The Influence of In3+ on the Crystal Growth and Visible Band Photorefraction of Uranium-Doped Lithium Niobate Single Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Growth
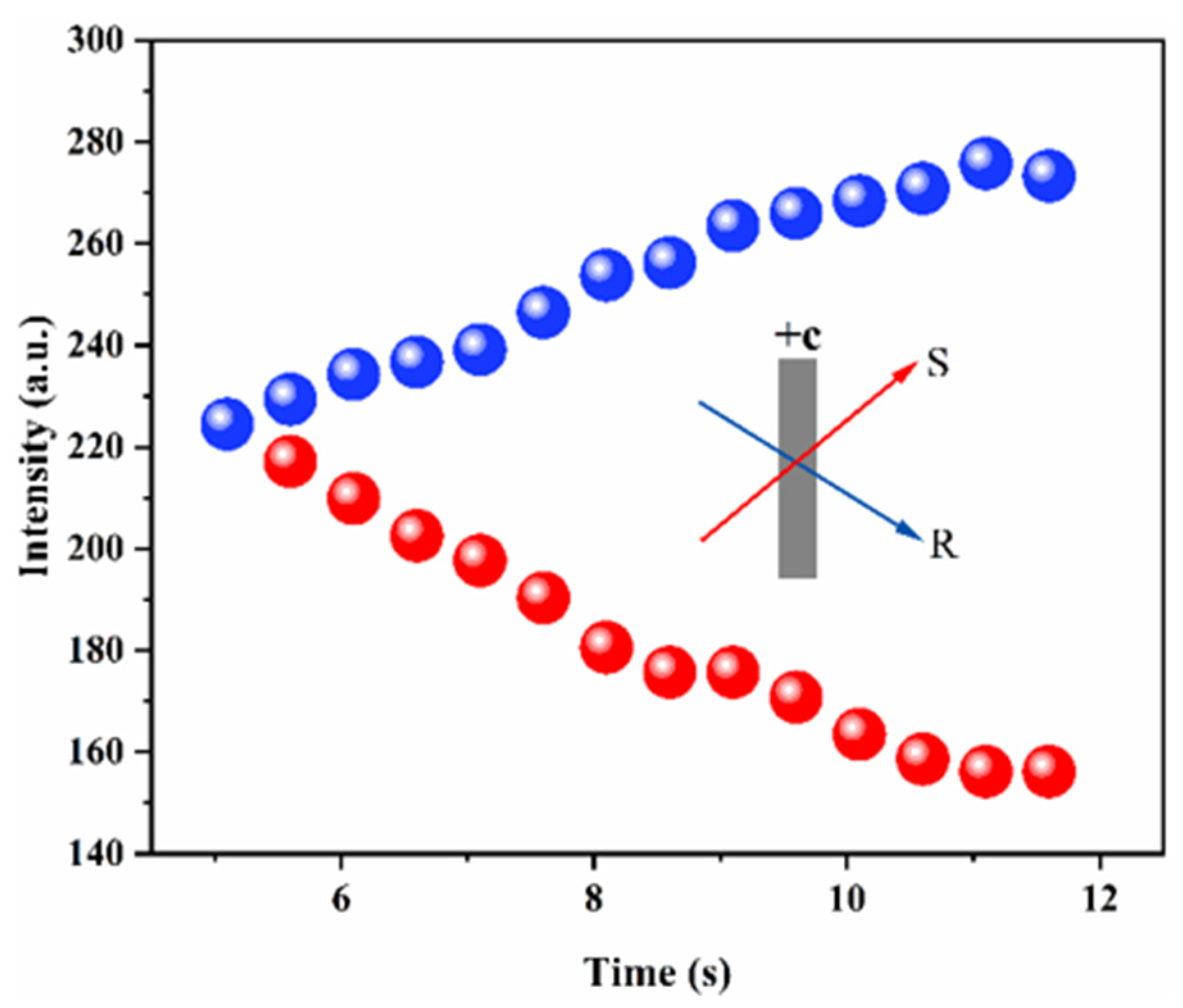
3.2. Photorefractive Properties
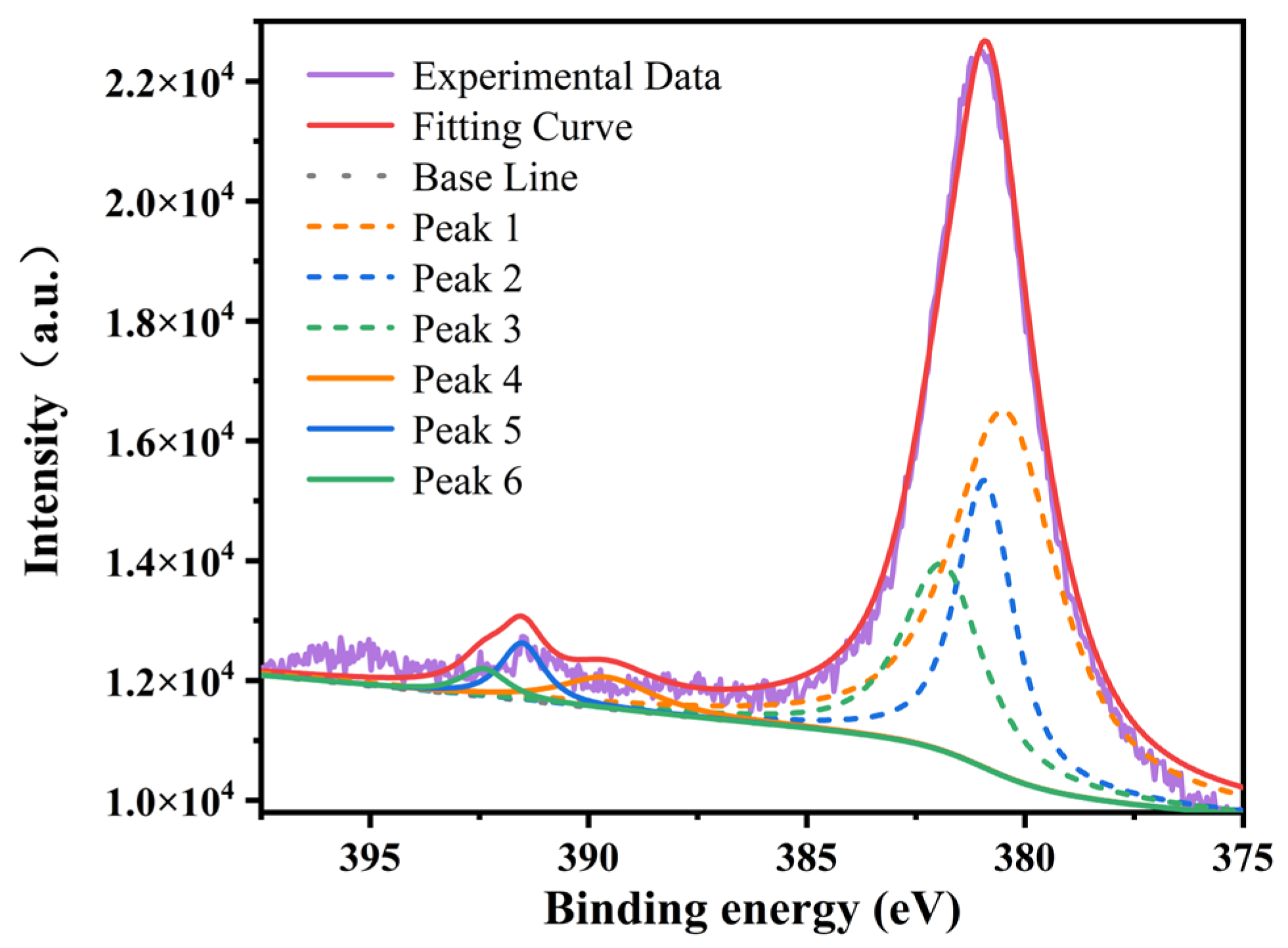
3.3. Valence States of Uranium Ions
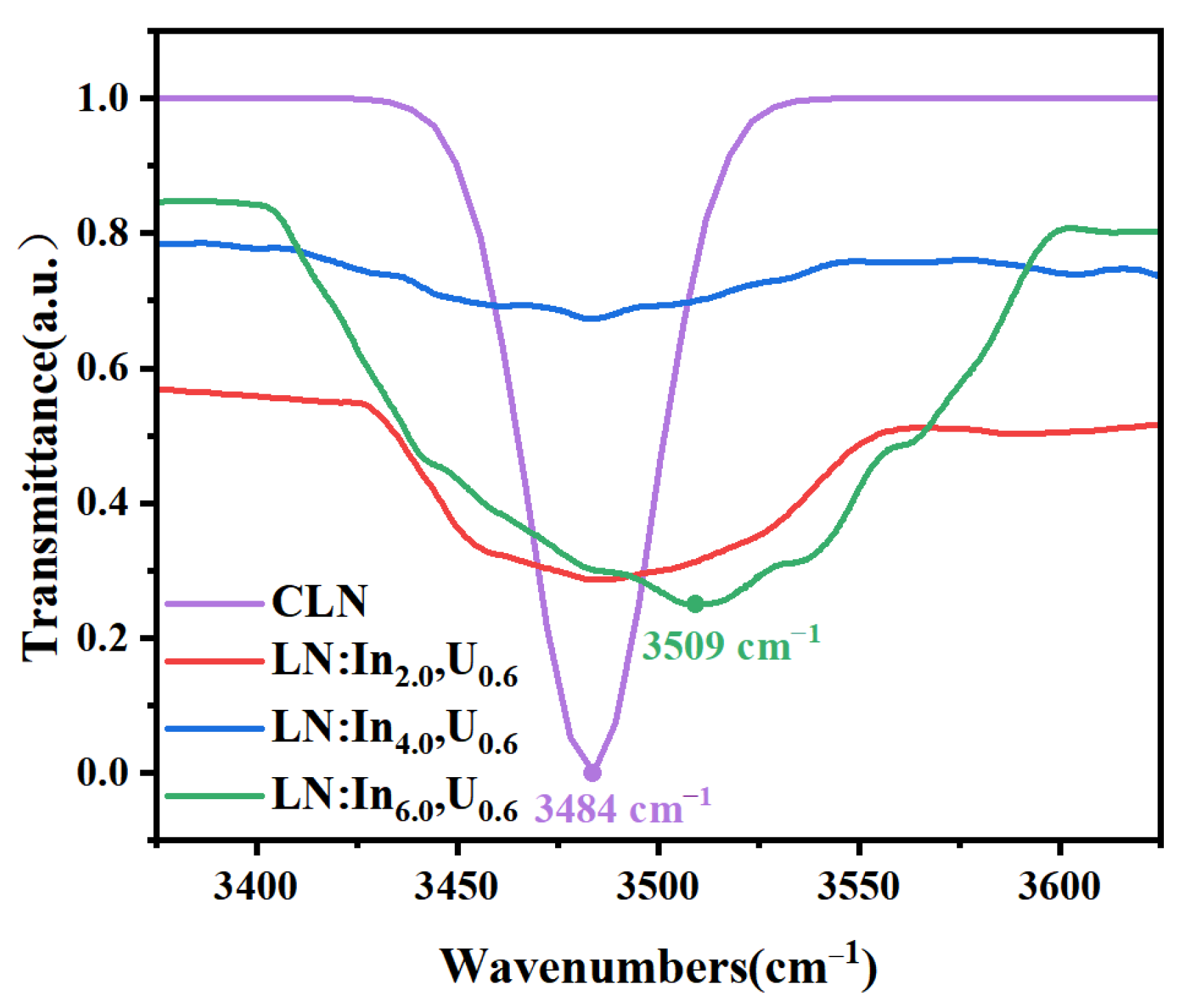
3.4. Real Threshold Concentration of Indium in LN: In, U
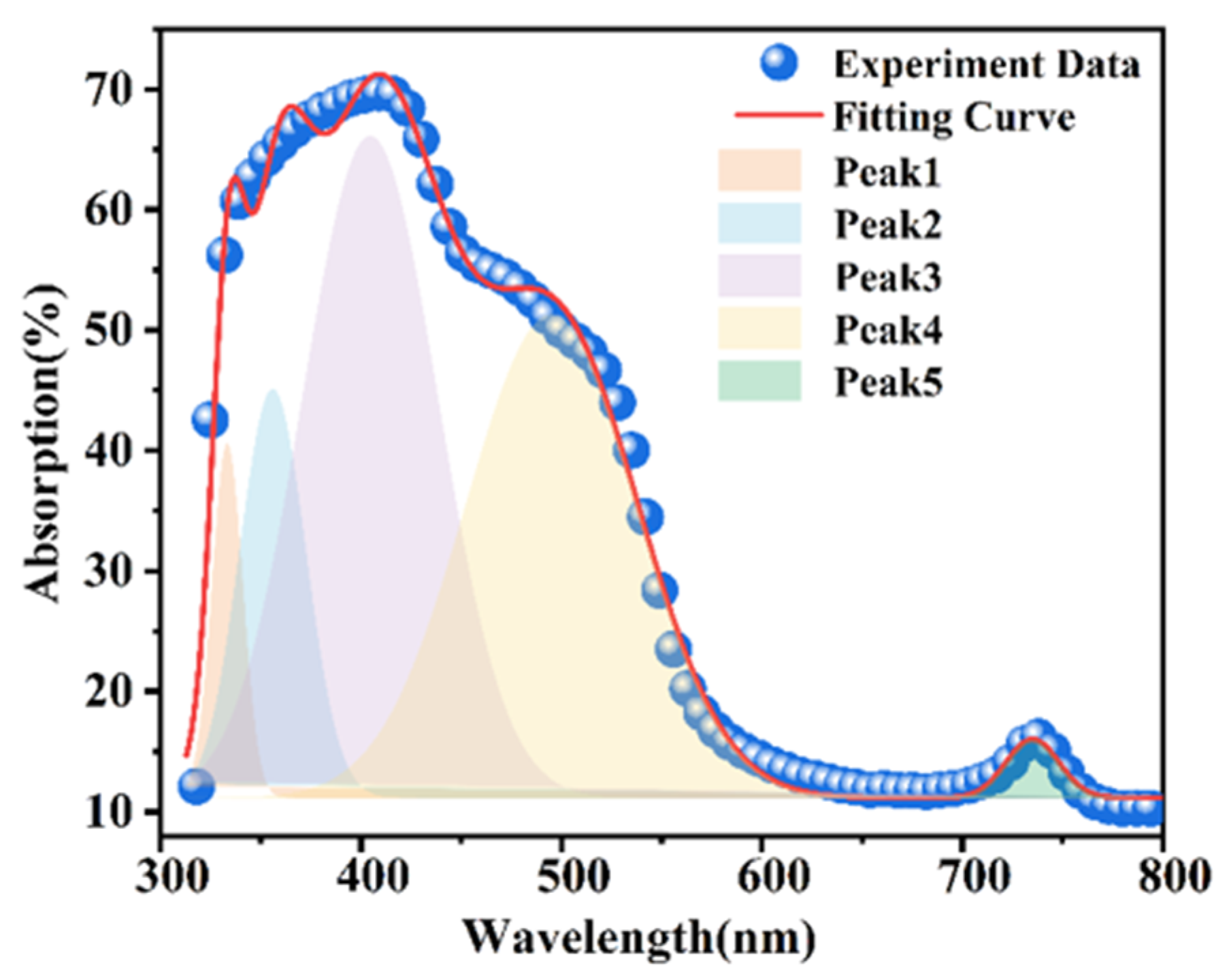
3.5. Absorption Difference Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, Y.; Bo, F.; Wang, W.; Zheng, D.; Liu, H.; Zhang, G.; Rupp, R.; Xu, J. Recent Progress in Lithium Niobate: Optical Damage, Defect Simulation, and On-Chip Devices. Adv. Mater. 2019, 32, 1806452. [Google Scholar] [CrossRef] [PubMed]
- Broderick, N.G.R.; Bratfalean, R.T.; Monro, T.M.; Richardson, D.J. Temperature and wavelength tuning of second-, third-, and fourth-harmonic generation in a two-dimensional hexagonally poled nonlinear crystal. J. Opt. Soc. Am. B 2002, 19, 2263–2272. [Google Scholar] [CrossRef]
- Wang, F.; Sun, D.; Liu, Q.; Song, Y.; Zhang, F.; Zhou, W.; Sang, Y.; Wang, D.; Liu, H. Growth of Large Size Near-Stoichiometric Lithium Niobate Single Crystals with Low Coercive Field for Manufacturing High Quality Periodically Poled Lithium Niobate. Opt. Mater. 2022, 125, 112058. [Google Scholar] [CrossRef]
- Bazzan, M.; Sada, C. Optical Waveguides in Lithium Niobate: Recent Developments and Applications. Appl. Phys. Rev. 2015, 2, 040603. [Google Scholar] [CrossRef]
- Feng, H.; Ge, T.; Guo, X.; Wang, B.; Zhang, Y.; Chen, Z.; Zhu, S.; Zhang, K.; Sun, W.; Huang, C.; et al. Integrated Lithium Niobate Microwave Photonic Processing Engine. Nature 2024, 627, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Boes, A.; Chang, L.; Langrock, C.; Yu, M.; Zhang, M.; Lin, Q.; Lončar, M.; Fejer, M.; Bowers, J.; Mitchell, A. Lithium Niobate Photonics: Unlocking the Electromagnetic Spectrum. Science 2023, 379, eabj4396. [Google Scholar] [CrossRef] [PubMed]
- Leah, B. Now Entering, Lithium Niobate Valley. Available online: https://otd.harvard.edu/news/now-entering-lithium-niobate-valley (accessed on 22 February 2024).
- Tian, T.; Chen, Y.; Zhang, J.; Wang, S.; Yuan, W.; Liu, H.; Chu, Y.; Mao, C.; Xu, W.; Zheng, D. Photorefraction of uranium-doped lithium niobate crystals at multiple visible wavelengths. CrystEngComm 2023, 25, 1207. [Google Scholar] [CrossRef]
- Wang, S.; Shan, Y.; Zheng, D.; Liu, S.; Bo, F.; Liu, H.; Kong, Y.; Xu, J. The real-time dynamic holographic display of LN:Bi,Mg crystals and defect-related electron mobility. Opto-Electron. Adv. 2022, 5, 210135. [Google Scholar] [CrossRef]
- Kling, A.; Marques, J.G. Unveiling the Defect Structure of Lithium Niobate with Nuclear Methods. Crystals 2021, 11, 501. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Peng, C.; Lu, Z.; Luo, X.; Xue, D. Microstructure and Defect Characteristics of Lithium Niobate with Different Li Concentrations. Inorg. Chem. Front. 2021, 8, 4006–4013. [Google Scholar] [CrossRef]
- Phillips, W.; Amodei, J.J.; Staebler, D.L. Optical and holographic storage properties of transition metal doped lithium niobate. RCA Rev. 1972, 33, 94–109. [Google Scholar]
- Mcmillen, D.; Hudson, T.; Wagner, J.; Singleton, J. Holographic recording in specially doped lithium niobate crystals. Opt. Express 1998, 2, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liu, S.; Kong, Y.; Chen, S.; Rupp, R.; Xu, J. Fast photorefractive response of vanadium-doped lithium niobate in the visible region. Opt. Lett. 2012, 37, 1841–1843. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Kong, Y.; Liu, S.; Li, W.; Wu, L.; Chen, S.; Xu, J. Photorefraction of molybdenum-doped lithium niobate crystals. Opt. Lett. 2012, 37, 2679–2681. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Yuan, W.; Liu, W.; Shen, H.; Zhang, Y.; Chu, Y.; Ma, Y.; Xu, J. Crystal Growth and Spectroscopic Properties of Uranium Dioxide Doped LiNbO3 with Multiband Absorption. J. Cryst. Growth 2021, 565, 126132. [Google Scholar] [CrossRef]
- Kong, Y.; Wen, J.; Wang, H. New doped lithium niobate crystal with high resistance to photorefraction-LiNbO3: In. Appl. Phys. Lett. 1995, 66, 280–281. [Google Scholar] [CrossRef]
- Zhang, T.; Geng, T.; Sun, W. Effect of In ions on photorefractive properties in Fe: In: LiNbO3 crystals. Proc. SPIE 2007, 6595, 65950P-2. [Google Scholar]
- Zhu, L.; Zheng, D.; Liu, H.; Saeed, S.; Wang, S.; Liu, S.; Chen, S.; Kong, Y.; Xu, J. Enhanced photorefractive properties of indium co-doped LiNbO3: Mo crystals. AIP Adv. 2018, 8, 095316. [Google Scholar] [CrossRef]
- Tian, T.; Yan, X.; Kong, Y.; Liu, H.; Zheng, D.; Liu, S.; Chen, S.; Xu, J.; Xu, J. Improvement in the Photorefractive Response Speed and Mechanism of Pure Congruent Lithium Niobate Crystals by Increasing the Polarization Current. Crystals 2017, 7, 368. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.M.; Shin, J.; Char, K. LaInO3/BaSnO3 Polar Interface on MgO Substrates. APL Mater. 2018, 6, 096104. [Google Scholar] [CrossRef]
- Lebib, A.; Ben Amara, E.; Beji, L. Structural and Luminescent Characteristics of Porous GaAs. J. Lumin. 2017, 188, 337–341. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Mao, C.; Chen, M.; Chu, Y.; Li, Y. Structure, Luminescence Properties and Anti-Thermal Quenching of a Novel Eu3+-Activated Red Phosphor Based on the Negative Thermal Expansion Material In0.5Sc1.5(MoO4)3. J. Alloys Compd. 2024, 973, 172887. [Google Scholar]
- Zheng, D.; Wang, W.; Wang, S.; Qu, D.; Liu, H.; Kong, Y.; Liu, S.; Chen, S.; Rupp, R.; Xu, J. Real-Time Dynamic Holographic Display Realized by Bismuth and Magnesium Co-Doped Lithium Niobate. Appl. Phys. Lett. 2019, 114, 241903. [Google Scholar] [CrossRef]
- Kushwaha, S.; Sreedhar, B.; Padmaja, P. XPS, EXAFS, and FTIR as tools to probe the unexpected adsorption-coupled reduction of U(VI) to U(V) and U(IV) on borassus flabellifer-based adsorbents. ACS J. Surf. Colloids 2012, 28, 16038–16048. [Google Scholar] [CrossRef]
- Fu, X.; Zhong, Y.; Wang, X.; Liu, K.; Zhao, Z.; Liu, C. Investigation of surface oxidation of uranium monocarbide by in situ XPS technique. Acta. Chim. Sinica 2005, 3, 648–652. [Google Scholar]
- Allen, G.C.; Tucker, P.M.; Tyler, J.W. The behaviour of uranium oxides in low partial pressures of O2 studied using X-ray photoelectron spectroscopy. Vacuum 1982, 32, 481–486. [Google Scholar] [CrossRef]
- Bagus, P.S.; Nelin, C.J.; Ilton, E.S. Theoretical modeling of the uranium 4f XPS for U (VI) and U(IV) oxides. J. Chem. Phys. 2013, 139, 1549. [Google Scholar]
- Liu, K.; Luo, L.; Luo, L.; Long, Z.; Hong, Z.; Yang, H.; Wu, S. Initial oxidation behaviors of nitride surfaces of uranium by XPS analysis. Appl. Surf. Sci. 2013, 280, 268–272. [Google Scholar] [CrossRef]
- Donald, S.B.; Davisson, M.L.; Dai, Z.R.; Roberts, S.K.; Nelson, A.J. Relative impact of H2O and O2 in the oxidation of UO2 powders from 50 to 300 °C. J. Nucl. Mater. 2017, 496, 353–361. [Google Scholar] [CrossRef]
- Mårtensson, N.; Malmquist, P.Å.; Svensson, S.; Johansson, B. The electron spectrum of UF6 recorded in the gas phase. J. Chem. Phys. 1984, 80, 5458. [Google Scholar] [CrossRef]
- McEachern, R.J.; Taylor, P. A review of the oxidation of uranium dioxide at temperatures below 400 °C. J. Nucl. Mater. 1998, 254, 87–121. [Google Scholar]
- Skomurski, F.N.; Shuller, L.C.; Ewing, R.C.; Becker, U. Corrosion of UO2 and ThO2: A quantum-mechanical investigation. J. Nucl. Mater. 2008, 375, 290–310. [Google Scholar] [CrossRef]
- Kong, Y.; Deng, J.; Zhang, W.; Wen, J.; Zhang, G.; Wang, H. OH− Absorption Spectra in Doped Lithium Niobate Crystals. Phys. Lett. A 1994, 196, 128–132. [Google Scholar] [CrossRef]
- Smyth, D.M. Defects and transport in LiNbO3. Ferroelectrics 1983, 50, 93–102. [Google Scholar] [CrossRef]
- Hargreaves, W.A. High-Resolution measurements of absorption, fluorescence, and crystal-field splittings of solutions of divalent, trivalent, and tetravalent Uranium ions in fluoride crystals. Phys. Rev. 1967, 156, 331–342. [Google Scholar]
- Ashkin, A.; Boyd, G.D.; Dziedzic, J.M.; Smith, R.G.; Ballman, A.A.; Levinstein, J.J.; Nassau, K. Optical-induced refractive index inhomogeneities in LiNbO3 and LiTaO3. Appl. Phys. Lett. 1966, 9, 72–74. [Google Scholar] [CrossRef]
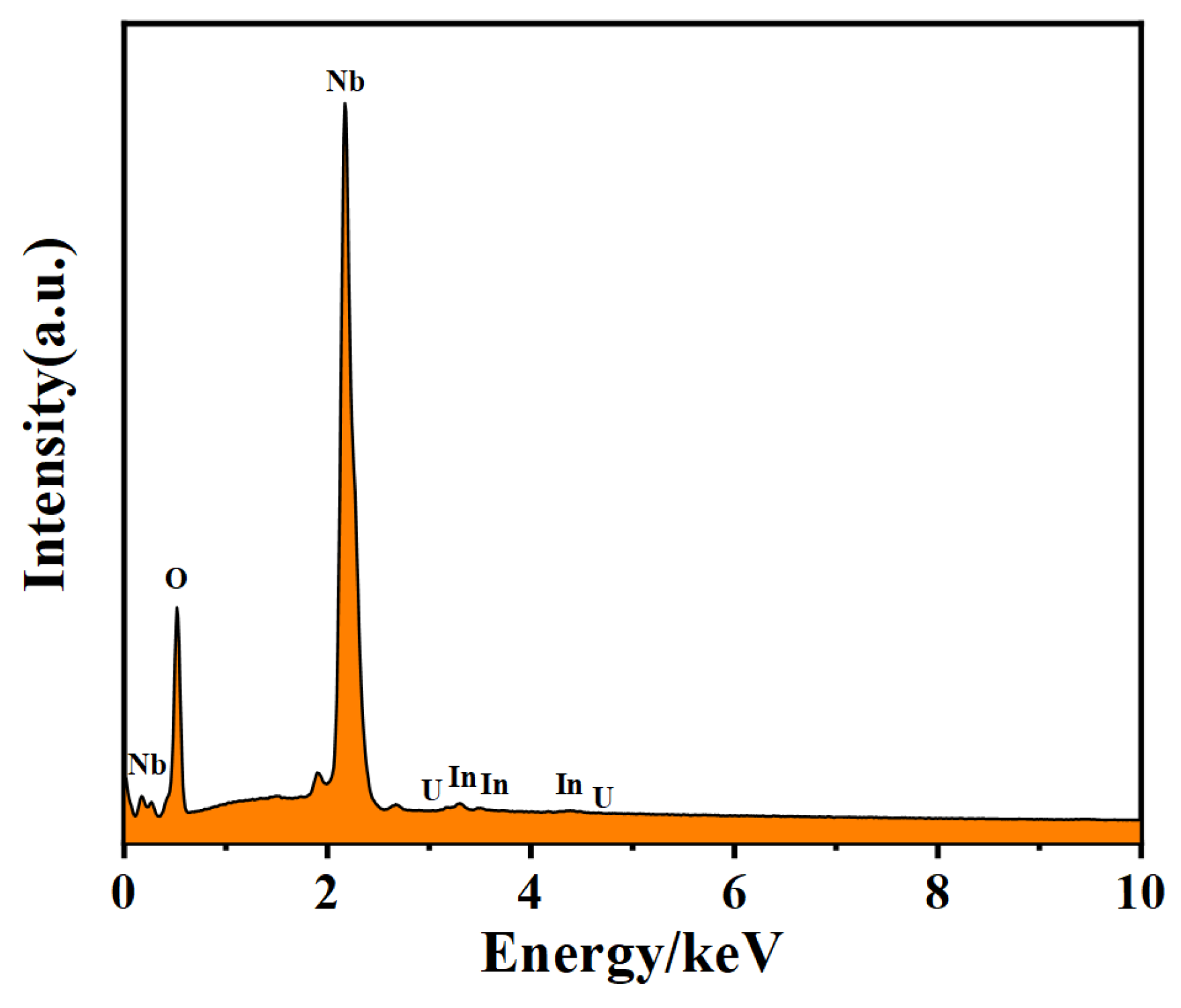
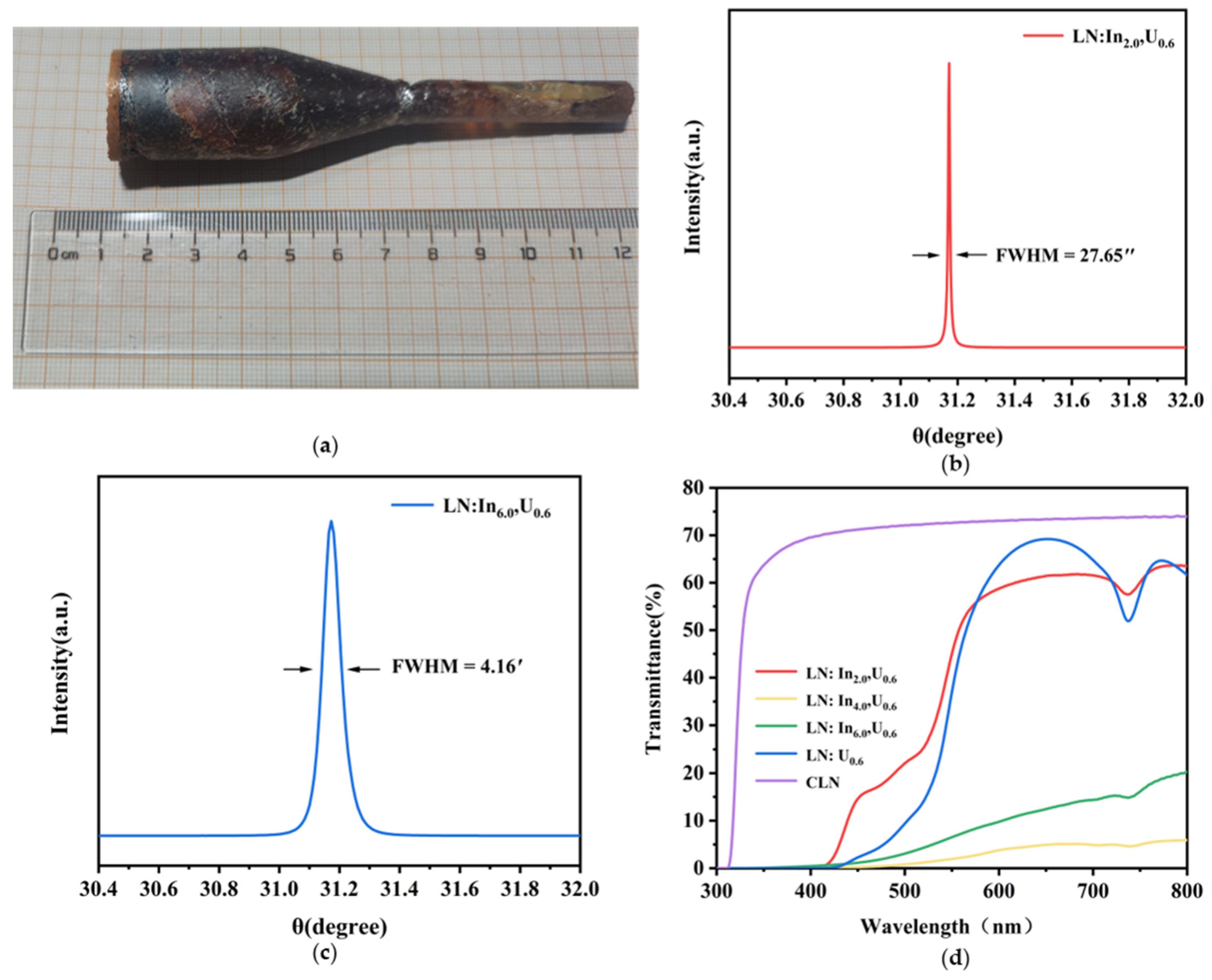

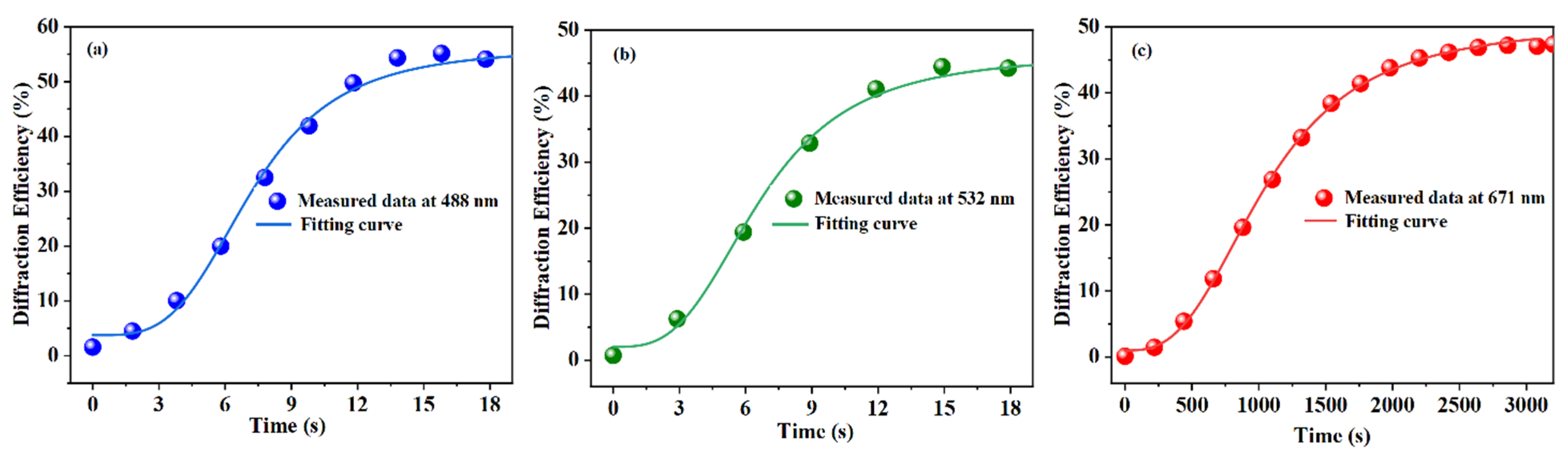
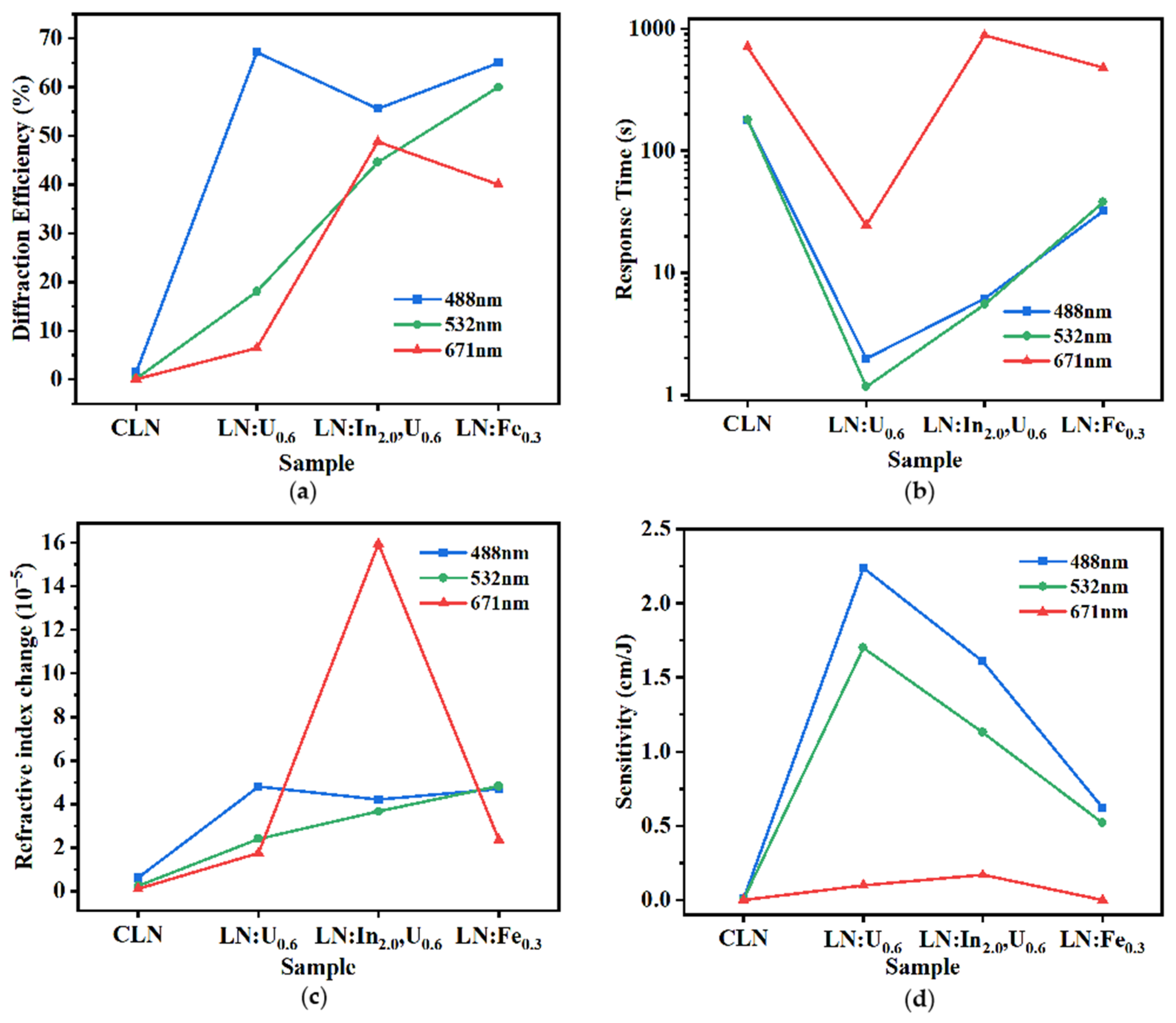
| Sample | LN: In2.0, U0.6 | LN: In4.0, U0.6 | LN: In6.0, U0.6 | |||
|---|---|---|---|---|---|---|
| Ion | In | U | In | U | In | U |
| Effective segregation coefficient (keff) | 1.00 | 0.98 | 0.93 | 0.89 | 0.84 | 0.84 |
| Formula | LN: In2.0, U0.6 |
|---|---|
| Space group | R3c |
| a (Å) | 5.15847 (8) |
| b (Å) | 5.15852 (2) |
| c (Å) | 13.87605 (12) |
| A (°) | 90 |
| β (°) | 90 |
| γ (°) | 120 |
| V (Å3) | 319.7733 (16) |
| Rp (%) | 4.699% |
| Rwp (%) | 6.354% |
| Gof | 1.973 |
| LN: In2.0, U0.6 | Site | x | Y | z | Ueq | Occupancy |
|---|---|---|---|---|---|---|
| Li1 | Li | 0.00000 | 0.00000 | 0.27901 | 0.02912 | 0.968 |
| In | Li | 0.00000 | 0.00000 | 0.27901 | 0.02912 | 0.021 |
| Nb1 | Nb | 0.00000 | 0.00000 | 0.00000 | 0.00799 | 0.983 |
| Nb2 | Li | 0.00000 | 0.00000 | 0.27901 | 0.02912 | 0.011 |
| O | O | 0.32090 | 0.04151 | 0.89544 | 0.01405 | 1.000 |
| U | Nb | 0.00000 | 0.00000 | 0.00000 | 0.00799 | 0.006 |
| Fitting Parameters | U4f7/2 | U4f5/2 | ||
|---|---|---|---|---|
| Binding Energy (eV) | FWHM | Binding Energy (eV) | FWHM | |
| U4+ | 380.53 | 2.9 | 389.63 | 6.4 |
| U5+ | 380.93 | 1.5 | 391.53 | 1.3 |
| U6+ | 381.93 | 2.1 | 392.43 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, T.; Xu, W.; Fang, C.; Chen, Y.; Liu, H.; Chu, Y.; Shen, H.; Xu, J. The Influence of In3+ on the Crystal Growth and Visible Band Photorefraction of Uranium-Doped Lithium Niobate Single Crystals. Crystals 2024, 14, 380. https://doi.org/10.3390/cryst14040380
Tian T, Xu W, Fang C, Chen Y, Liu H, Chu Y, Shen H, Xu J. The Influence of In3+ on the Crystal Growth and Visible Band Photorefraction of Uranium-Doped Lithium Niobate Single Crystals. Crystals. 2024; 14(4):380. https://doi.org/10.3390/cryst14040380
Chicago/Turabian StyleTian, Tian, Wenjie Xu, Chenkai Fang, Yuheng Chen, Hongde Liu, Yaoqing Chu, Hui Shen, and Jiayue Xu. 2024. "The Influence of In3+ on the Crystal Growth and Visible Band Photorefraction of Uranium-Doped Lithium Niobate Single Crystals" Crystals 14, no. 4: 380. https://doi.org/10.3390/cryst14040380
APA StyleTian, T., Xu, W., Fang, C., Chen, Y., Liu, H., Chu, Y., Shen, H., & Xu, J. (2024). The Influence of In3+ on the Crystal Growth and Visible Band Photorefraction of Uranium-Doped Lithium Niobate Single Crystals. Crystals, 14(4), 380. https://doi.org/10.3390/cryst14040380







