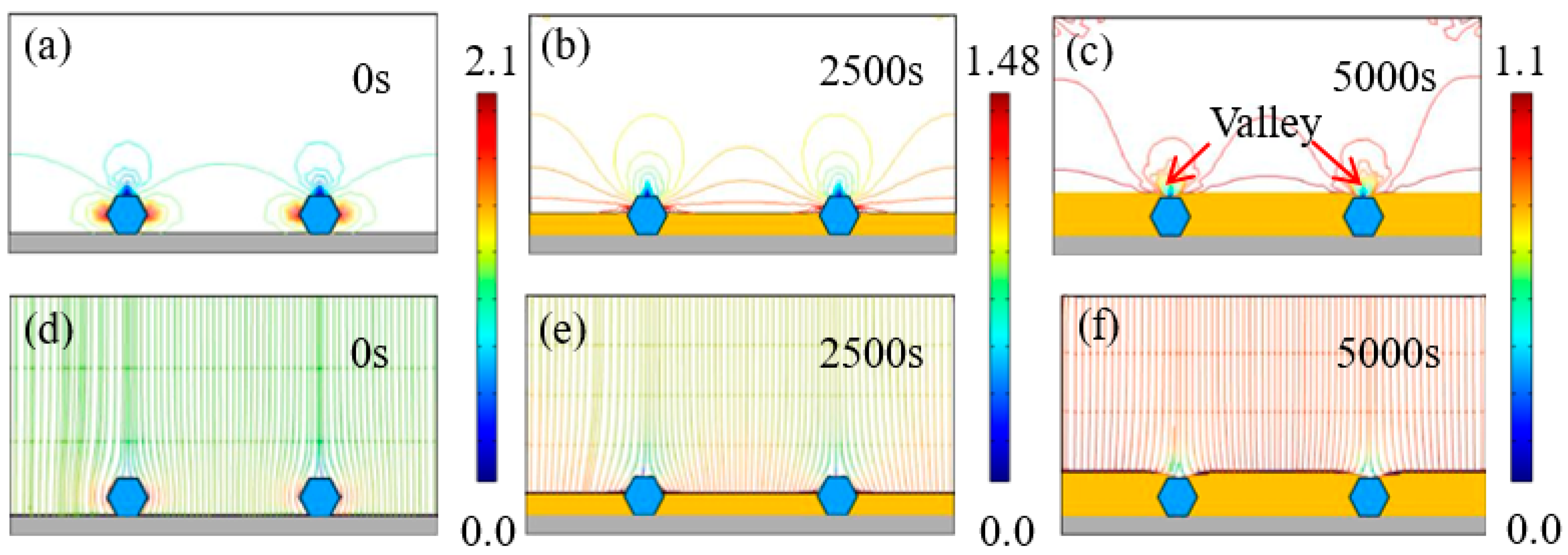
Figure 6 depicts the evolution of time-dependent electrodeposition conditions for the Ni–diamond coating at a concentration of 4 g/L. Similar to the coating at 2 g/L, the distribution of current density lines and electric field lines shows concentration on both sides of the diamond particles. The maximum current density gradually decreases from 2.1 to 1.1 A/dm² over the time span from 0 to 5000 s. Simultaneously, the Ni deposits progressively grow and eventually fully envelop the co-deposited diamond particles, resulting in the formation of valleys above the particles.
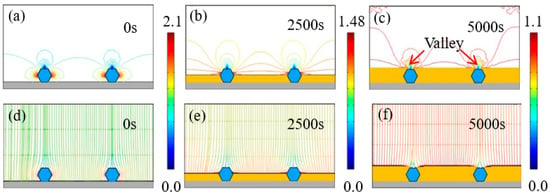
Figure 6.
(a–c) Contours of current density versus time at the diamond concentration of 4 g/L, and (d–f) corresponding electric field lines near the diamond particles at 0, 2500, and 5000 s.
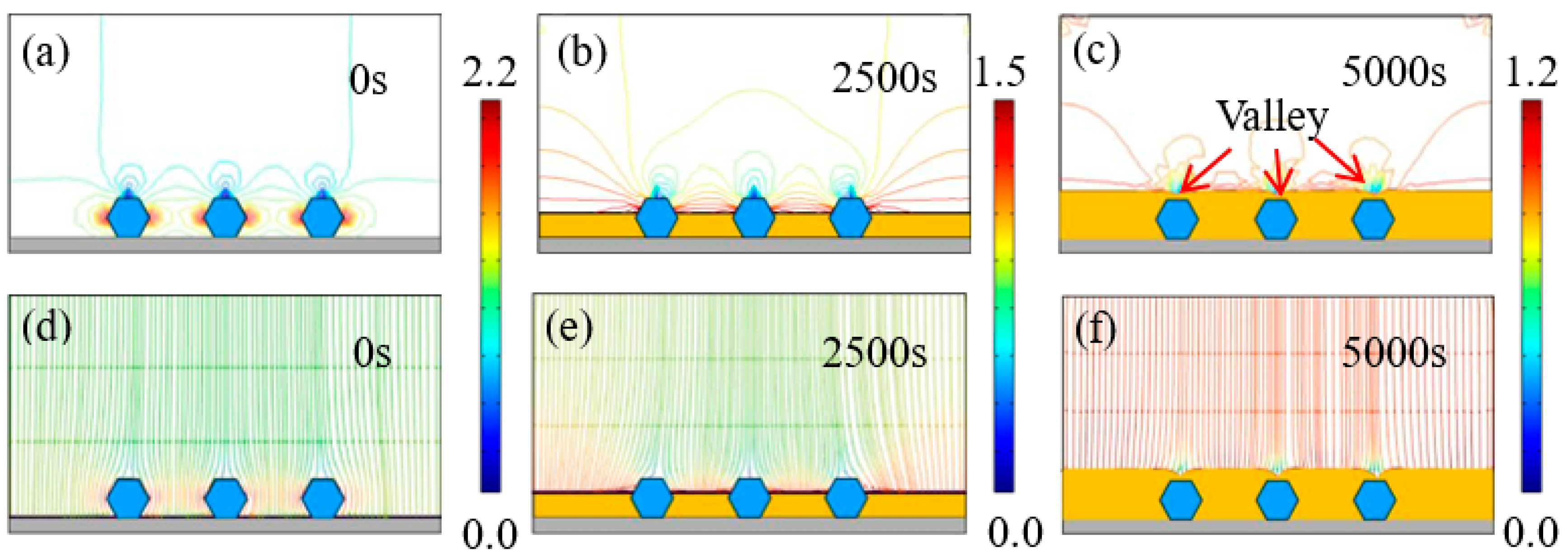
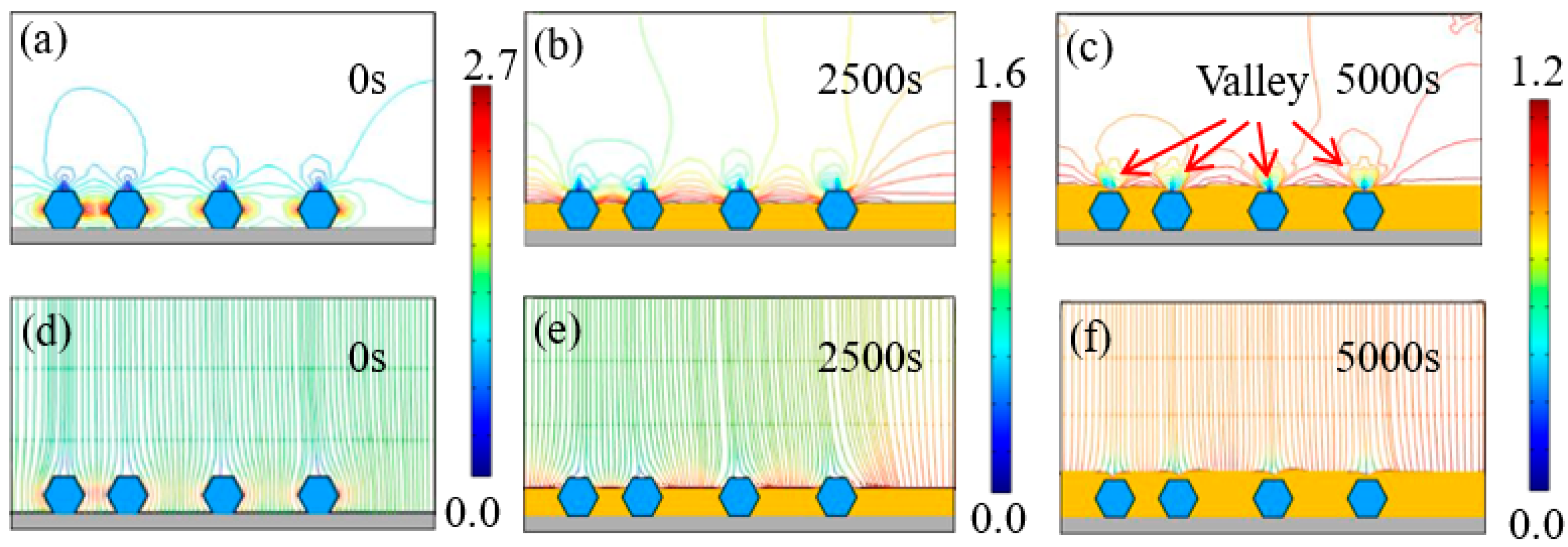
Figure 7 and Figure 8 exhibit the time-dependent electrodeposition condition evolution of the Ni–diamond coatings at 8 and 16 g/L, respectively. It can be observed that the distribution of the current density lines and the electric field lines on the cathodes of the coatings is similar to that of the coating prepared at the low concentration of diamond particles. The largest current density appeared on both sides of the diamond particles, which were 2.2 and 2.7 A/dm2 for the coating prepared at 8 and 16 g/L at 0 s, respectively. With the time increasing to 5000 s, the value of the largest current density decreased to 1.2 A/dm2 for both coatings. Simultaneously, the diamond particles were fully covered by the Ni deposits, and the valley was formed above each particle.
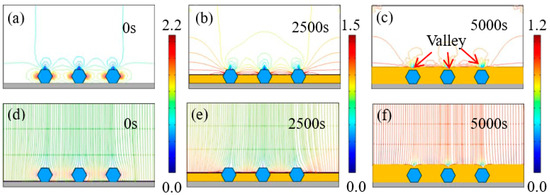
Figure 7.
(a–c) Contours of current density versus time at a diamond concentration of 8 g/L, and (d–f) corresponding electric field lines in the vicinity of diamond particles at 0, 2500, and 5000 s.
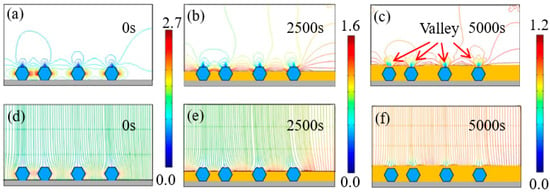
Figure 8.
(a–c) Contours of current density versus time at a diamond concentration of 16 g/L, and (d–f) corresponding electric field lines near the diamond particles at 0, 2500, and 5000 s.
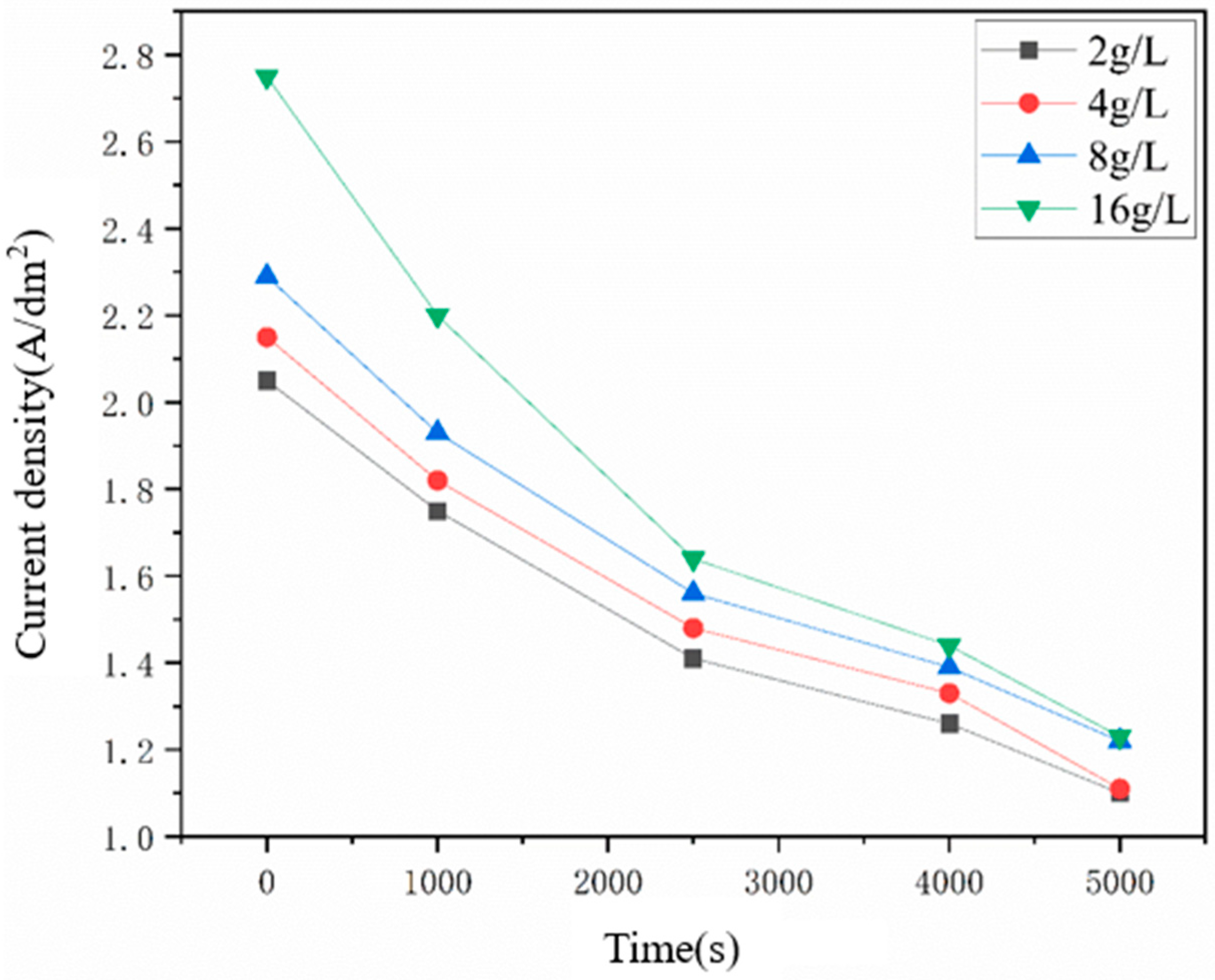
Additionally, the Ni deposits were uniformly formed at the locations without particles as shown in the above figures, indicating that the diamond particles could affect the growth of Ni grains near the particles, which was more obvious with the increasing number of captured diamond particles. Figure 9 represents the dependence of the concentrated current density on the electrodeposition duration of Ni–diamond coatings prepared at different diamond concentrations. Higher numbers of captured diamond particles on the cathode led to an increase in the value of the concentrated current density at the same electrodeposition duration, which was essentially caused by the reduced cathode area, due to the non-conductivity of the particles. The concentration of current density at 16 g/L was significantly greater than that at 2 g/L, which brought in the strengthened effect of the captured particles on the growth of Ni grains. Moreover, the concentrated current densities of all the coatings are greater than the initial current densities (1 A/dm2) at the whole durations. With the electrodeposition time increasing, all the concentrated current density decreased, which was attributed to the elimination of the small cathode surface area problem.
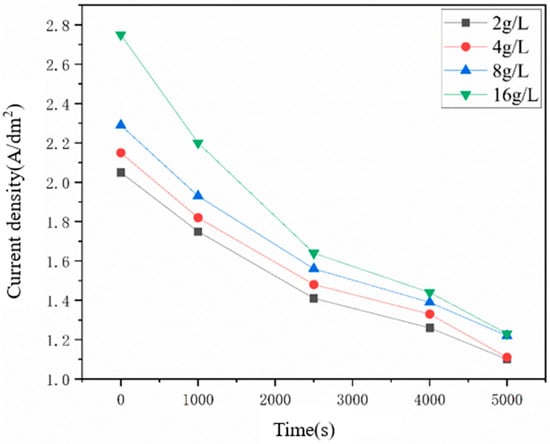
Figure 9.
Dependence of the concentrated current density on the electrodeposition time.
The concentration of the current density near the particles was responsible for the tailoring effect of the particles, which could be expressed as follows [28]:
where is the average crystallite size, and is a constant; where is the current density, and (A/cm2) is the exchange current density, (cm3/mol) is the molar volume of the sediment, z is the valence number of the reduced ion, F (A s/mol) is the Faraday constant, and and are constants. The corresponding value of n is typically greater than 1 in the electrodeposition of the Ni deposits with a Watts electrolyte. Herein, the Ni grain size was inversely correlated with the current density. Considering the distribution of electrodeposition conditions with time in the above figures, the current density was concentrated around the diamond particles, which reduced the Ni grain sizes and disturbed the [200] fiber texture of the Ni deposits. Moreover, the co-deposited diamond particles could also hinder the growth of Ni grains under the particles. Therefore, with the increase in the concentration of diamond particles in the electrolyte, the tailoring effect of the particles was strengthened due to the increased concentration of diamond particles co-deposited into the Ni deposits, which improved the current density concentration and consequently, decreased the grain size and [200] fiber texture of the Ni deposits.
3.4. Wear Resistance of the Ni–Diamond Coatings
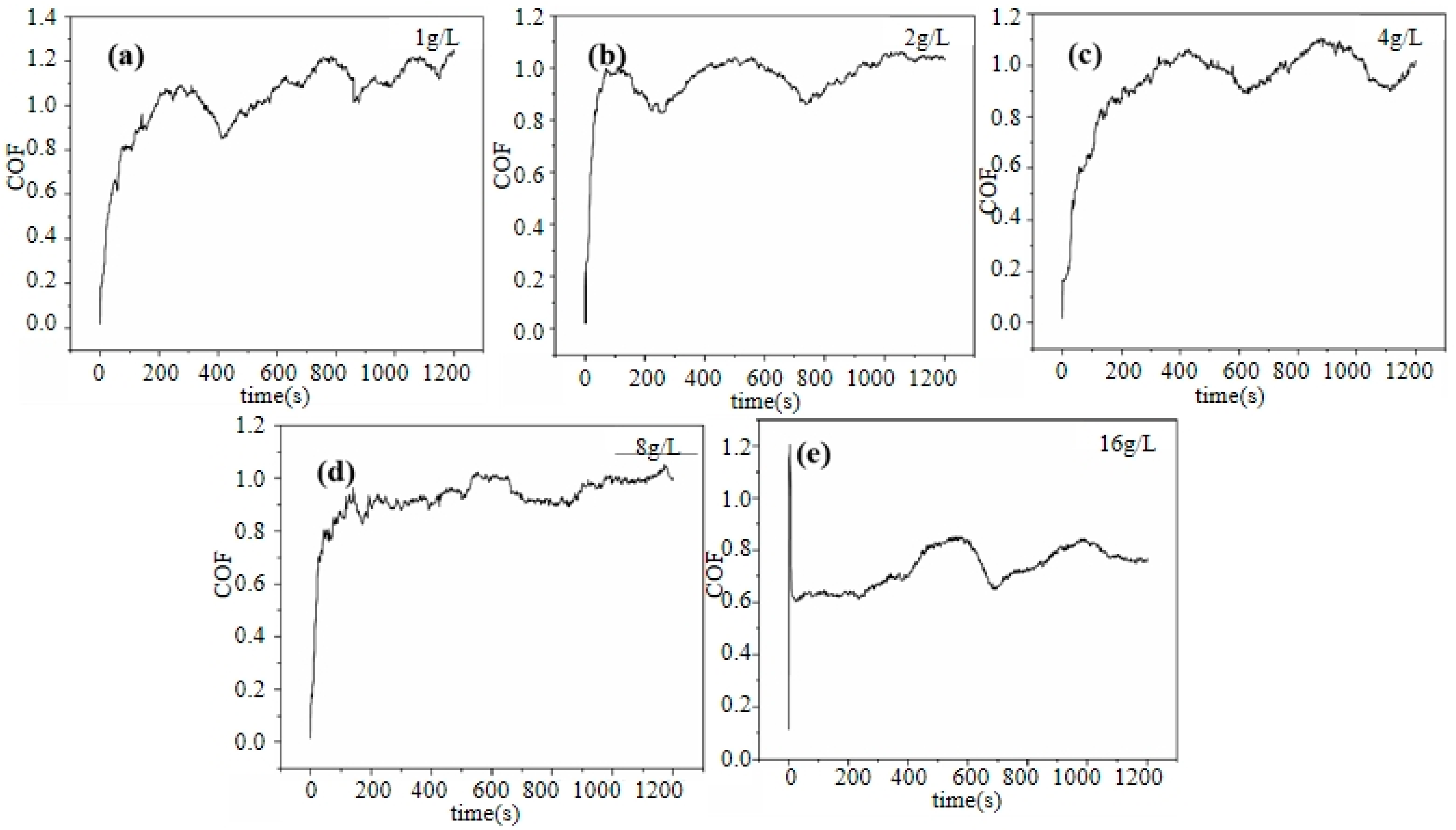
Figure 10 shows the coefficient of friction (COF) curves of the Ni–diamond coatings prepared at various concentrations of diamond particles in the electrolyte. The coefficient of friction (COF) curves for all coatings exhibited distinct stages: a running-in stage followed by a stable wear stage. At the running-in stage, the COFs of all the coatings rapidly increased. Afterwards, the COFs of all the coatings tended to be gradually stabilized, accompanied by a few slight fluctuations at the stable wear stage. Furthermore, with the increase in concentration of the diamond particles in the electrolyte, the average COF of the coating was significantly reduced; the average COF of 1 g/L was ca. 1.03, while the average COF of 16 g/L was reduced to ca. 0.73. This was attributed to the tailoring effect of the co-deposited diamond particles on the microstructures of the coatings, which normally enhanced the micro-hardness of the coating. As a result, the abrasive dust of the coating prepared at a high concentration of particles strongly decreased, so that the COF of the coating was reduced [29]. Moreover, the falling off of the co-deposited particles on the surface of the coating could work as lubricants during wearing. Therefore, the increased amount of co-deposited diamond particles could cause the COF reduction in the coating with an increase in the concentration of diamond particles in the electrolyte [30,31,32,33,34].
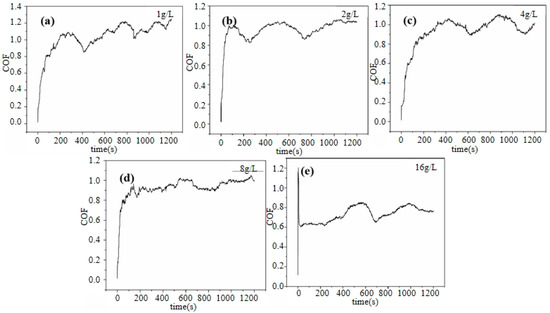
Figure 10.
COF curves of the Ni–diamond coatings prepared at (a) 1, (b) 2, (c) 4, (d) 8, and (e) 16 g/L.
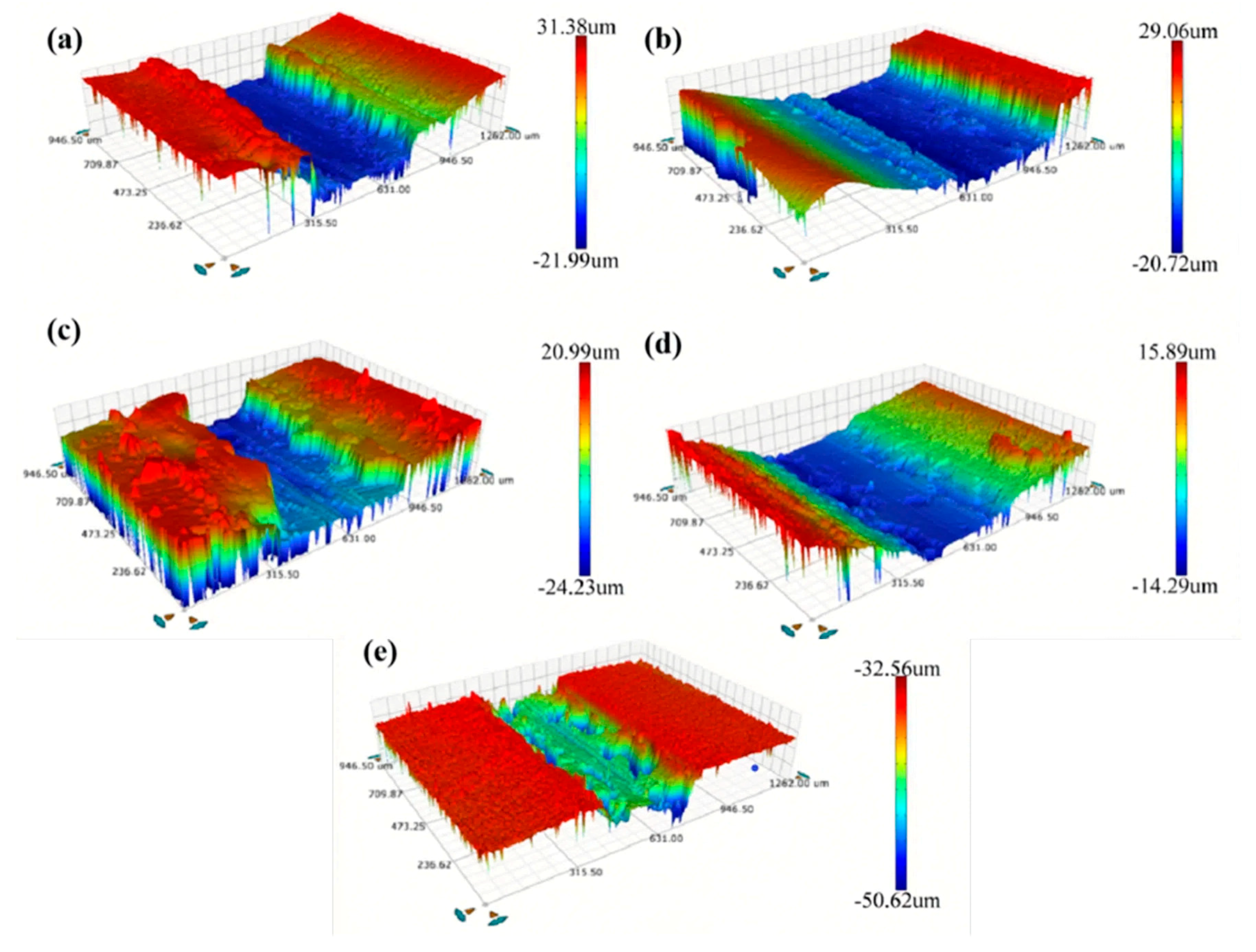
Figure 11 shows the worn scratch profiles of the Ni–diamond coatings prepared with different concentrations of diamond particles. It is observed that the profiles of worn scratches are different among the coatings, which can be caused by the different microstructures of the coatings with various contents of co-deposited particles. The coatings prepared at 2, 8, and 16 g/L exhibited a kind of normal profile of worn scratches, while the coatings prepared at 1 and 4 g/L possessed a kind of deep and narrow profile of worn scratches. This could be attributed to the low mechanical property of the Ni deposits and the inhomogeneous distribution of the diamond particles in the Ni deposits, which caused the massive exfoliation in the Ni deposits during wearing. The corresponding profile information of the coatings is listed in Table 1. As the concentration of diamond particles increased, the depth and width of the worn scratch on the coating decreased notably. For instance, the depth and width of the coating prepared with a concentration of 1 g/L measured 53 and 631.2 μm, respectively, while for the coating prepared at 16 g/L, these measurements were reduced to 18 and 378.6 μm, respectively.
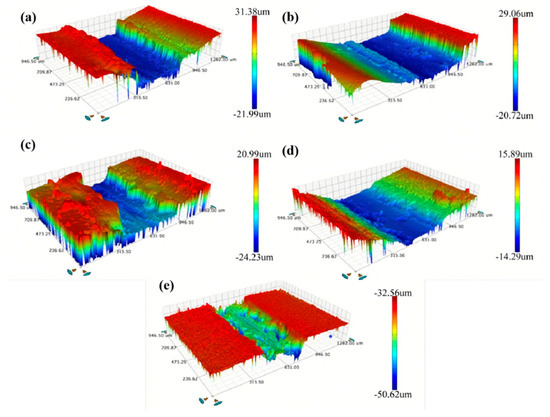
Figure 11.
Profiles of the worn scratches of the Ni–diamond coatings prepared at the particle concentrations of (a) 1, (b) 2, (c) 4, (d) 8, and (e) 16 g/L.

Table 1.
Worn scratch profile information of the Ni–diamond coatings with increasing concentrations of diamond particles.
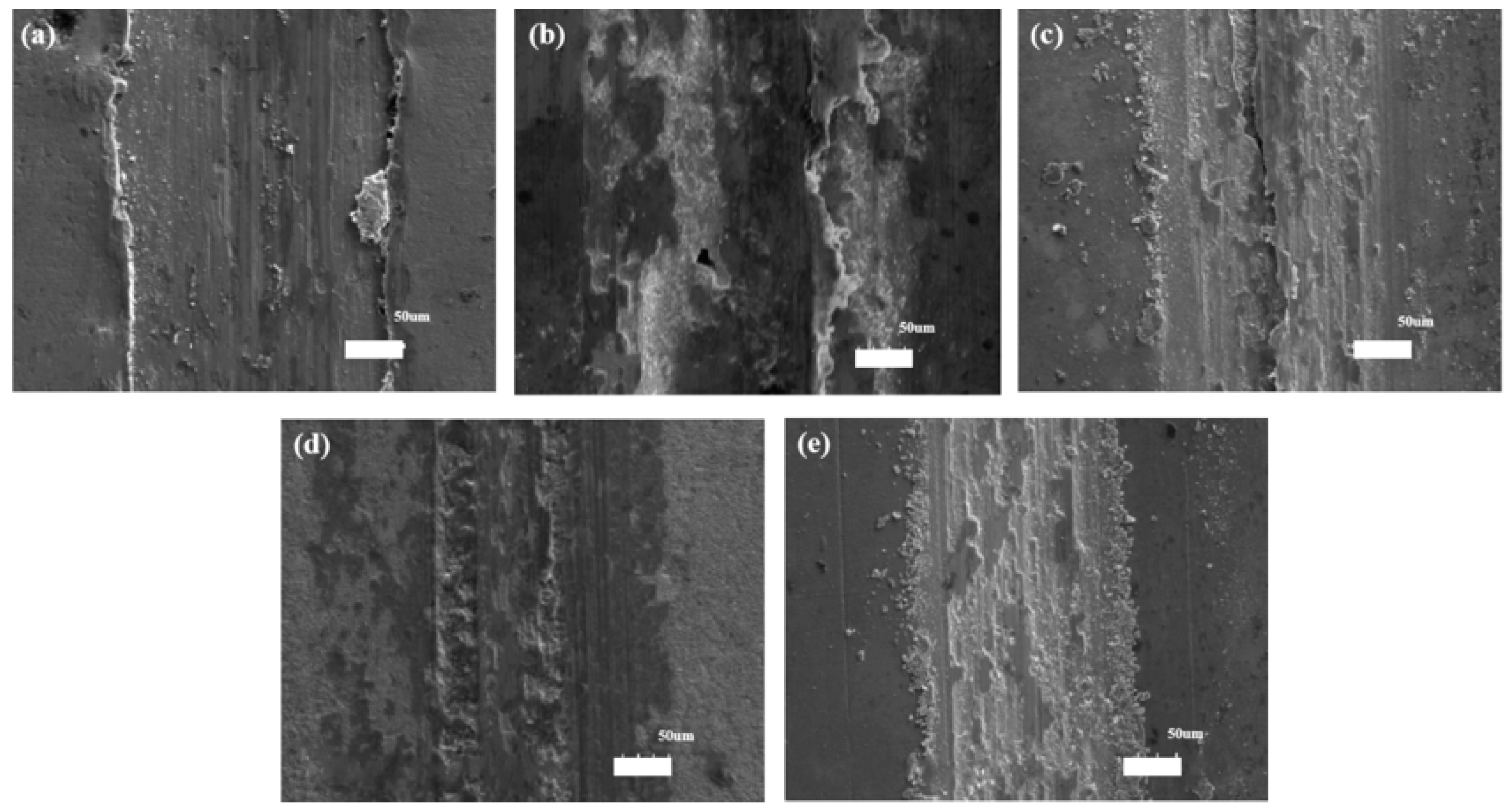
The results of COFs and worn scratches of the Ni–diamond coatings suggested that the wear resistance of the coating was gradually improved with increasing concentrations of diamond particles in the electrolyte. To further reveal the wear behaviors of the coatings, the SEM morphologies of the worn scratches of the coatings are exhibited in Figure 12. The furrows are visible in the worn scratches of all the coatings, indicating an abrasive wear behavior of the coatings. Moreover, some avulsion occurred within and at the side of the scratches, indicating adhesive wear behavior. Especially, the avulsion occurred at the side of the scratches of the coatings prepared at 1 and 4 g/L, which led to the scratch profiles of the coatings as shown in Figure 11. Meanwhile, the mixed wear behaviors of the coatings brought in the fluctuation of COF, as shown in Figure 10.
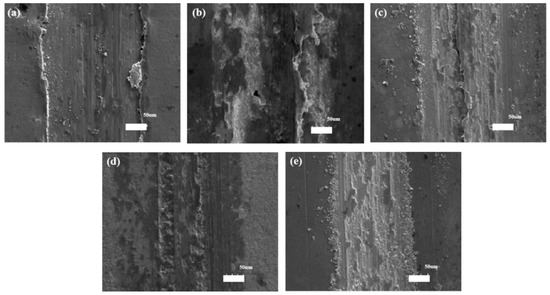
Figure 12.
SEM morphologies of the worn scratches of the Ni–diamond coatings prepared at (a) 1, (b) 2, (c) 4, (d) 8, and (e) 16 g/L.
The above wear behaviors of the coatings were attributed to the tailoring effect of the diamond particles on the microstructures of the coatings. The higher concentration of co-deposited diamond particles led to increased current density around them, thereby influencing the microstructure of the coatings, including grain size refinement, and modifying the crystallographic texture of the Ni deposits. As a result, the tailored microstructure and the reinforcing role of the co-deposited diamond particles decreased the amount of abrasive dust and the COF of the coating in the mixed wear behaviors of abrasive wear and adhesive wear.
4. Conclusions
The Ni–diamond coatings were electrodeposited at various diamond particle concentrations (1, 2, 4, 8, and 16 g/L) in the electrolyte. The study investigated microstructure evolution mechanisms and wear behaviors, yielding the following conclusions:
- The surface morphology and microstructure of the Ni–diamond coating was significantly influenced by the co-deposited diamond particles. Increased particle concentration disrupted the poly-pyramid surface structure, decreased grain size, and weakened the [200] fiber texture of the Ni deposits. The coating produced at 16 g/L exhibited the smallest grain size and the least pronounced [200] fiber texture.
- The co-deposition behaviors of the diamond particles into the Ni deposits were examined using COMSOL5.6, which illustrated the electrodeposition condition evolution near the captured particles on the cathode. The current density was concentrated around particle edges, consequently leading to the refinement of grain size and [200] fiber texture near the particles, which suggested the tailoring effect of the co-deposited diamond particles.
- The wear resistance of the coatings improved with higher diamond particle concentrations in the electrolyte, leading to reduced COF and wear loss. The mixture of abrasive wear and adhesive wear was the wear behavior of the Ni–diamond coatings, which was attributed to the tailoring effect of the co-deposited diamond particles.
- Nickel–diamond composite coatings or other composite coatings are widely used in aerospace, energy and environmental protection. Its excellent performance makes it excel in various harsh environments. With the continuous development of science and technology, the application prospects of nickel-based composite plating will be broader, bringing higher levels of convenience and safety to human production and life.
Author Contributions
Software, Z.Y.; Validation, K.G.; Formal analysis, W.C. and J.Z.; Investigation, S.Z., W.L. and Y.L.; Resources, Z.P. and Y.Z.; Data curation, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
Authors gratefully acknowledge the financial support provided by Shanghai High-level Local University Innovation Team (Maritime safety & technical support), the Science & Technology Commission of Shanghai Municipality and Shanghai Engineering Research Center of Ship Intelligent Maintenance and Energy Efficiency under (Grant 20DZ2252300).
Data Availability Statement
The raw data provided in the study was provided by Chen Zhixiang’s master’s (Shanghai Maritime University Merchant Marine College, Shanghai, China) thesis. The data presented in this study are available on request from the corresponding author due to confidentiality reasons.
Acknowledgments
The authors gratefully acknowledge the support from the National Natural Science Foundation of China (No. 52272353).
Conflicts of Interest
Wen Cai is employed by the company Shanghai Waigaoqiao Shipbuilding Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhao, Y.; Jiang, C.; Xu, Z.; Cai, F.; Zhang, Z.; Fu, P. Microstructure and corrosion behavior of Ti nanoparticles reinforced Ni-Ti composite coatings by electrodeposition. Mater. Des. 2015, 85, 39–46. [Google Scholar] [CrossRef]
- LWang, L.; Zhao, Y.; Jiang, C.; Ji, V.; Chen, M.; Zhan, K.; Moreira, F. Investigation on microstructure and properties of electrodeposited Ni-Ti-CeO2 composite coating. J. Alloys Compd. 2018, 754, 93–104. [Google Scholar]
- Zhang, Z.; Jiang, C.; Cai, F.; Fu, P.; Ma, N.; Ji, V. Two stages for the evolution of crystallite size and texture of electrodeposited Ni-ZrC composite coating. Surf. Coat. Technol. 2015, 261, 122–129. [Google Scholar] [CrossRef]
- Jiang, X.; Yang, Y.; Qin, Z.; Jin, T.; Zhu, B.; Ren, L.; Jiang, L.; Liu, M.; Deng, Z.; Wei, G.; et al. Characterization of high absorbance and high-emissivity black NiCe oxide composite coatings produced via photo-assisted electrodeposition. Surf. Coat. Technol. 2023, 454, 129173. [Google Scholar] [CrossRef]
- Piyush, P.; Kumar, P.K.; Rita, M. A review on mechanical, tribological and electrochemical performance of ceramic particle-reinforced Ni-based electrodeposited composite coatings. J. Mater. Sci. 2022, 57, 19179–19211. [Google Scholar]
- Pandey, U.; Singh, A.K.; Sharma, C. Development of anti-corrosive novel nickel-graphene oxide-polypyrrole composite coatings on mild steel employing electrodeposition technique. Synth. Met. 2022, 290, 117135. [Google Scholar] [CrossRef]
- Xiao, Q.; Zou, J.; Chen, B.; Luo, J.; Lu, Y.; Chen, W.; Zhang, X.; Liang, T. Influence of Ti3C2Tx addition on the corrosion behaviour and tribological properties of electrodeposited Ni-W- Ti3C2Tx nanocomposite coatings. Mater. Sci. Technol. 2022, 38, 703–715. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Mei, T.; Miao, Y. Fabrication of Ni-B/TiC-Y2O3 nanocomposites by one-step electrodeposition at different duty cycle and evaluation of structural, surface and performance as protective coating. J. Alloys Compd. 2020, 823, 153888. [Google Scholar] [CrossRef]
- Almonti, D.; Baiocco, G.; Della Millia, M.; Mingione, E.; Menna, E.; Rubino, G.; Salvi, D.; Stamopoulos, A.; Ucciardello, N. Morphological and functional characterization of electroplated Ni-graphene composite coatings. J. Phys. Conf. Ser. 2024, 2692, 012668. [Google Scholar] [CrossRef]
- Verma, K.; Cao, H.; Mandapalli, P.; Wille, R. Modeling and simulation of electrophoreticdeposition coatings. J. Comput. Sci. 2020, 41, 101075. [Google Scholar] [CrossRef]
- Shetty, A.R.; Hegde, A.C. Electrofabrication of Ni-Co-CNT Composite Coatings for Hydrogen Energy. Nano Hybrids Compos. 2017, 17, 149–155. [Google Scholar] [CrossRef]
- Liu, J.H.; Pei, Z.L.; Shi, W.B.; Liu, Y.D.; Gong, J.; Sun, C. Studies on preparation, microstructure, mechanical properties and corrosion resistance of NiMo/micron-sized diamond composite coatings. Surf. Coat. Technol. 2020, 385, 125451. [Google Scholar] [CrossRef]
- Xu, X.; Li, Q.; Liu, X. Comparison of preparation process and wear resistance of nickel-diamond composite coatings. Met. Funct. Mater. 2022, 29, 100–105. [Google Scholar]
- Huang, C.A.; Shen, C.H.; Yang, S.W.; Liao, C.W.; Lai, P.L. Fabrication and evaluation of electroplated diamond grinding rods strengthened with Cr-C deposit. Int. J. Adv. Manuf. Technol. 2020, 110, 2541–2550. [Google Scholar] [CrossRef]
- Jung, D.J.; Kim, H.J.; Lee, K.A. Characteristics of Ni-coated diamond Metal Composite Coatings by Cold Spray Deposition. J. Korean Inst. Met. Mater. 2009, 47, 550–557. [Google Scholar]
- Qiu, H.; Dingwen, W.; Shaohui, Y. The microstructure, wear and electrochemical properties of electrodeposited Ni-diamond composite coatings: Effect of diamond concentration. Mater. Today Commun. 2023, 34, 105476. [Google Scholar]
- Wang, D.; Liu, M.; Zhu, Y.; Li, F. Influence of Double-Pulse Electrodeposition Parameters on the Performance of Nickel/Nanodiamond Composite Coatings. Coatings 2021, 11, 1068. [Google Scholar] [CrossRef]
- Li, B.; Mei, T.; Chu, H.; Wang, J.; Du, S.; Miao, Y.; Zhang, W. Ultrasonic-assisted electrodeposition of Ni/diamond composite coatings and its structure and electrochemical properties. Ultrason. Sonochemistry 2021, 73, 105475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, L.; Qiu, W. Enhancement of the wear resistance of Ni-diamond composite coatings via glycine modification. Diam. Relat. Mater. 2020, 109, 108086. [Google Scholar] [CrossRef]
- Awasthi, S.; Goel, S.; Pandey, C.P.; Balani, K. Multi-length scale tribology ofelectrophoretically deposited nickel-diamond coatings. JOM 2017, 69, 227–235. [Google Scholar] [CrossRef]
- Bao, H.; Li, Q.; Jia, H.; Yang, G. Mechanical properties comparison of Ni-diamond composite coatings fabricated by different methods. Mater. Res. Express 2019, 6, 106425. [Google Scholar] [CrossRef]
- Hong, Q.; Zhou, R.; Guo, X.; Wang, Z.; Yin, S. A novel strategy for improving the wear resistance of electrodeposited Ni-diamond composite coatings by diamond surface morphology modification. Diam. Relat. Mater. 2023, 137, 110093. [Google Scholar] [CrossRef]
- Wang, D.; Li, F.; Liu, M.; Zhang, W.; Yu, X.; Da, W. Effect of Nanodiamond Content in the Plating Solution on the Corrosion Resistance of Nickel-Nanodiamond Composite Coatings Prepared on Annealed 45 Carbon Steel. Coatings 2022, 12, 1558. [Google Scholar] [CrossRef]
- Zhang, W.; Mei, T.; Li, B.; Yang, L.; Du, S.; Miao, Y.; Chu, H. Effect of current density and agitation modes on the structural and corrosion behaviour of Ni/diamond composite coatings. J. Mater. Res. Technol. 2021, 12, 1473–1485. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.L.; Zhang, T.; Liu, W.G.; Li, W.X.; Zhou, Y.M. Preparation of Ni-P-diamond coatings with dry friction characteristics and abrasive wear resistance. Int. J. Refract. Met. Hard Mater. 2018, 70, 32–38. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, W.-Q.; Chen, Y.-L. Effect of current density on the corrosion resistance of Ni-W-Ti3C2Tx composite coatings. Rare Met. 2022, 46, 1298–1305. [Google Scholar]
- Liu, J.; Wu, R.; Zu, Y. Analysis of grain size of TiO2 at different calcination temperatures using Rietveld refinement, Scherrer and Williamson-Hall method. Chem. Manag. 2023, 13, 151–155. [Google Scholar]
- Jeong, D.H.; Gonzalez, F.; Palumbo, G.; Aust, K.T.; Erb, U. The effect of grain size on the wear properties of electrodeposited nanocrystalline nickel coatings. Scr. Mater. 2001, 44, 493–499. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Chen, J.; Zhou, X.; Zong, Y. Microstructure, Wear and Corrosion Behaviors of Electrodeposited Ni-Diamond Micro-Composite Coatings. Coatings 2022, 12, 1391. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Z. Study on wear resistance of electrodeposited Co-W-P/MoS2 composite coating on 45 steel. Weapon Mater. Sci. Eng. 2022, 45, 25–30. [Google Scholar]
- Kumar, A.; Varshney, D. Crystal structure refinement of Bi(1−x)NdxFeO3 multiferroic by the Rietveld method. Ceram. Int. 2012, 38, 3935–3942. [Google Scholar] [CrossRef]
- Liu, H.; Cui, G.; Shi, R.; Li, S.; Kou, Z. MoS2/CoCrNi self-lubricating composite coatings and high temperature tribological properties. Rare Met. Mater. Eng. 2020, 49, 4280–4289. [Google Scholar]
- Pandey, M.K.; Sahay, S.; Kar, A.K. Nickel concentration dependent mechanical and tribological properties of subsurface layer of electrodeposited Ni-C nanocomposite thin films. Thin Solid Film. 2023, 773, 139818. [Google Scholar]
- Dadvand, M.; Savadogo, O. Effect of pulse reverse current waveform on tribological and mechanical properties of electrodeposited nickel-tungsten alloys on brass substrate. Tribol. Mater. Surf. Interfaces 2022, 16, 281–291. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).