Study on the Synthesis of LTA-Type Molecular Sieves from Coal Gangue and Aluminum Ash and Its Adsorption Properties towards Cu2+
Abstract
1. Introduction
2. Experiment
2.1. Experimental Reagents
2.2. Preparation of Molecular Sieves
2.3. Adsorption Experiment of Cu2+
2.4. Characterization Testing
3. Results and Discussion
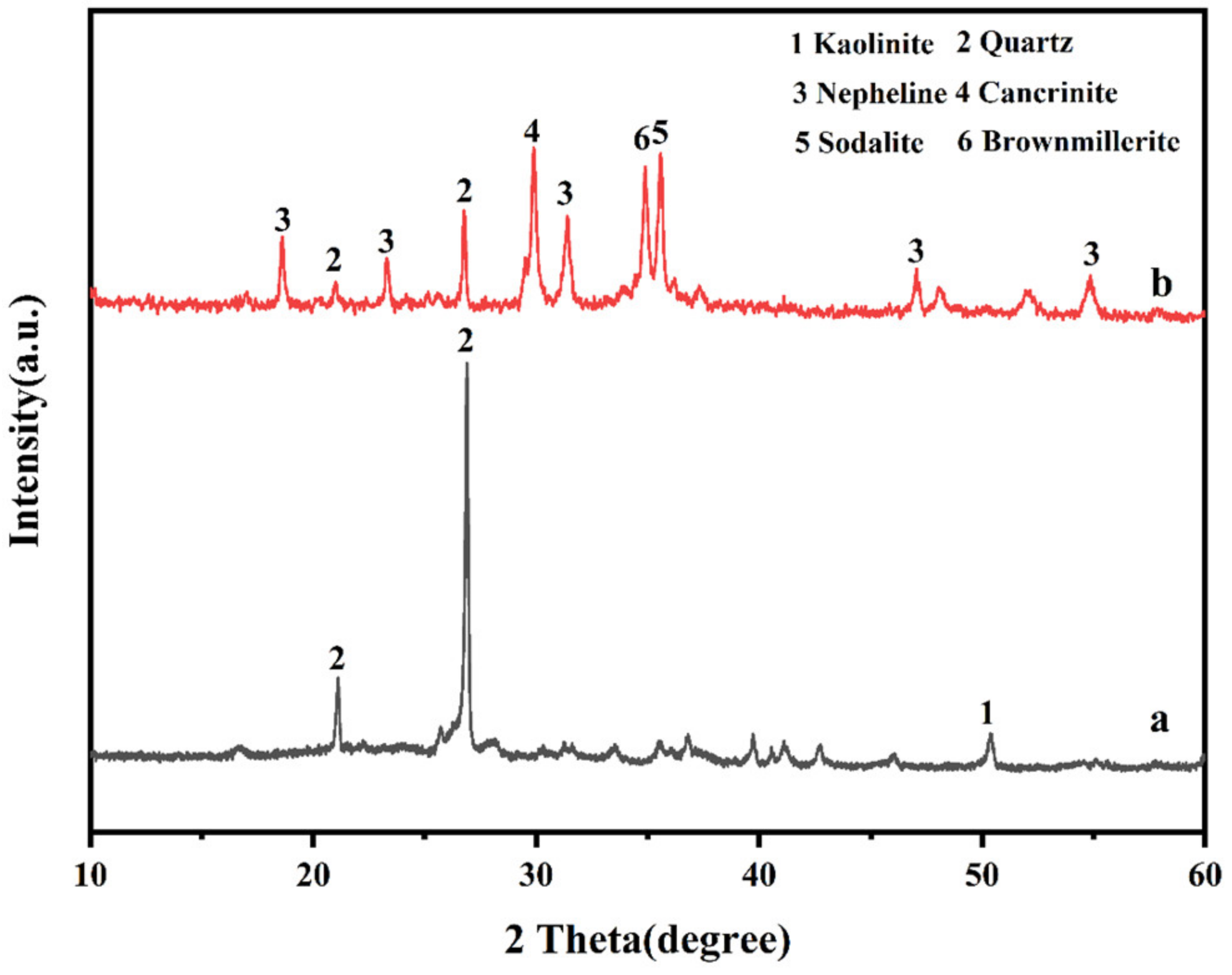
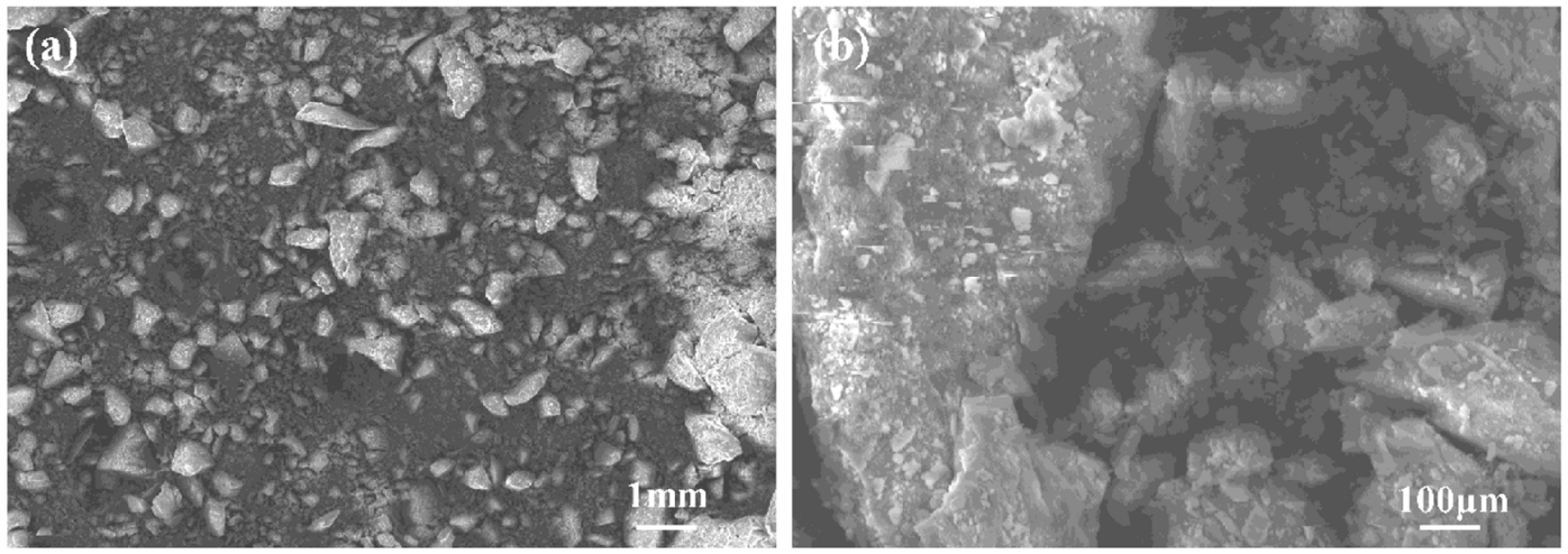
3.1. Analysis of Coal Gangue
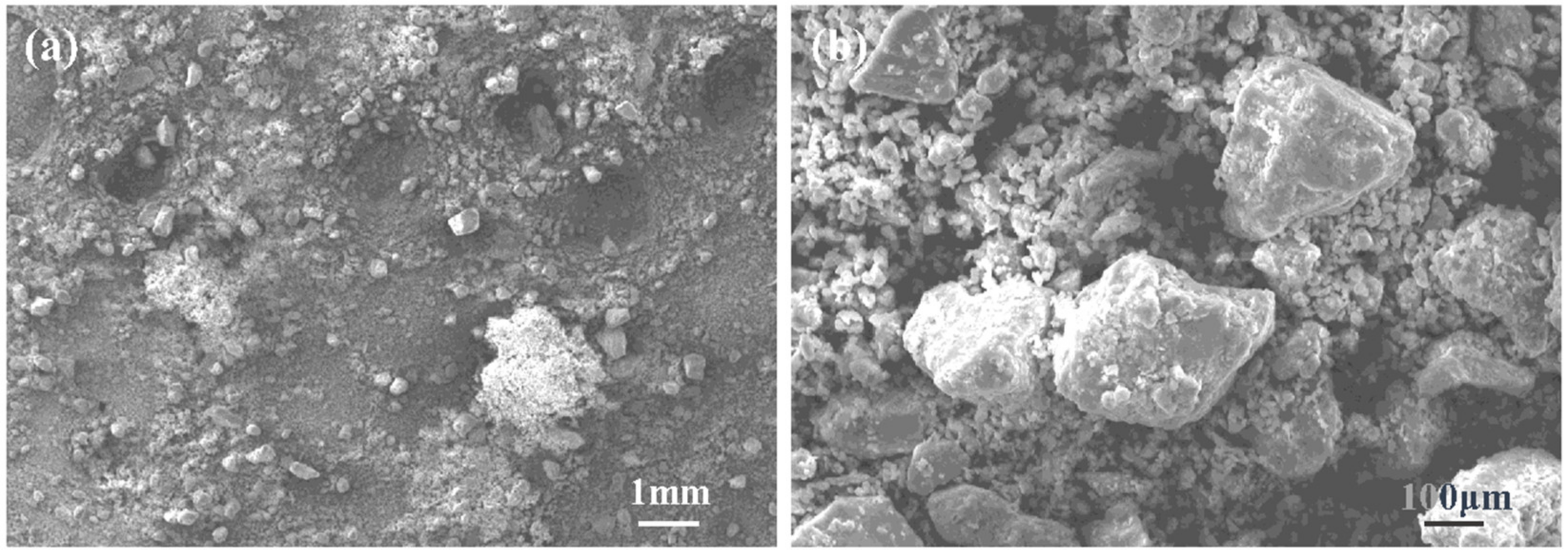
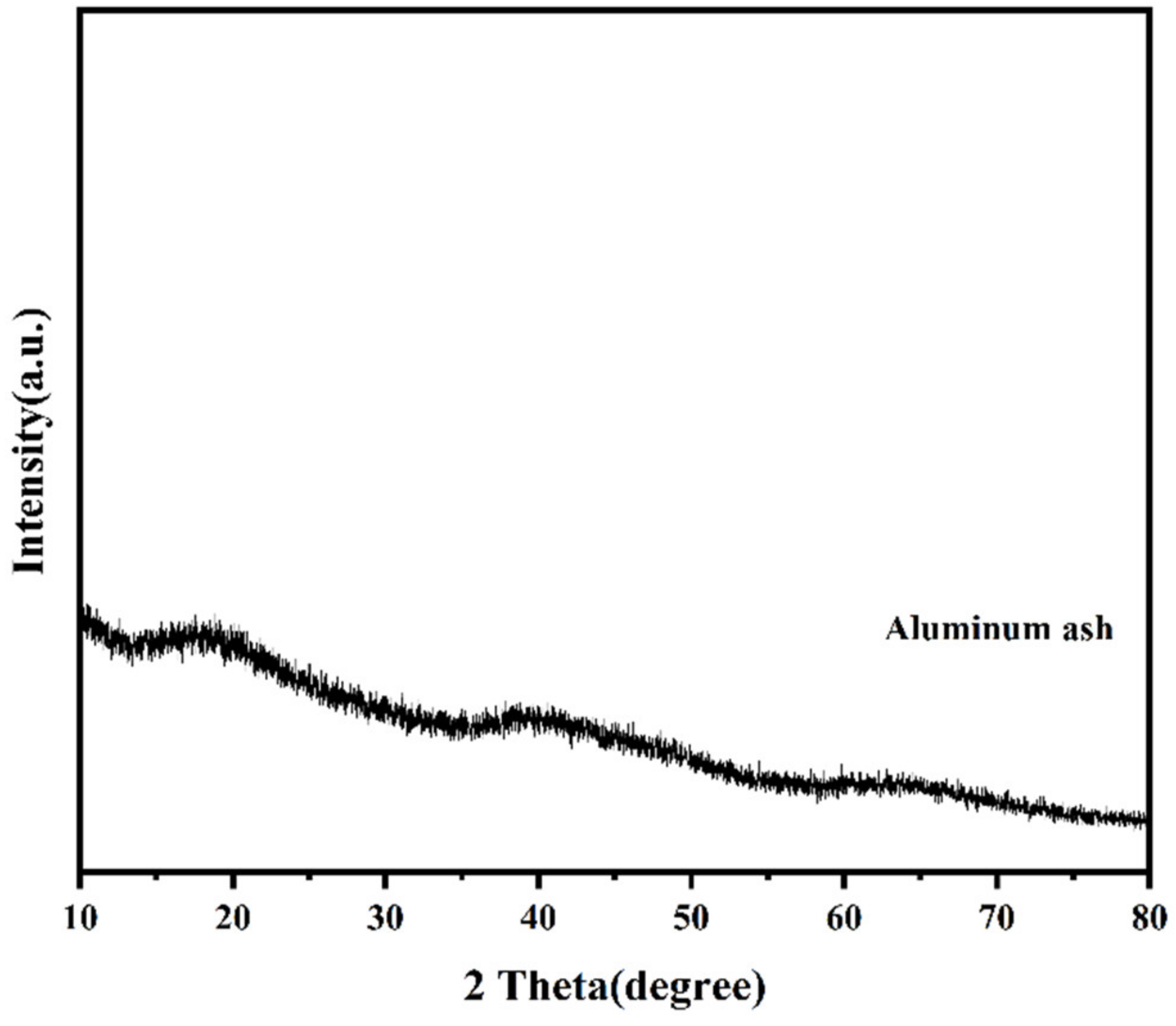
3.2. Analysis of Aluminum Ash
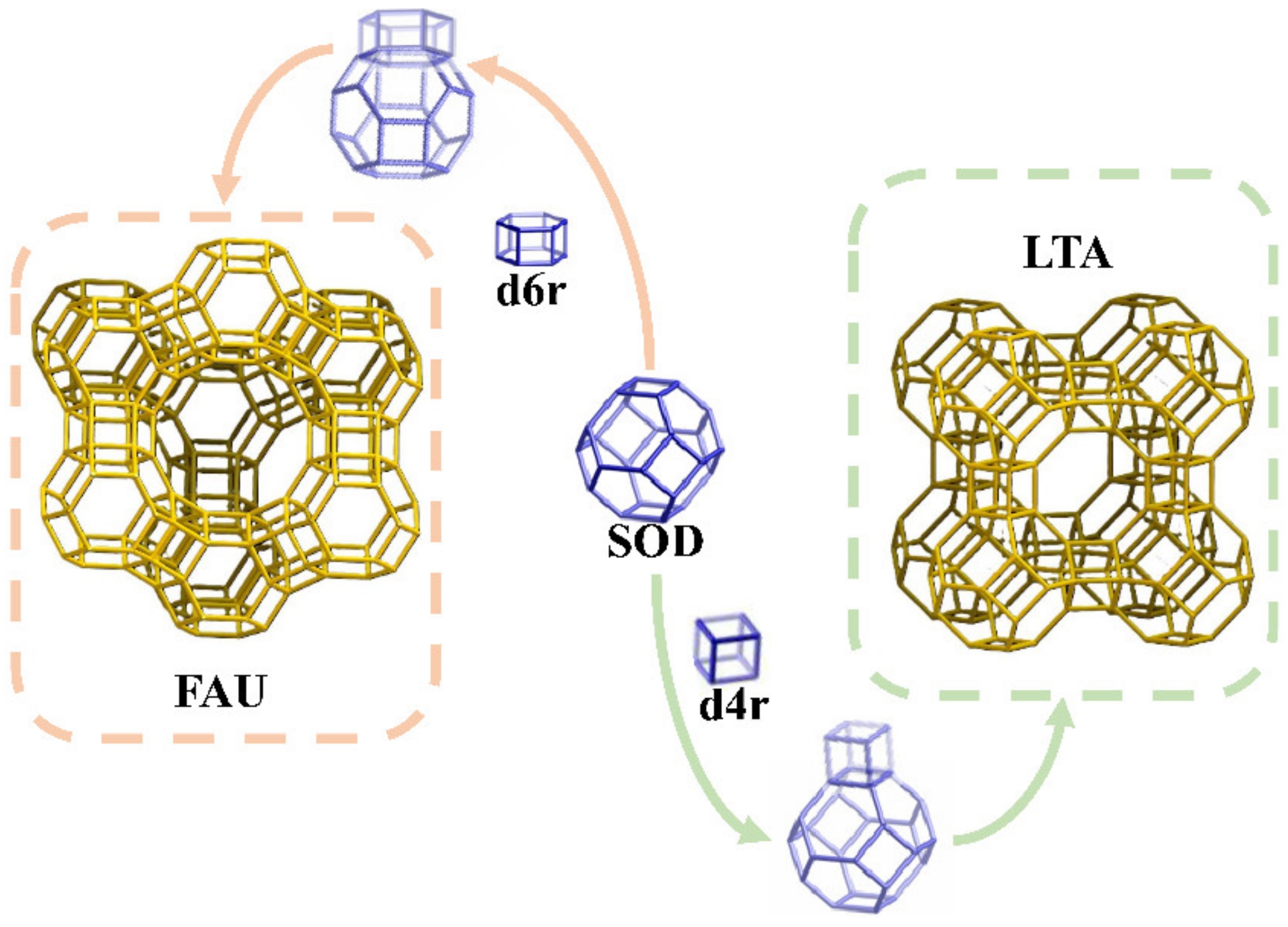
3.3. Coal Gangue and Aluminum Ash Hydrothermal Synthesis of LTA-Type Molecular Sieve
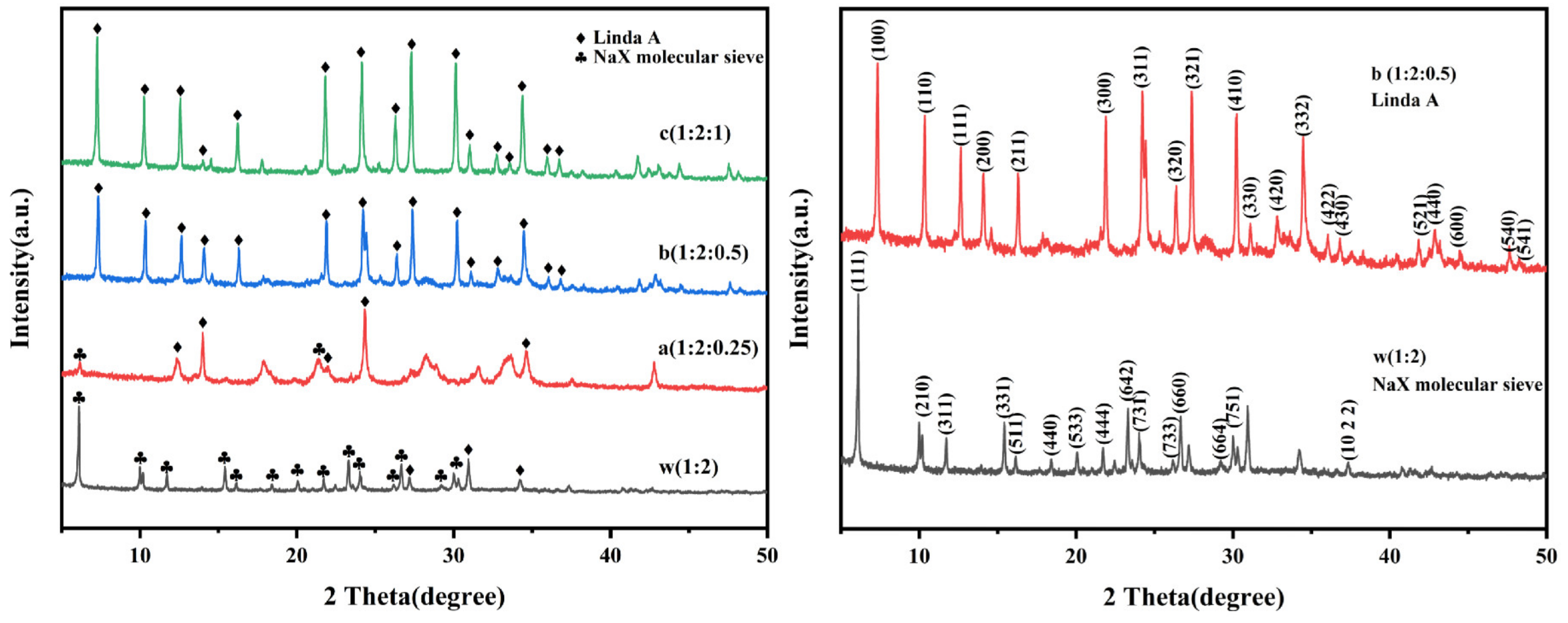
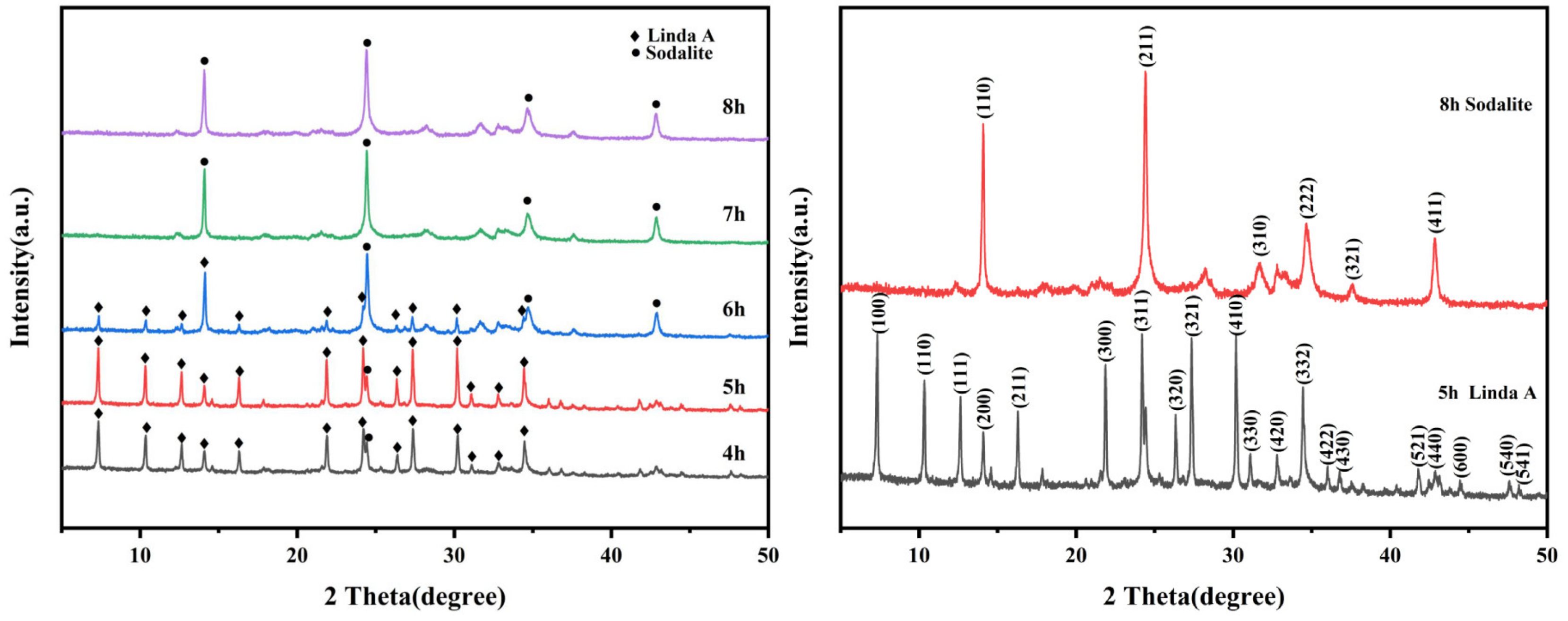
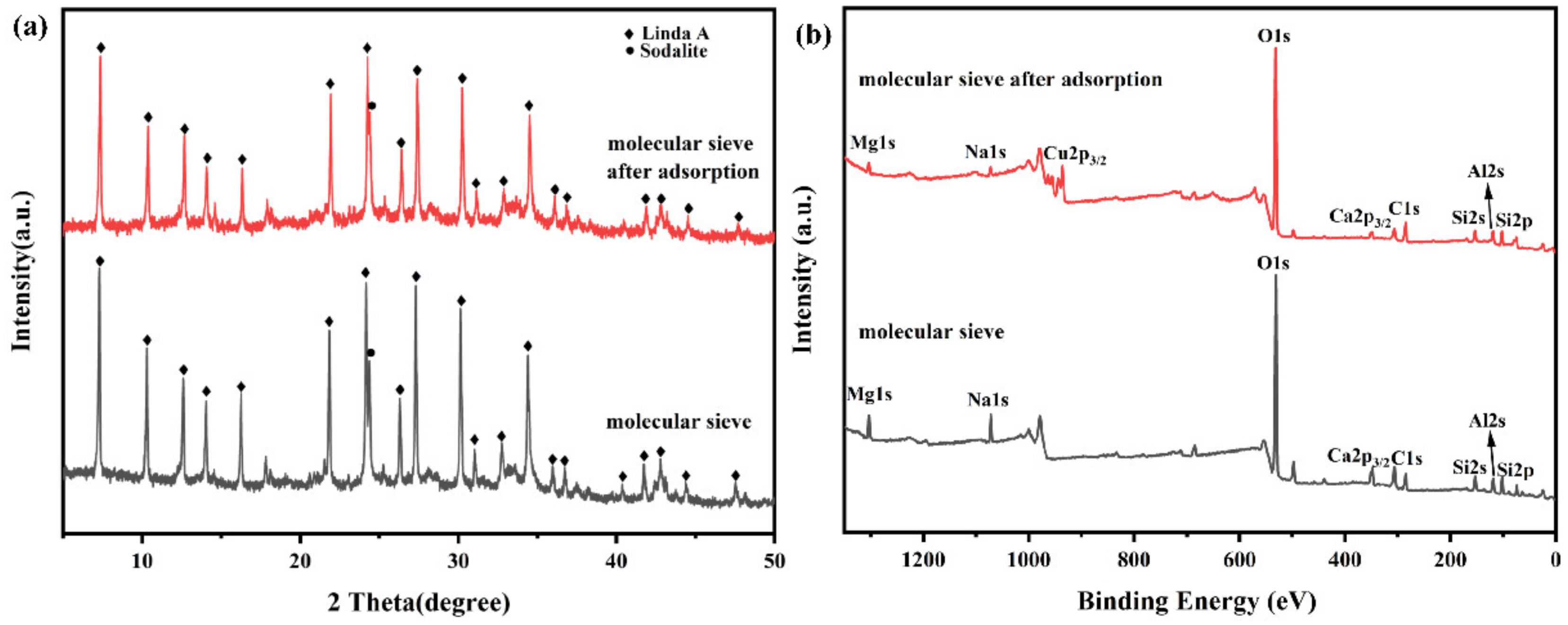
3.3.1. Analysis of Crystal Structure
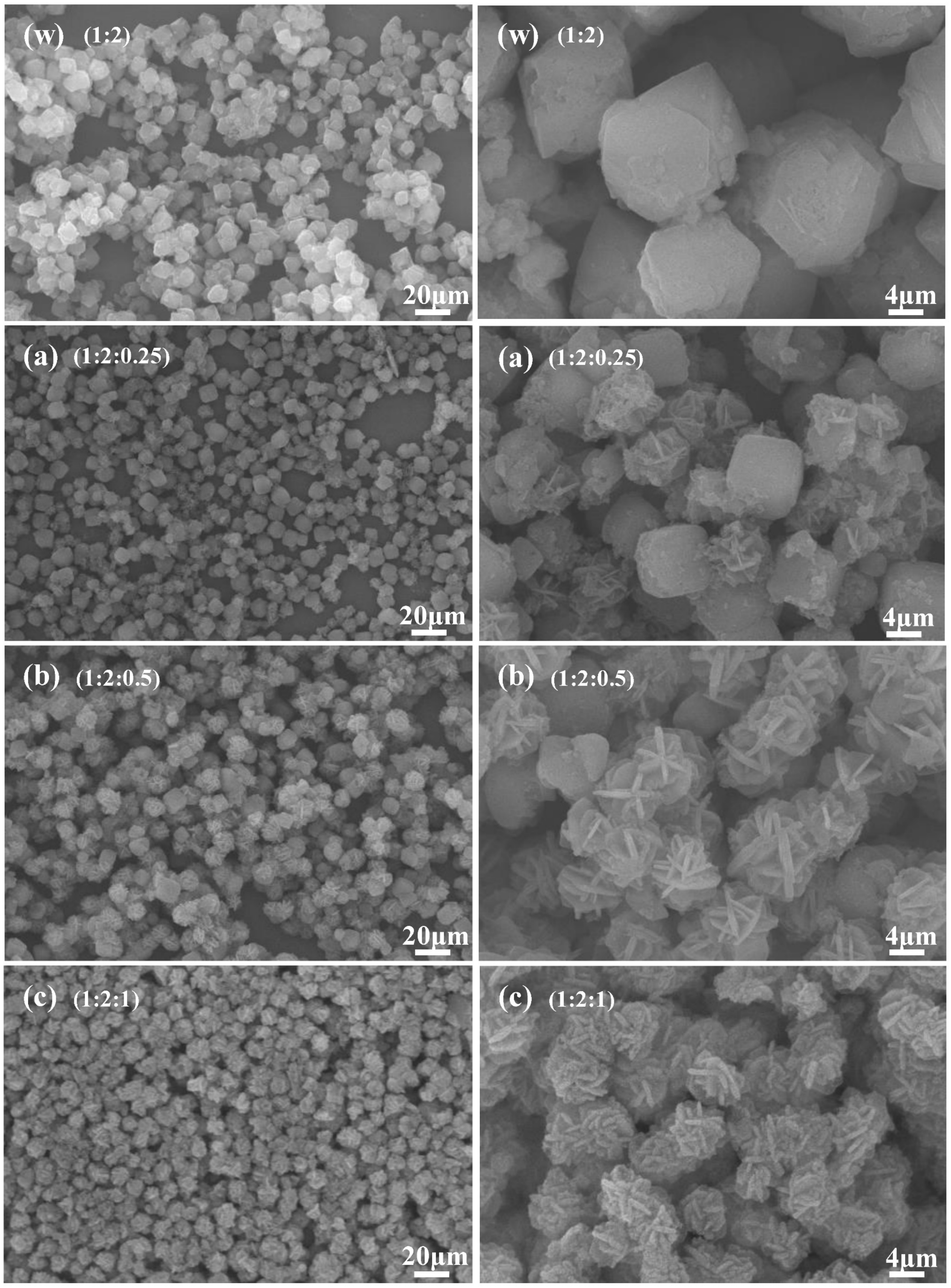
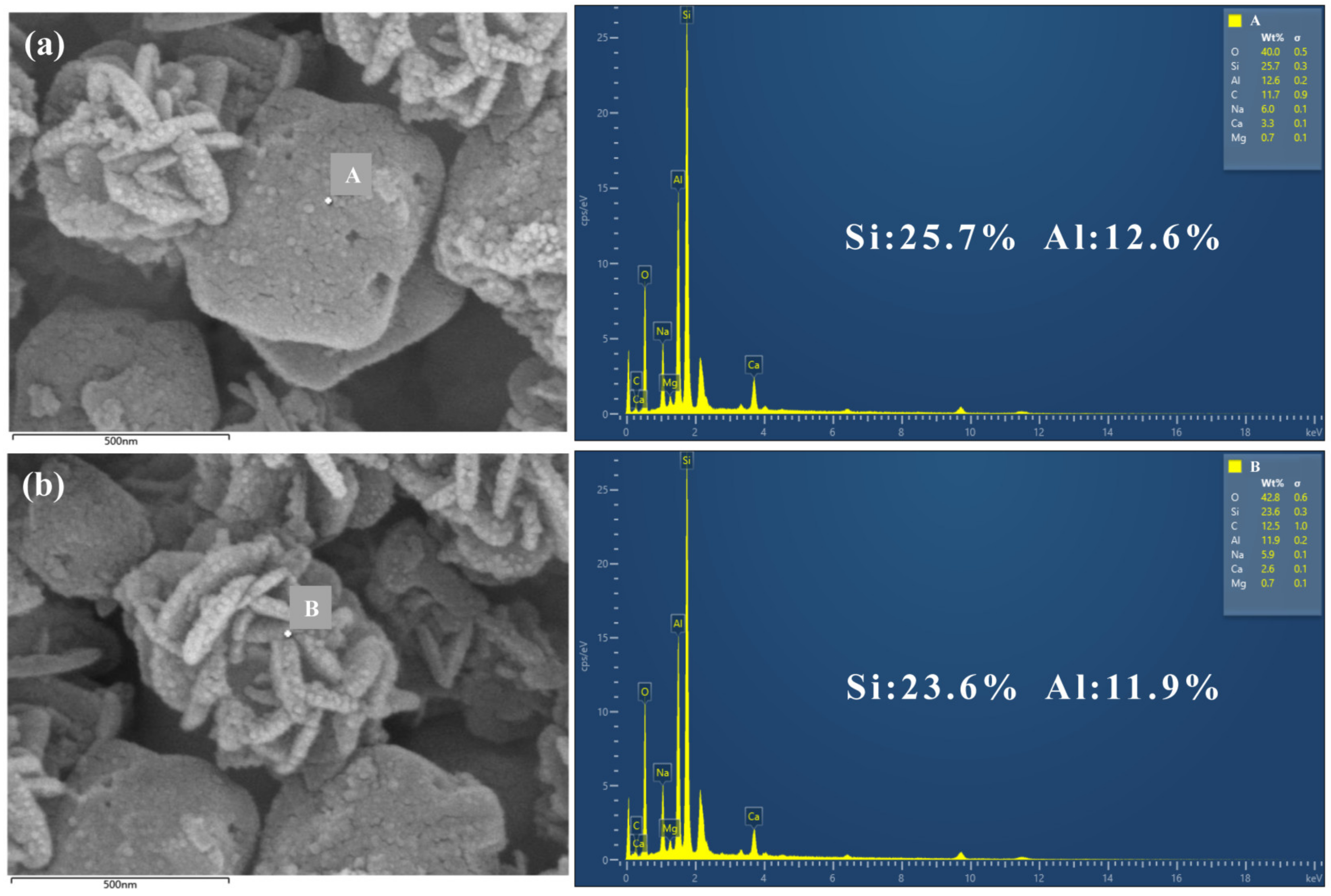
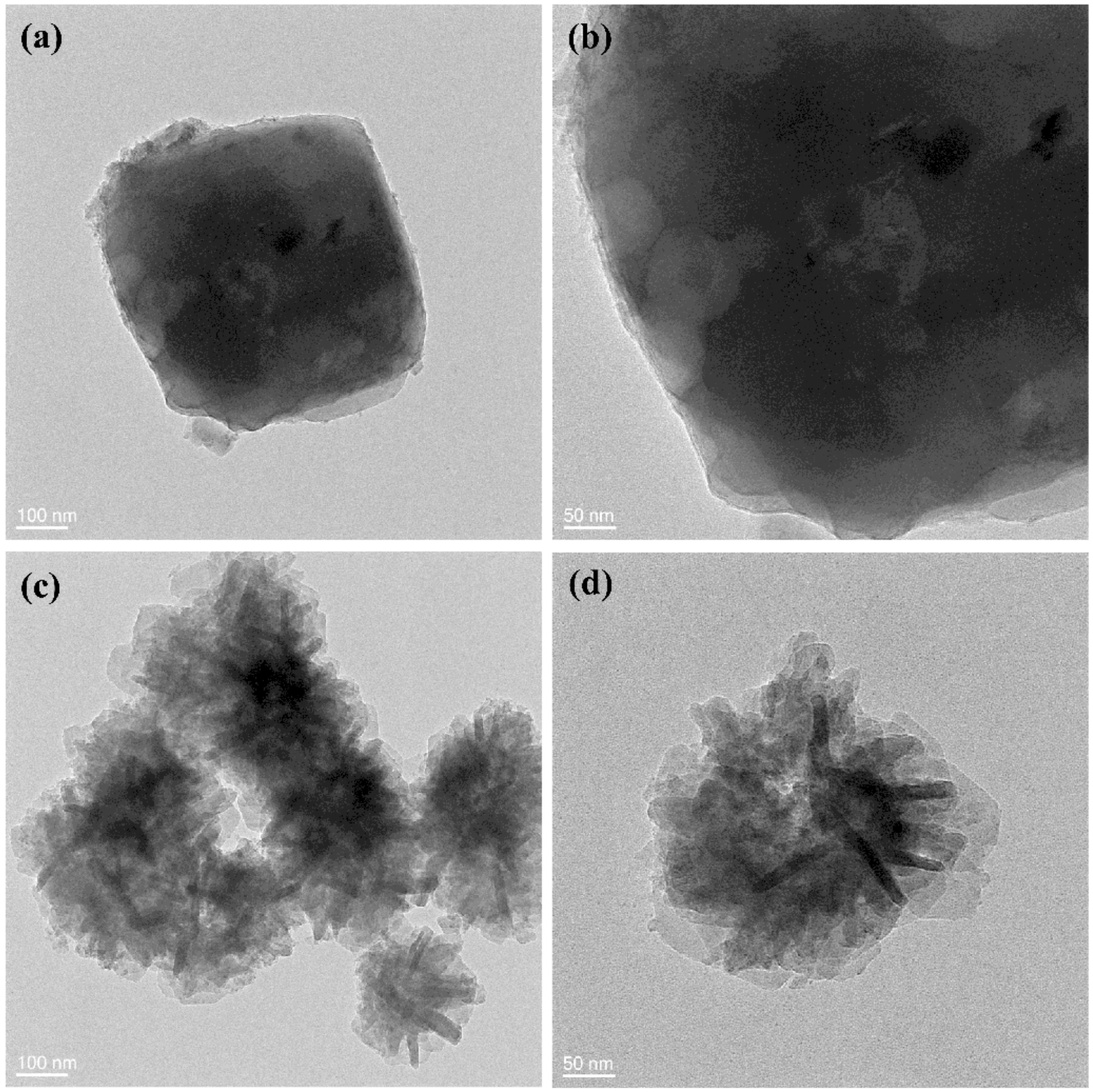
3.3.2. Morphological Analysis
3.4. Analysis of Adsorption Performance
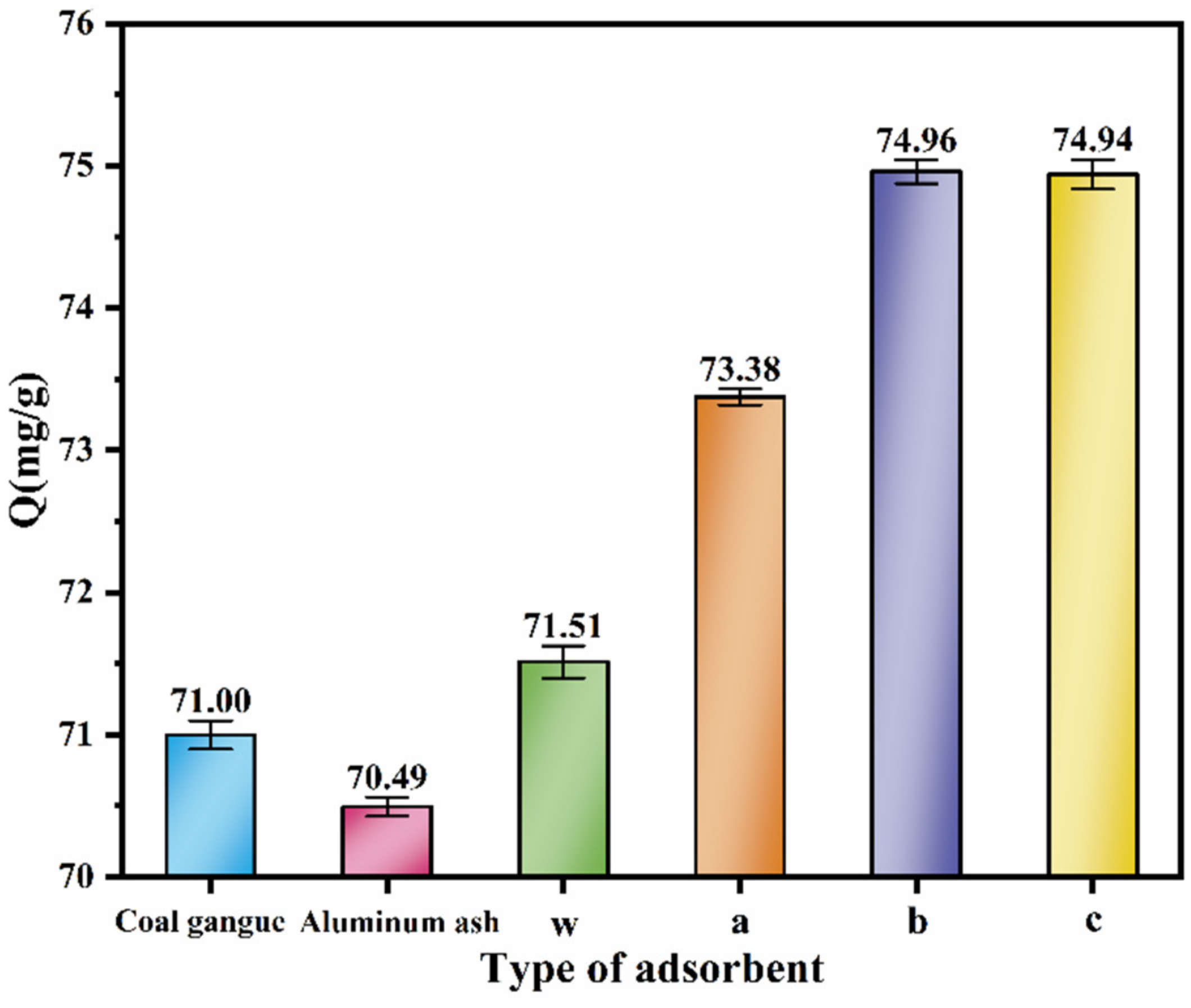
3.4.1. Comparative Analysis of Adsorption Performance between Prepared Product and Raw Materials
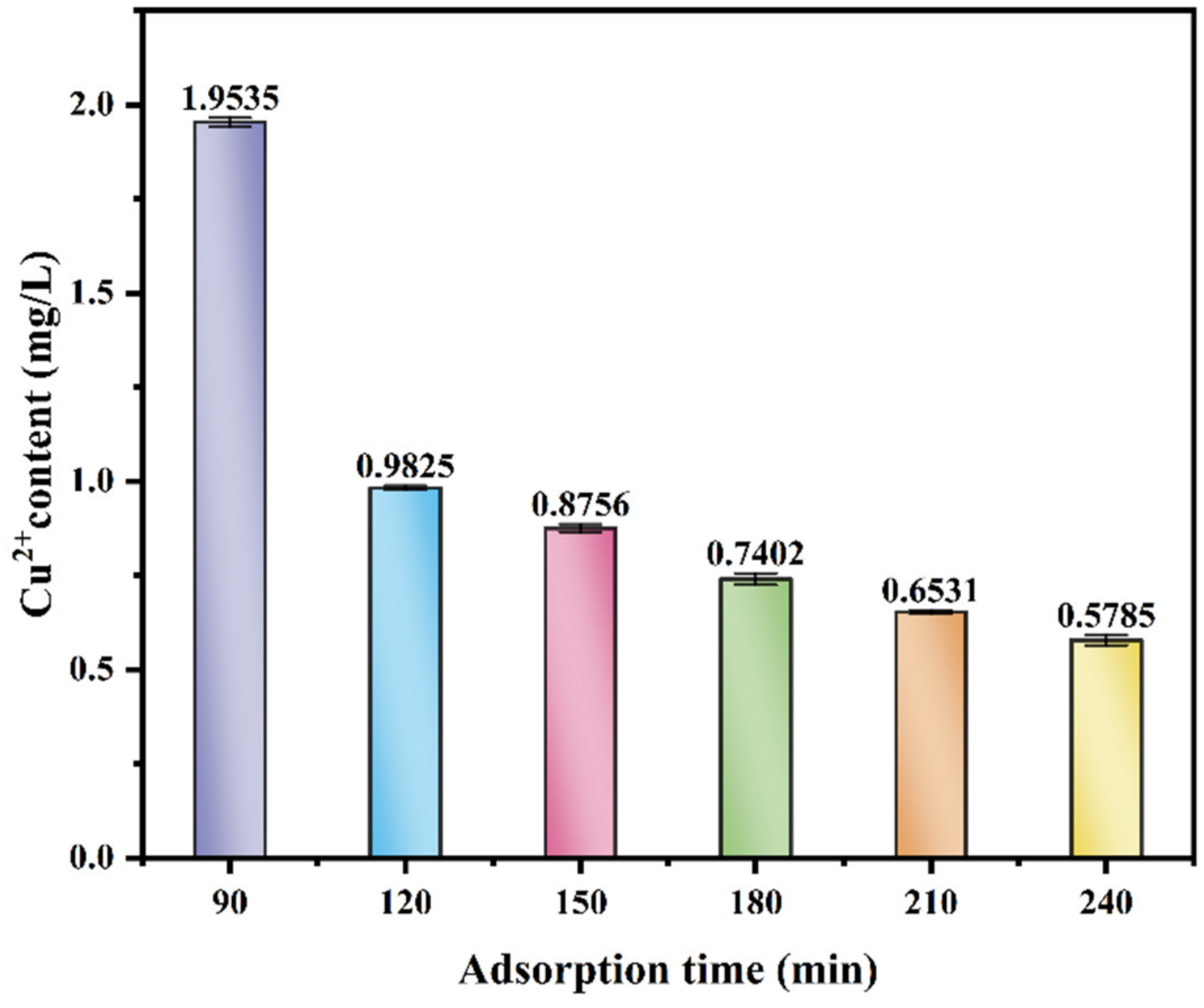
3.4.2. The Effect of Adsorption Time on the Adsorption Performance of Cu2+
3.4.3. The Effect of Adsorption Temperature on the Adsorption Performance of Cu2+
3.4.4. The Effect of Initial Concentration on the Adsorption Performance of Cu2+
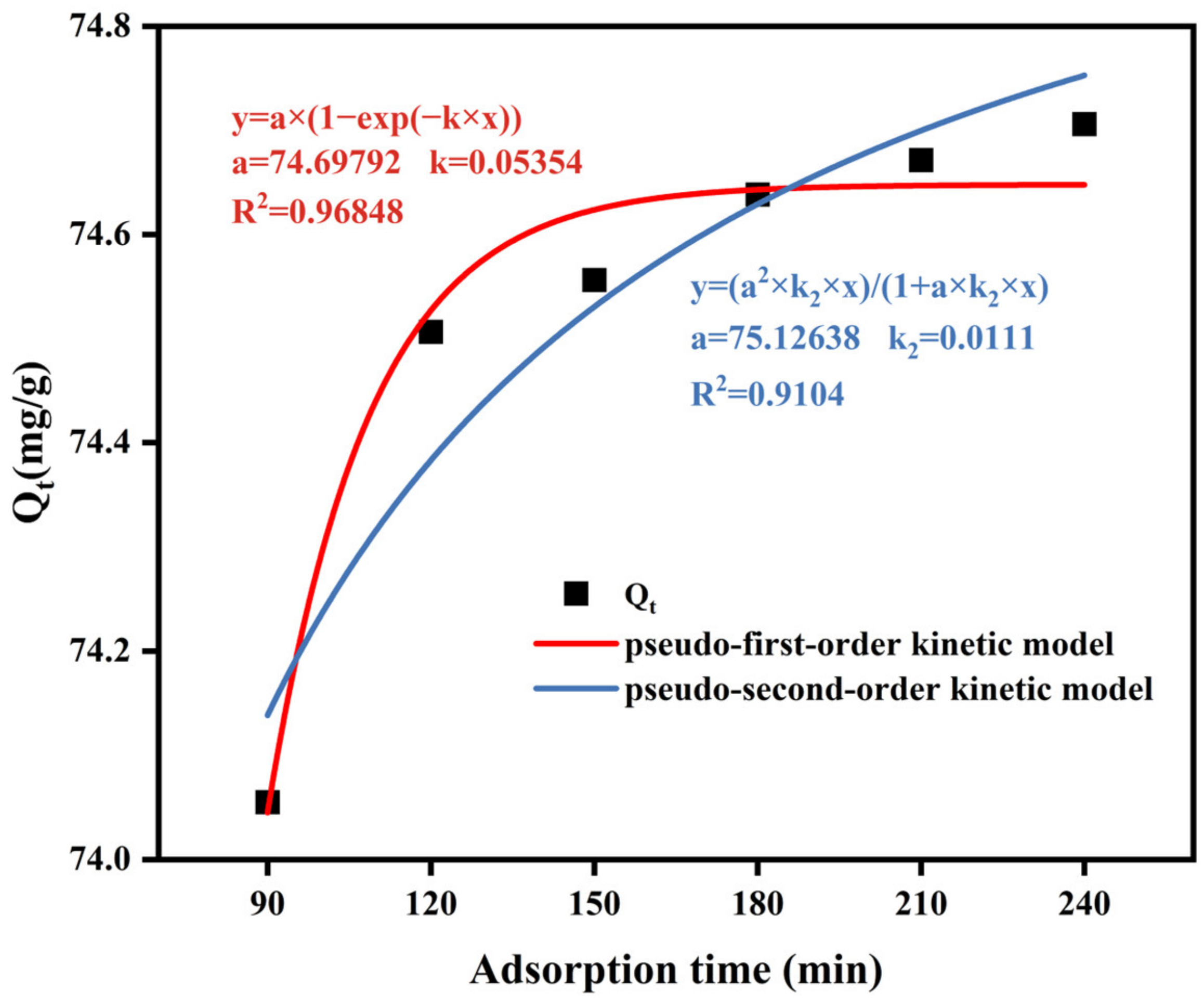
3.4.5. Adsorption Kinetics Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Development and Reform Commission of China. Guiding Opinions on the Comprehensive Utilization of Bulk Solid Waste during the 14th Five Year Plan Period. 2021. Available online: https://www.ndrc.gov.cn/xxgk/zcfb/tz/202103/t20210324_1270286.html (accessed on 12 March 2024).
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China. 2022 China Ecological Environment Status Bulletin. 2023. Available online: https://www.mee.gov.cn/hjzl/sthjzk/zghjzkgb/202305/P020230529570623593284.pdf (accessed on 12 March 2024).
- Ouyang, S.; Huang, Y.; Gao, H.; Guo, Y.; Wu, L.; Li, J. Study on the distribution characteristics and ecological risk of heavy metal elements in coal gangue taken from 25 mining areas of China. Environ. Sci. Pollut. Res. 2022, 29, 48285–48300. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, R.; Wang, Y.; Cheng, X.; Niu, R.; Ruan, H. Spatial distribution characteristics and risk assessment of soil heavy metal pollution around typical coal gangue hill located in Fengfeng Mining area. Environ. Geochem. Health 2023, 45, 7215–7236. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, J.; Huang, Y.; Song, T.; Gao, H.; Qiao, M. Research on Migration Law of Mn in Mudstone Floor in the Goaf under Coupling Conditions of Seepage and Stress. Pol. J. Environ. Stud. 2020, 29, 939–950. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, J.; Zhan, R.; He, W.; Tong, L.; Wan, P.; Bai, L. Control mechanism of the migration of heavy metal ions from gangue backfill bodies in mined-out areas. Front. Earth Sci. 2023, 10, 1090799. [Google Scholar] [CrossRef]
- Meng, F.; Yu, J.; Tahmasebi, A.; Han, Y. Pyrolysis and Combustion Behavior of Coal Gangue in O2/CO2 and O2/N2 Mixtures Using Thermogravimetric Analysis and a Drop Tube Furnace. Energy Fuels 2013, 27, 2923–2932. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Luo, J.; Zhu, W.; Fan, X.; Zhu, Y.; Zhang, Z. Mechanical properties and microscopic characteristics of steel fiber coal gangue concrete. Front. Mater. 2023, 10, 1211129. [Google Scholar] [CrossRef]
- Li, M.; Peng, Y.; Ding, L.; Zhang, J.; Ma, D.; Huang, P. Analysis of Surface Deformation Induced by Backfill Mining Considering the Compression Behavior of Gangue Backfill Materials. Appl. Sci. 2023, 13, 160. [Google Scholar] [CrossRef]
- Yang, J.; Tian, L.; Meng, L.; Wang, F.; Die, Q.; Yu, H.; Yang, Y.; Huang, Q. Thermal utilization techniques and strategies for secondary aluminum dross: A review. J. Environ. Manag. 2024, 351, 119939. [Google Scholar] [CrossRef]
- Cinarli, U.; Turan, A. Investigation of Alumina-Based Ceramic Production from Aluminum Black Dross. Min. Metall. Explor. 2021, 38, 257–267. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 2013, 261, 316–324. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Allahverdi, A. Hazardous aluminum dross characterization and recycling strategies: A critical review. J. Environ. Manag. 2018, 223, 452–468. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China. Statistical Bulletin on National Economic and Social Development of the People’s Republic of China in 2023. 2024. Available online: https://www.stats.gov.cn/sj/zxfb/202402/t20240228_1947915.html (accessed on 12 March 2024).
- Liu, Y.; Yang, J.; Shen, H.; Zhang, J.; Li, W.; Zhang, X.; Liu, J.; Liu, B.; Zhang, S. Synthesis of porous glass ceramics with hierarchical and interconnected pores from secondary aluminum dross and waste glass. Ceram. Int. 2022, 48, 34364–34373. [Google Scholar] [CrossRef]
- Yuan, H.; Luo, T.; Zhang, D.; Wang, Q. Harmless treatment and recycling of secondary aluminum dross: A review. J. Sustain. Cem.-Based Mater. 2023, 12, 1460–1473. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of valuable products from hazardous aluminum dross: A review. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Du, J.; Ma, A.; Wang, X.; Zheng, X. Review of the Preparation and Application of Porous Materials for Typical Coal-Based Solid Waste. Materials 2023, 16, 5434. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Li, L.; Li, J.; Jin, K.; Zhang, S.; Zhang, J.; Yan, W. Recent Progresses on the Synthesis of Zeolites from the Industrial Solid Wastes. Chem. J. Chin. Univ.-Chin. 2021, 42, 40–59. [Google Scholar] [CrossRef]
- Coronas, J. Present and future synthesis challenges for zeolites. Chem. Eng. J. 2010, 156, 236–242. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zheng, S.; Di, Y.; Sun, Z. A review of the synthesis and application of zeolites from coal-based solid wastes. Int. J. Miner. Metall. Mater. 2022, 29, 1–21. [Google Scholar] [CrossRef]
- Bieseki, L.; Penha, F.G.; Castella Pergher, S.B. Zeolite A Synthesis Employing a Brazilian Coal Ash as the Silicon and Aluminum Source and its Applications in Adsorption and Pigment Formulation. Mater. Res.-Ibero-Am. J. Mater. 2013, 16, 38–43. [Google Scholar] [CrossRef]
- Rigo, R.T.; Pergher, S.B.C.; Petkowicz, D.I.; dos Santos, J.H.Z. A new procedure for a zeolite synthesis from natural clays. Quim. Nova 2009, 32, 21–25. [Google Scholar] [CrossRef]
- Hu, C.; Yan, W.; Xu, R. Phase Transition Behavior of Zeolite Y under Hydrothermal Conditions. Acta Chim. Sin. 2017, 75, 679–685. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Liu, Z.; Wang, D. Insight into the Growth Mechanism of Low-Temperature Synthesis of High-Purity Lithium Slag-Based Zeolite A. Materials 2024, 17, 568. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Deng, H.; Li, L.; Zhang, Q.; Chen, G.; Sun, C.; He, H.; Yu, J. Fluoride-free and seed-free microwave-assisted hydrothermal synthesis of nanosized high-silica Beta zeolites for effective VOCs adsorption. Chem. Sci. 2023, 14, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Ke, G.; Shen, H.; Yang, P. Synthesis of X-Zeolite from Waste Basalt Powder and its Influencing Factors and Synthesis Mechanism. Materials 2019, 12, 3895. [Google Scholar] [CrossRef] [PubMed]
- Xing, E.; Shi, Y.; Xie, W.; Zhang, F.; Mu, X.; Shu, X. Size-controlled synthesis of MCM-49 zeolite from NaX for liquid-phase alkylation of benzene with ethylene. Microporous Mesoporous Mater. 2016, 236, 54–62. [Google Scholar] [CrossRef]
- Xing, E.; Shi, Y.; Zheng, A.; Zhang, J.; Gao, X.; Liu, D.; Xin, M.; Xie, W.; Zhang, F.; Mu, X.; et al. Transformation from NaA to MCM-49 Zeolite and Its Catalytic Alkylation Performance. Ind. Eng. Chem. Res. 2015, 54, 3123–3135. [Google Scholar] [CrossRef]
- Structure Commission of the International Zeolite Association (IZA-SC). Database of Zeolite Structures. Available online: https://www.iza-structure.org/databases/ (accessed on 14 March 2024).
- Maria Gomez, J.; Montes, I.; Diez, E.; Rodriguez, A. Mesoporous low silica X (MLSX) zeolite: Mesoporosity in loewenstein limit? Microporous Mesoporous Mater. 2022, 330, 111618. [Google Scholar] [CrossRef]
- GB 5749-2022; Standards for Drinking Water Quality. Standardization Administration of the People’s Republic of China, State Administration for Maket Regulation of China: Beijing, China, 2022.
- Iqbal, A.; Sattar, H.; Haider, R.; Munir, S. Synthesis and characterization of pure phase zeolite 4A from coal fly ash. J. Clean. Prod. 2019, 219, 258–267. [Google Scholar] [CrossRef]
- Sivalingam, S.; Sen, S. Optimization of synthesis parameters and characterization of coal fly ash derived microporous zeolite X. Appl. Surf. Sci. 2018, 455, 903–910. [Google Scholar] [CrossRef]
- Wang, P.; Sun, Q. Synthesis and characterisation of zeolite LTA with sheet structure. Micro Nano Lett. 2020, 15, 433–436. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, S.; Wei, S.; Zhang, S.; Zhang, J.; Gu, J. Green and efficient synthesis of mesoporous sodalite assisted by persulfate or peroxymonosulfate. Microporous Mesoporous Mater. 2022, 346, 112321. [Google Scholar] [CrossRef]
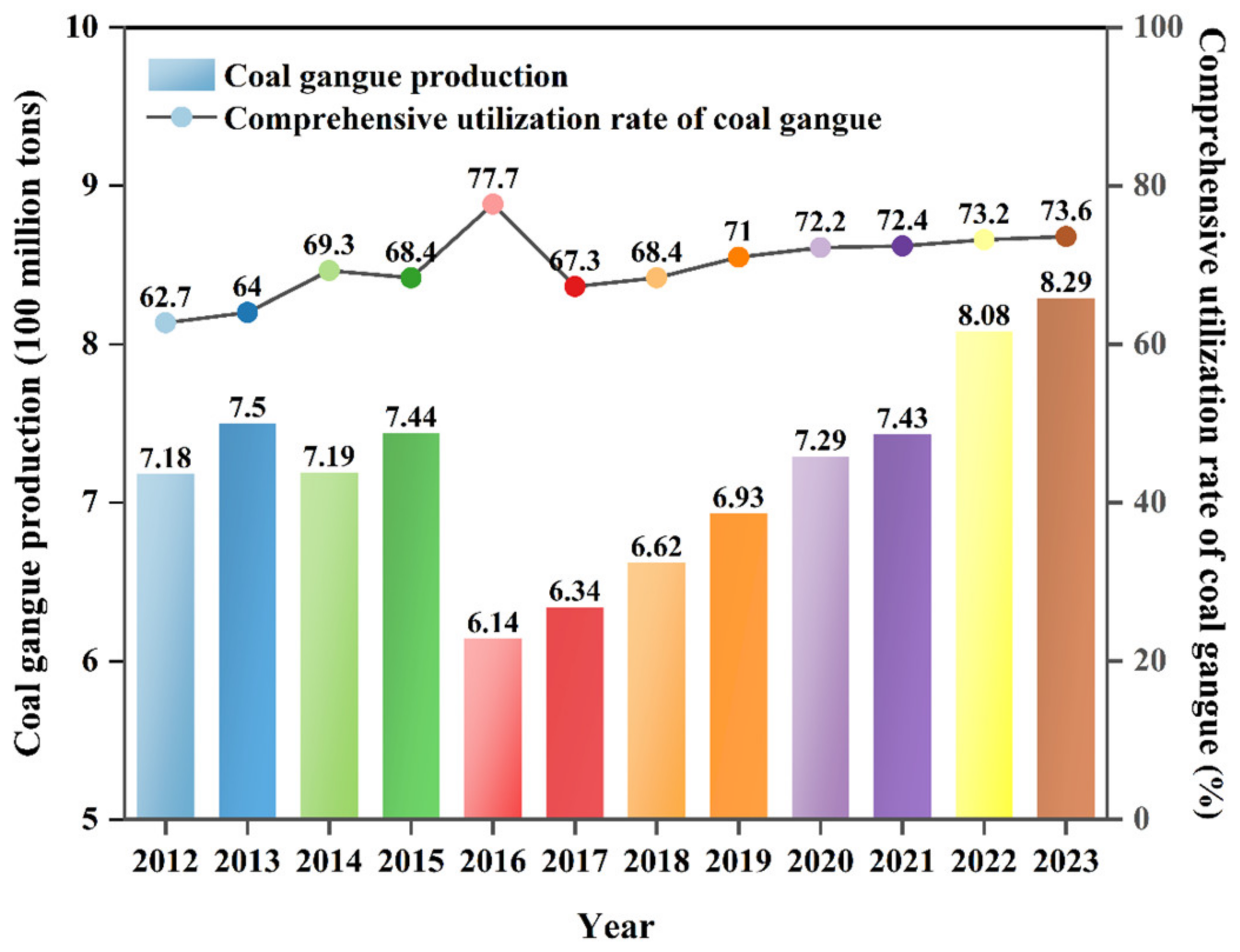
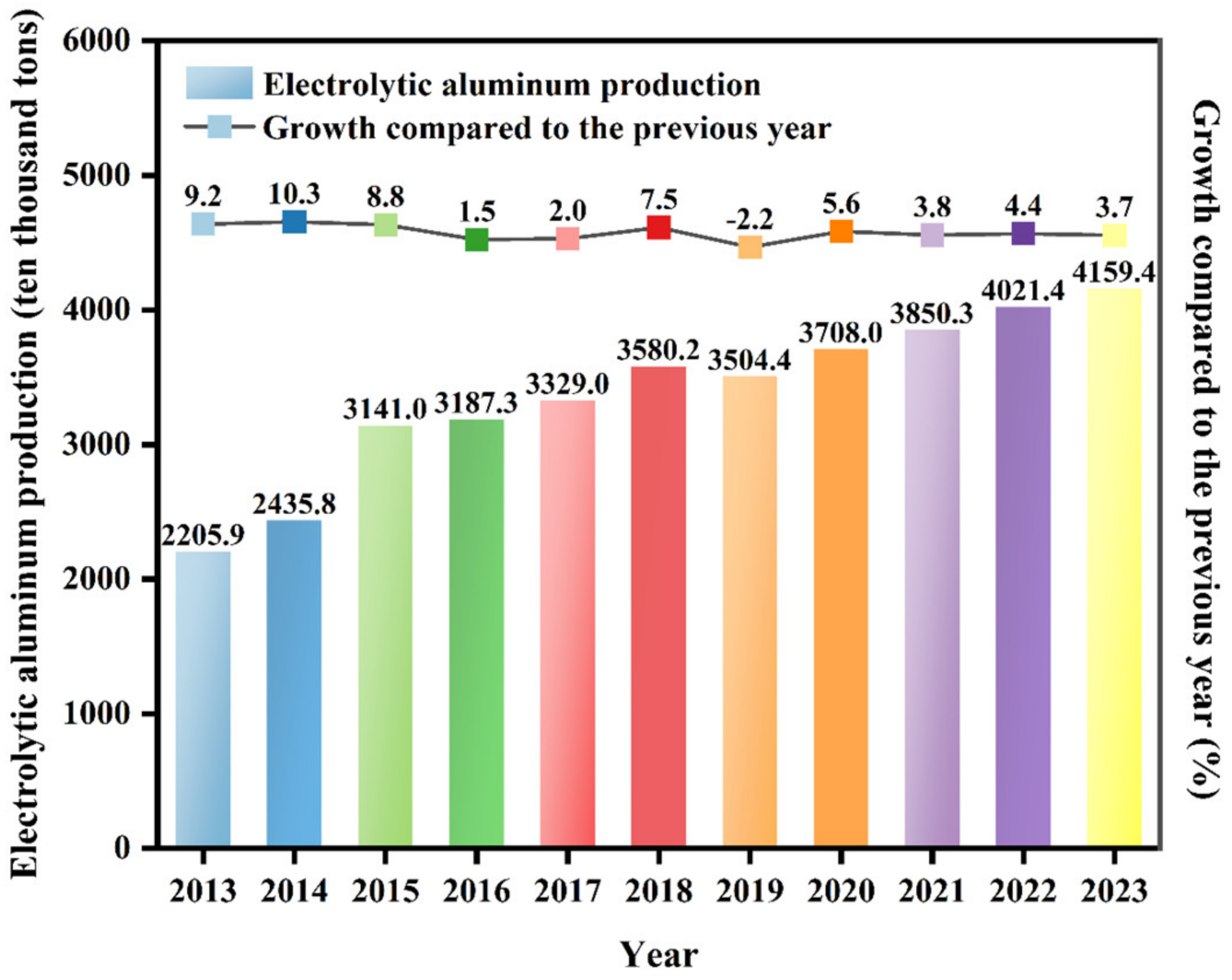
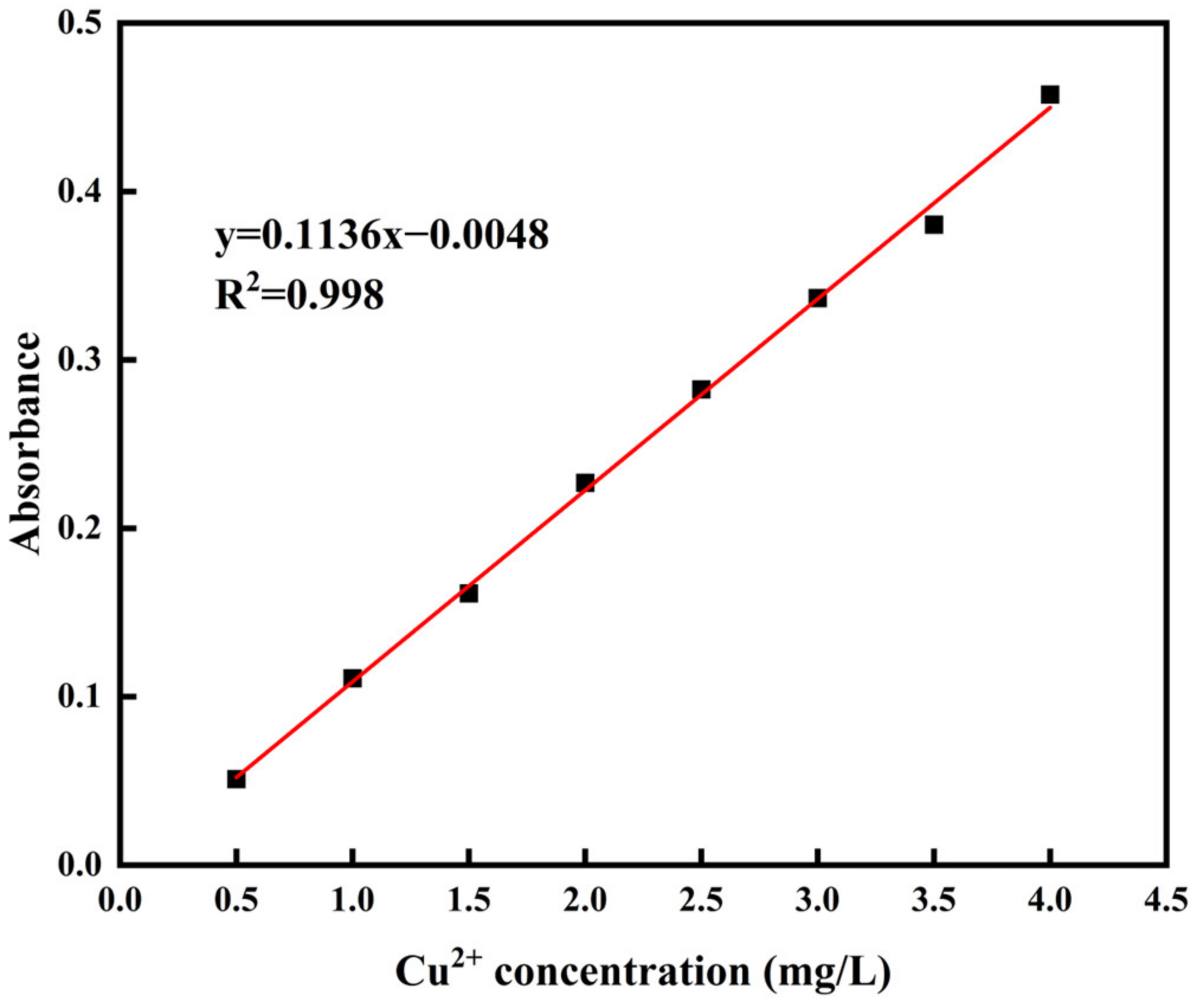
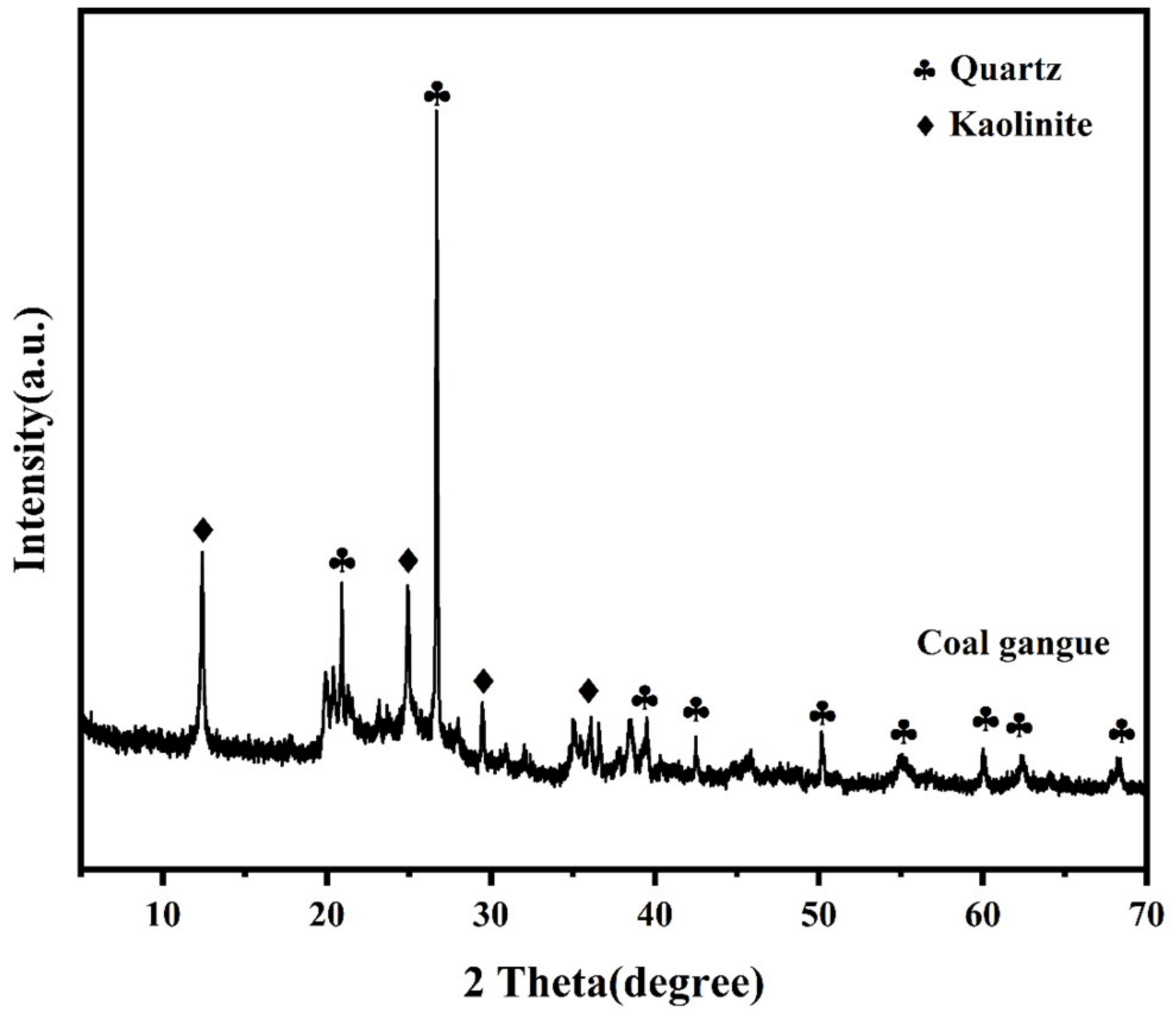





| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | MnO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 57.00 | 28.29 | 5.34 | 2.61 | 0.85 | 1.46 | 0.77 | 1.11 | 0.07 | 0.06 |
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | CuO | Na2O | NiO | ZnO |
|---|---|---|---|---|---|---|---|---|---|
| 1.56 | 79.08 | 0.61 | 2.33 | 1.20 | 11.43 | 0.28 | 1.59 | 0.16 | 0.01 |
| Al | O | N | S | P | Si | Mg | Zn |
|---|---|---|---|---|---|---|---|
| 41.686 | 41.233 | 10.686 | 2.062 | 1.960 | 1.571 | 0.802 | 0.007 |
| Number | m (Coal Gangue:NaOH:Aluminum Ash) | ||
|---|---|---|---|
| Coal Gangue | NaOH | Aluminum Ash | |
| w | 1:2 (alkali fusion) | — | |
| a | 1:2 (alkali fusion) | 0.25 | |
| b | 1:2 (alkali fusion) | 0.5 | |
| c | 1:2 (alkali fusion) | 1 | |
| Element | Na | Mg | Ca | Si | Al | Cu | |
|---|---|---|---|---|---|---|---|
| Sample | |||||||
| a | 13.953 | 8.953 | 3.953 | 22.324 | 14.927 | — | |
| b | 1.556 | 3.776 | 2.631 | 23.898 | 11.313 | 17.292 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Xu, W.; Cai, J.; Yu, Q.; Min, J. Study on the Synthesis of LTA-Type Molecular Sieves from Coal Gangue and Aluminum Ash and Its Adsorption Properties towards Cu2+. Crystals 2024, 14, 379. https://doi.org/10.3390/cryst14040379
Wang Q, Xu W, Cai J, Yu Q, Min J. Study on the Synthesis of LTA-Type Molecular Sieves from Coal Gangue and Aluminum Ash and Its Adsorption Properties towards Cu2+. Crystals. 2024; 14(4):379. https://doi.org/10.3390/cryst14040379
Chicago/Turabian StyleWang, Qingping, Wei Xu, Jingyi Cai, Qingbo Yu, and Jing Min. 2024. "Study on the Synthesis of LTA-Type Molecular Sieves from Coal Gangue and Aluminum Ash and Its Adsorption Properties towards Cu2+" Crystals 14, no. 4: 379. https://doi.org/10.3390/cryst14040379
APA StyleWang, Q., Xu, W., Cai, J., Yu, Q., & Min, J. (2024). Study on the Synthesis of LTA-Type Molecular Sieves from Coal Gangue and Aluminum Ash and Its Adsorption Properties towards Cu2+. Crystals, 14(4), 379. https://doi.org/10.3390/cryst14040379






