Crystallization of Secondary Phase on Super-Duplex Stainless Steel SAF2507: Advanced Li-Ion Battery Case Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
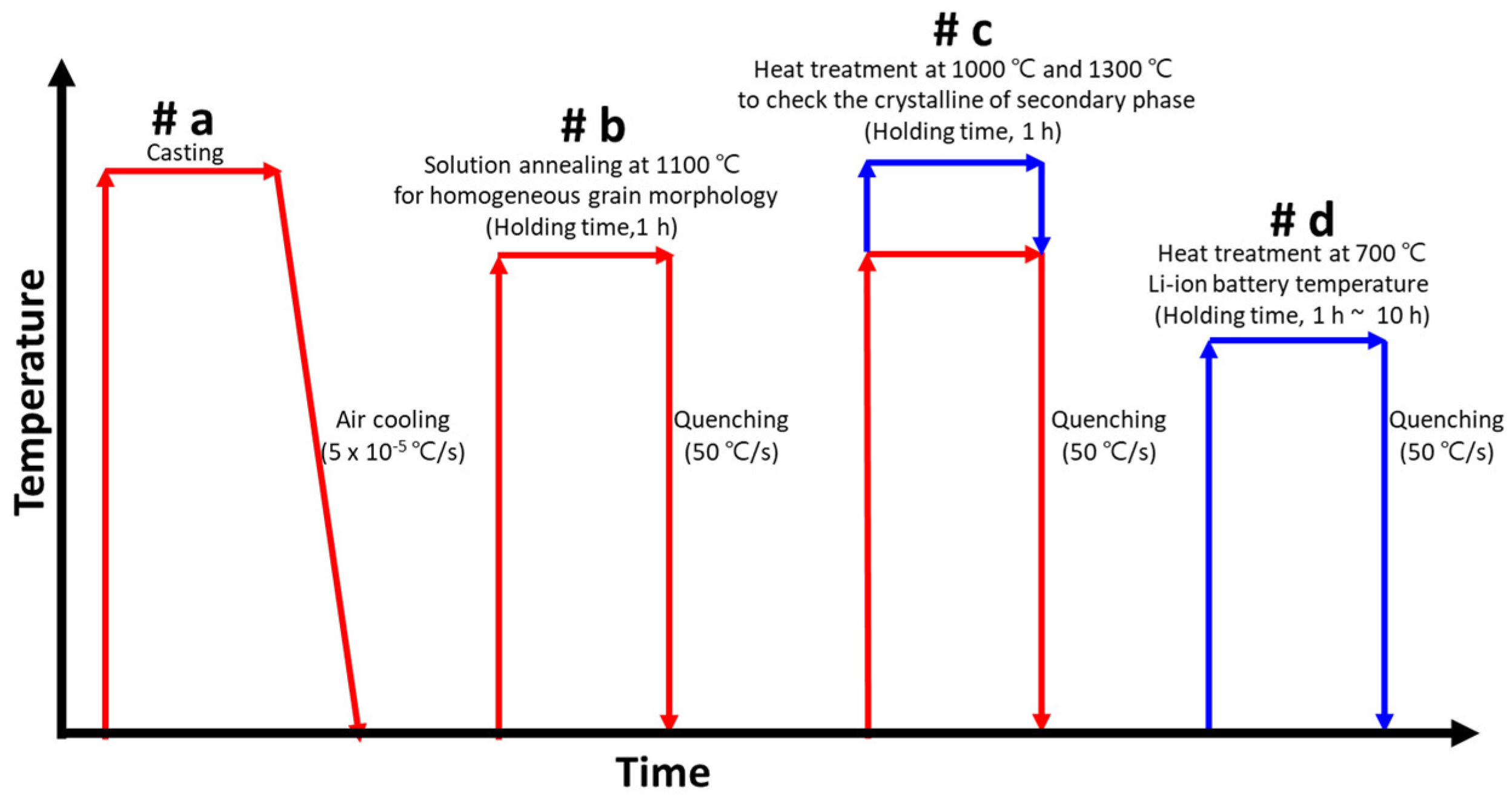
2.2. Heat Treatment
2.3. Microstructure and Phase
2.4. Chemical Composition
3. Results
3.1. Heat Treatment
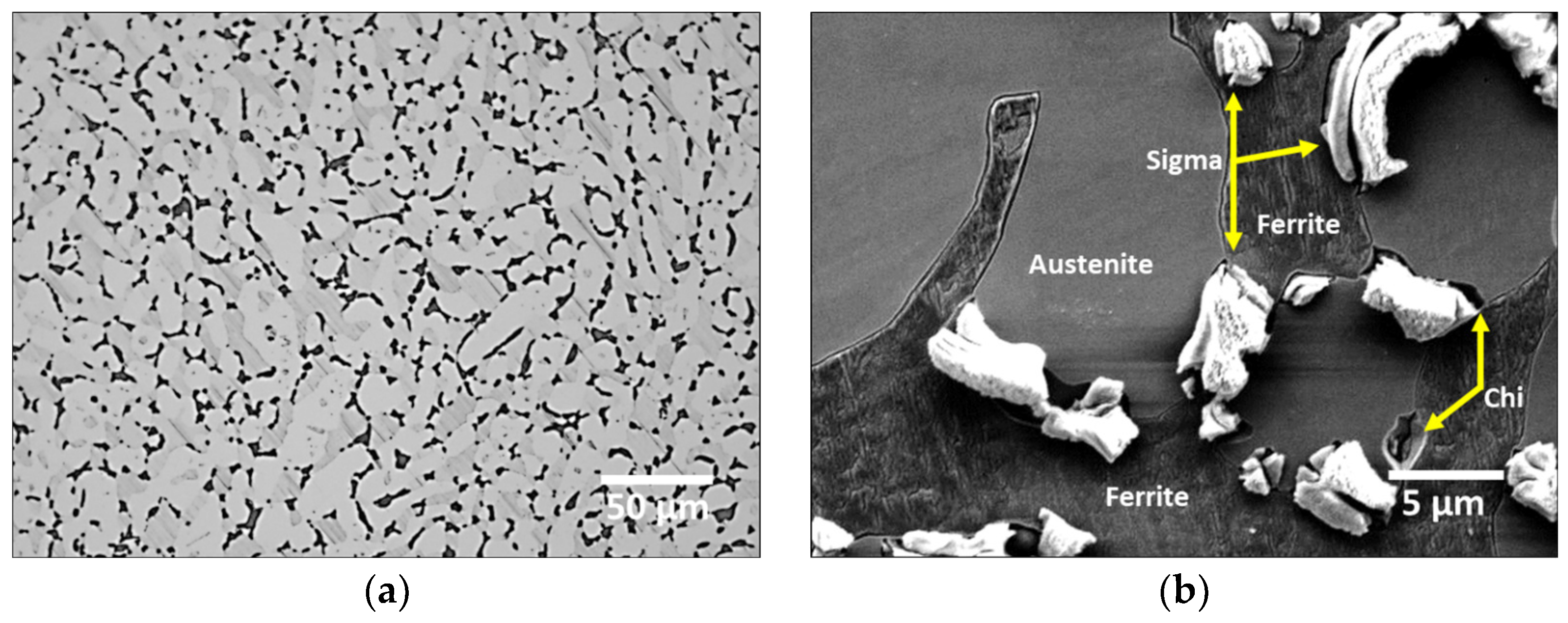
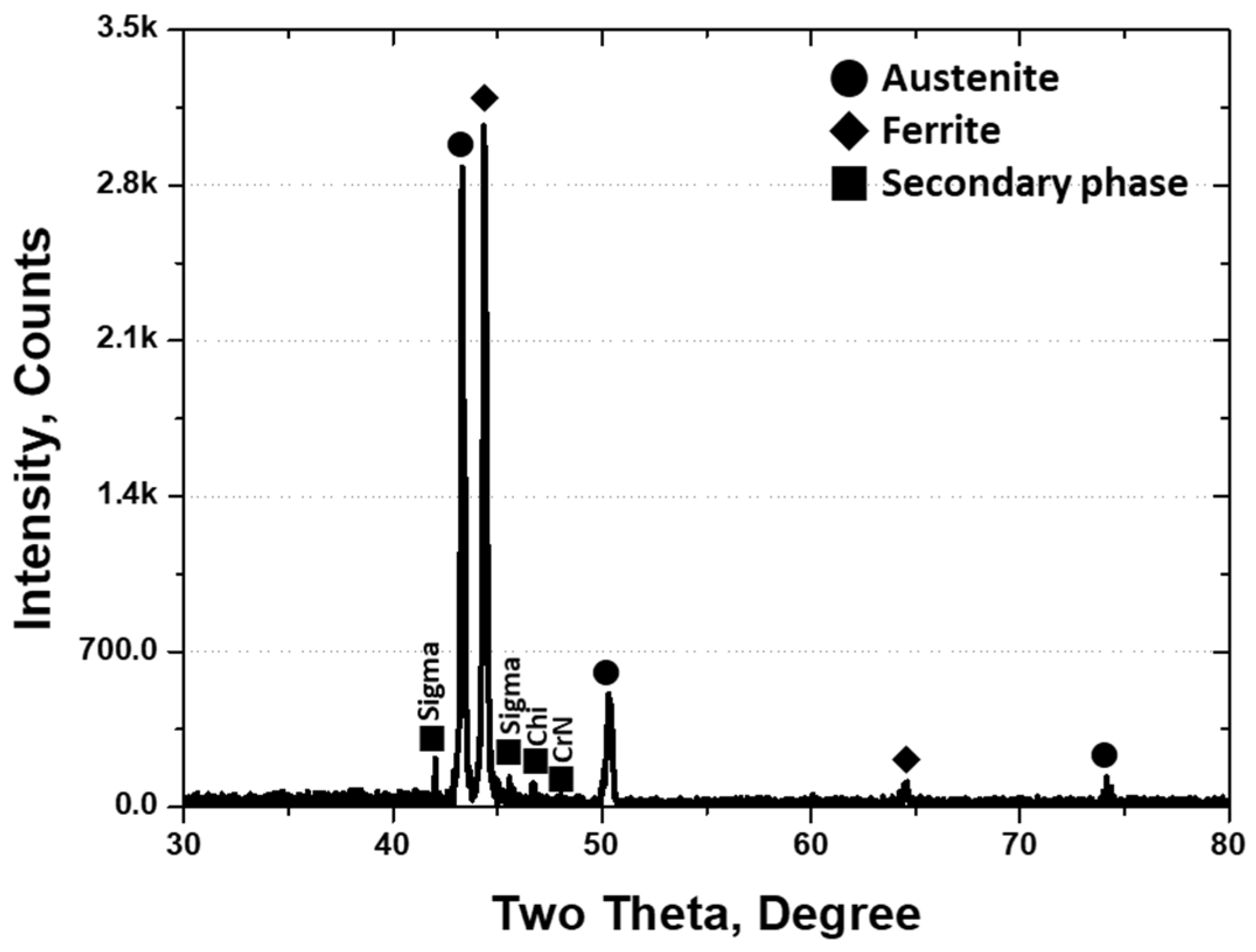
3.2. Crystallization of Secondary Phase
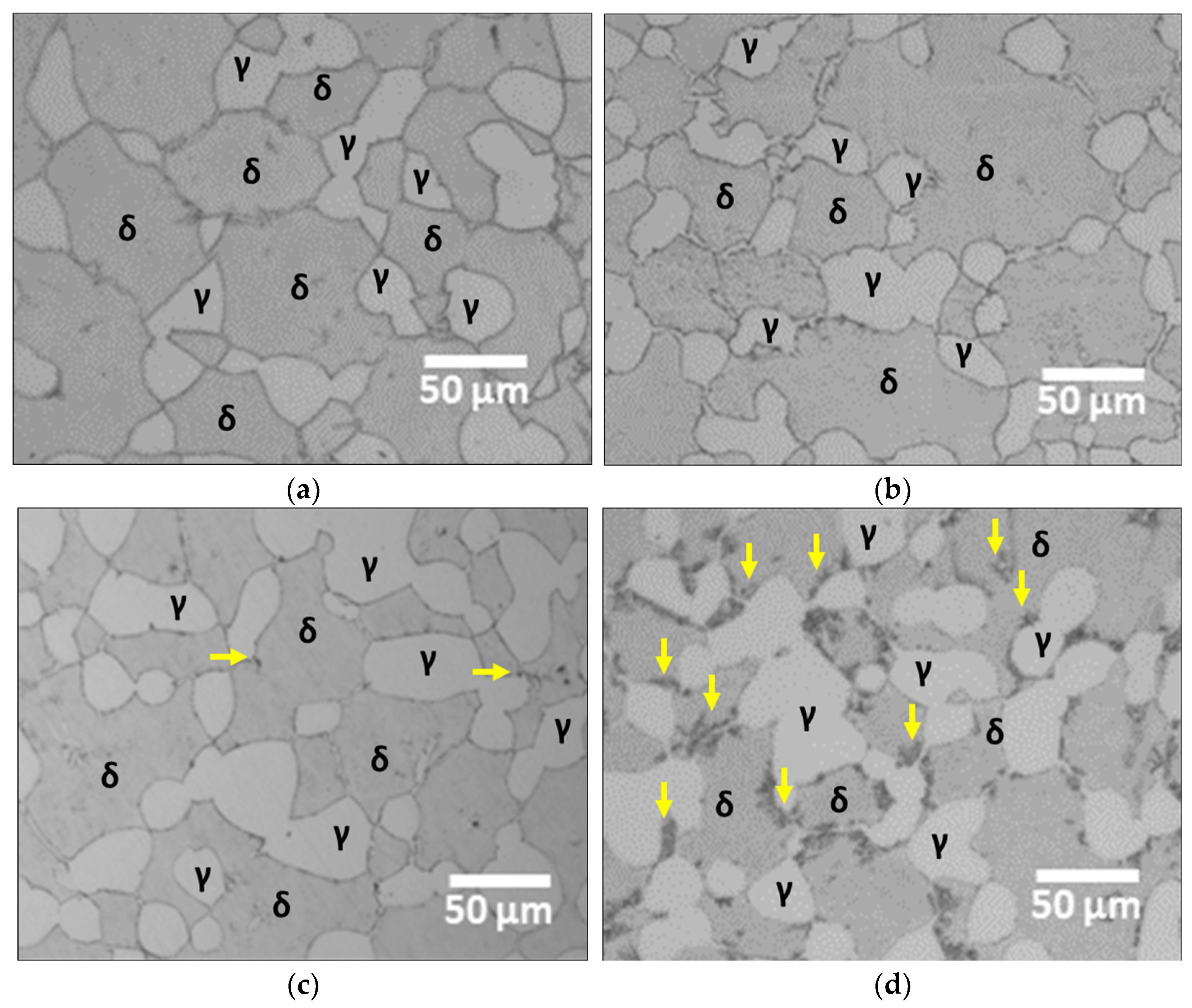
3.3. Secondary Phase at 700 °C
3.4. Crystallization Behavior of the Secondary Phase
4. Discussion
5. Conclusions
- SAF2507 precipitated at 1000 °C. Secondary-phase crystallization was initiated when the transformation of austenite from ferrite occurred at the austenite boundary. Because ferrite possessed a higher Cr content (26.6 wt.%) than austenite (23.3%), the transformation of ferrite to austenite resulted in the transfer of Cr (>3%) to ferrite. This led to the crystallization of the secondary phase owing to the unallocated Cr content. The crystallized secondary phase grew along the austenite boundaries.
- SAF2507 was heat-treated over time at the operating temperature of Li-ion batteries (700 °C). After 5 h of treatment, secondary-phase crystallization was observed; this reached 8.1% after 10 h of heat treatment. The crystallization of the secondary phase was initiated at the boundaries of austenite owing to the differences in crystallization and the high energy required for phase transformation.
- SAF2507 exhibited excellent strength and corrosion resistance. The crystallization of the secondary phase occurred after heat treatment for 5 h at 700 °C. As the operating time of a Li-ion battery is less than 5 h, secondary-phase crystallization is unlikely to occur. The safety of using Li-ion batteries at operating times beyond 5 h at 700 °C due to improved performance is expected to be maintained before the crystallization of the secondary phase occurs. Therefore, the use of SAF2507 as the material for Li-ion battery cases has the potential to enhance safety against high-temperature fever and damage.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mayyas, A.; Steward, D.; Mann, M. The Case for Recycling: Overview and Challenges in the Material Supply Chain for Automotive Li-Ion Batteries. Sustain. Mater. Technol. 2019, 19, e00087. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, X. Li-Ion Battery Individual Electrode State of Charge and Degradation Monitoring Using Battery Casing through Auto Curve Matching for Standard CCCV Charging Profile. Appl. Energy 2022, 321, 119367. [Google Scholar] [CrossRef]
- Tudoroiu, R.-E.; Zaheeruddin, M.; Tudoroiu, N.; Radu, S.M.; Chammas, H. Investigations of Different Approaches for Controlling the Speed of an Electric Motor with Nonlinear Dynamics Powered by a Li-Ion Battery-Case Study. In Electric Vehicles—Design, Modelling and Simulation; IntechOpen: London, UK, 2023. [Google Scholar]
- Klink, J.; Hebenbrock, A.; Grabow, J.; Orazov, N.; Nylén, U.; Benger, R.; Beck, H.-P. Comparison of Model-Based and Sensor-Based Detection of Thermal Runaway in Li-Ion Battery Modules for Automotive Application. Batteries 2022, 8, 34. [Google Scholar] [CrossRef]
- Tudoroiu, N.; Zaheeruddin, M.; Tudoroiu, R.-E.; Radu, M.S.; Chammas, H. Investigations on Using Intelligent Learning Techniques for Anomaly Detection and Diagnosis in Sensors Signals in Li-Ion Battery—Case Study. Inventions 2023, 8, 74. [Google Scholar] [CrossRef]
- Park, J.; Fatima, S.A. A DFT Study of TiC3 as Anode Material for Li-Ion Batteries. Appl. Surf. Sci. 2023, 638, 158024. [Google Scholar] [CrossRef]
- Kale, R.B.; More, S.S.; Khupse, N.D.; Kalubarme, R.S.; Kulkarni, M.V.; Rane, S.B.; Kale, B.B. High-Voltage Ionic Liquid-Based Flexible Solid Polymer Electrolyte for High-Performance Li-Ion Batteries. Sustain. Energy Fuels 2023, 7, 2934–2942. [Google Scholar] [CrossRef]
- Hoosain, S.E.; Tshabalala, L.C.; Skhosana, S.; Freemantle, C.; Mndebele, N. Investigation of the Properties of Direct Energy Deposition Additive Manufactured 304 Stainless Steel. S. Afr. J. Ind. Eng. 2021, 32, 258–263. [Google Scholar] [CrossRef]
- Yoo, Y.-R.; Choi, S.-H.; Kim, Y.-S. Effect of Laser Peening on the Corrosion Properties of 304L Stainless Steel. Materials 2023, 16, 804. [Google Scholar] [CrossRef] [PubMed]
- Acharyya, S.G.; Khandelwal, A.; Kain, V.; Kumar, A.; Samajdar, I. Surface Working of 304L Stainless Steel: Impact on Microstructure, Electrochemical Behavior and SCC Resistance. Mater. Charact. 2012, 72, 68–76. [Google Scholar] [CrossRef]
- Speidel, M.O. Nitrogen Containing Austenitic Stainless Steels. Mater. Werkst. Entwickl. Fert. Prüfung Eig. Anwendungen Tech. Werkst. 2006, 37, 875–880. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Lee, T.-H.; Bae, J.-H.; Chun, D.W. Molybdenum Effects on Pitting Corrosion Resistance of FeCrMnMoNC Austenitic Stainless Steels. Metals 2018, 8, 653. [Google Scholar] [CrossRef]
- Nishimoto, M.; Muto, I.; Sugawara, Y. Review―Understanding and Controlling the Electrochemical Properties of Sulfide Inclusions for Improving the Pitting Corrosion Resistance of Stainless Steels. Mater. Trans. 2023, 64, 2051–2058. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super Duplex Stainless Steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Solution Annealing on Austenite Morphology and Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2021, 16, 210813. [Google Scholar] [CrossRef]
- Martins, M.; Casteletti, L.C. Sigma Phase Morphologies in Cast and Aged Super Duplex Stainless Steel. Mater. Charact. 2009, 60, 792–795. [Google Scholar] [CrossRef]
- Baghdadchi, A.; Hosseini, V.A.; Valiente Bermejo, M.A.; Axelsson, B.; Harati, E.; Högström, M.; Karlsson, L. Wire Laser Metal Deposition of 22% Cr Duplex Stainless Steel: As-Deposited and Heat-Treated Microstructure and Mechanical Properties. J. Mater. Sci. 2022, 57, 9556–9575. [Google Scholar] [CrossRef]
- Moniruzzaman, F.N.U.M.; Shakil, S.I.; Shaha, S.K.; Kacher, J.; Nasiri, A.; Haghshenas, M.; Hadadzadeh, A. Study of Direct Aging Heat Treatment of Additively Manufactured PH13–8Mo Stainless Steel: Role of the Manufacturing Process, Phase Transformation Kinetics, and Microstructure Evolution. J. Mater. Res. Technol. 2023, 24, 3772–3787. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Heat Energy Input on Electrochemical Properties of Solution-Annealed Super-Duplex Stainless Steel UNS S 32750 Laser Welding. Int. J. Electrochem. Sci. 2022, 17, 220339. [Google Scholar] [CrossRef]
- Sung, C.; Kim, K.; Chung, W.; Shin, B.-H. Electrochemical Properties of UNS S 32750 and UNS S 32760 Annealed Super Duplex Stainless Steels. Int. J. Electrochem. Sci. 2022, 17, 220526. [Google Scholar] [CrossRef]
- Valiente Bermejo, M.A.; Thalavai Pandian, K.; Axelsson, B.; Harati, E.; Kisielewicz, A.; Karlsson, L. Microstructure of Laser Metal Deposited Duplex Stainless Steel: Influence of Shielding Gas and Heat Treatment. Weld. World 2021, 65, 525–541. [Google Scholar] [CrossRef]
- Paulraj, P.; Garg, R. Effect of Intermetallic Phases on Corrosion Behavior and Mechanical Properties of Duplex Stainless Steel and Super-Duplex Stainless Steel. Adv. Sci. Technol. Res. J. 2015, 9, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Topolska, S.; Łabanowski, J. Effect of Microstructure on Impact Toughness of Duplex and Superduplex Stainless Steels. J. Achiev. Mater. Manuf. Eng. 2009, 36, 142–149. [Google Scholar]
- Linton, V.M.; Laycock, N.J.; Thomsen, S.J.; Klumpers, A. Failure of a Super Duplex Stainless Steel Reaction Vessel. Eng. Fail Anal. 2004, 11, 243–256. [Google Scholar] [CrossRef]
- Tehovnik, F.; Arzensek, B.; Arh, B.; Skobir, D.; Pirnar, B.; Zuzek, B. Microstructure Evolution in SAF 2507 Super Duplex Stainless Steel. Mater. Technol. 2011, 45, 339–345. [Google Scholar]
- Elhoud, A.M.; Renton, N.C.; Deans, W.F. Hydrogen Embrittlement of Super Duplex Stainless Steel in Acid Solution. Int. J. Hydrogen Energy 2010, 35, 6455–6464. [Google Scholar] [CrossRef]
- Vongsilathai, S.; Lothongkum, A.W.; Lothongkum, G. Corrosion Behavior of a New 25Cr-3Ni-7Mn-0.66 N Duplex Stainless Steel in Artificial Seawater. Mater. Test. 2021, 63, 505–511. [Google Scholar] [CrossRef]
- Shin, B.-H.; Park, J.; Jeon, J.; Heo, S.; Chung, W. Effect of Cooling Rate after Heat Treatment on Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Anti-Corros. Methods Mater. 2018, 65, 492–498. [Google Scholar] [CrossRef]
- Shin, B.-H.; Park, J.; Kim, S.; Ok, J.-W.; Kim, D.-I.; Yoon, J.-H. Study of Electroless Nickel Plating on Super Duplex Stainless Steel for Lithium-Ion Battery Cases: Electrochemical Behaviour and Effects of Plating Time. Metals 2024, 14, 307. [Google Scholar] [CrossRef]
- Pettersson, N.; Pettersson, R.F.A.; Wessman, S. Precipitation of Chromium Nitrides in the Super Duplex Stainless Steel 2507. Metall. Mater. Trans. A 2015, 46, 1062–1072. [Google Scholar] [CrossRef]
- Oh, S.; Kim, D.; Kim, K.C.; Kim, D.-I.; Chung, W.; Shin, B.-H. Electrochemical Properties of Electroless Ni Plated Super Duplex Stainless in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2023, 18, 100287. [Google Scholar] [CrossRef]
- Nilsson, J.O.; Wilson, A. Influence of Isothermal Phase Transformations on Toughness and Pitting Corrosion of Super Duplex Stainless Steel SAF 2507. Mater. Sci. Technol. 1993, 9, 545–554. [Google Scholar] [CrossRef]
- Kannan, A.R.; Shanmugam, N.S.; Rajkumar, V.; Vishnukumar, M. Insight into the Microstructural Features and Corrosion Properties of Wire Arc Additive Manufactured Super Duplex Stainless Steel (ER2594). Mater. Lett. 2020, 270, 127680. [Google Scholar] [CrossRef]
- Maurya, A.K.; Pandey, C.; Chhibber, R. Effect of Filler Metal Composition on Microstructural and Mechanical Characterization of Dissimilar Welded Joint of Nitronic Steel and Super Duplex Stainless Steel. Arch. Civ. Mech. Eng. 2022, 22, 90. [Google Scholar] [CrossRef]
- Valeriano, L.D.C.; Correa, E.O.; Mariano, N.A.; Robin, A.L.M.; Machado, M.A.G. Influence of the Solution-Treatment Temperature and Short Aging Times on the Electrochemical Corrosion Behaviour of Uns S32520 Super Duplex Stainless Steel. Mater. Res. 2019, 22, e20180774. [Google Scholar] [CrossRef]
- Kim, D.; Chung, W.; Shin, B.-H. Effects of the Volume Fraction of the Secondary Phase after Solution Annealing on Electrochemical Properties of Super Duplex Stainless Steel UNS S32750. Metals 2023, 13, 957. [Google Scholar] [CrossRef]
- Shin, B.-H.; Kim, D.; Park, S.; Hwang, M.; Park, J.; Chung, W. Precipitation Condition and Effect of Volume Fraction on Corrosion Properties of Secondary Phase on Casted Super-Duplex Stainless Steel UNS S32750. Anti-Corros. Methods Mater. 2018, 66, 61–66. [Google Scholar] [CrossRef]
- Shin, B.-H.; Kim, D.; Kim, D.-I.; Lee, W.; Kwon, S.-H. Effect of Microstructure on Electroless Ni Plating Behavior on Super Duplex Stainless Steel SAF2507 in Li-Ion Batteries. Coatings 2023, 13, 1807. [Google Scholar] [CrossRef]


| Elements | C | N | Mn | Ni | Cr | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| Chemical composition, wt.% | 0.01 | 0.27 | 0.8 | 6.8 | 25.0 | 3.8 | 0.2 | Bal |
| Elements | C | N | Mn | Ni | Cr | Mo | Fe | PRE |
|---|---|---|---|---|---|---|---|---|
| Austenite | 0.01 | 0.51 | 1.1 | 7.9 | 23.3 | 3.2 | Bal | 42.0 |
| Ferrite | 0.01 | 0.05 | 0.8 | 5.5 | 26.6 | 4.4 | Bal | 42.1 |
| Element | Cr | Mo | Ni | Mn | Fe |
|---|---|---|---|---|---|
| Sigma | 30.9 ± 1.5 | 8.9 ± 2.1 | 4.5 ± 0.8 | 0.5 ± 0.1 | 56.3 |
| Chi | 22.1 ± 0.9 | 2.2 ± 0.5 | 9.5 ± 1.2 | 1.0 ± 0.2 | 65.2 |
| Heat Treatment Time | 0 h | 1 h | 5 h | 10 h |
|---|---|---|---|---|
| Austenite | 25.5 ± 2.3% | 25.4 ± 2.1% | 27.1 ± 2.0% | 31.6 ± 1.9% |
| Ferrite | 74.5 ± 2.3% | 74.6 ± 2.1% | 71.9 ± 2.0% | 60.3 ± 2.2% |
| Secondary phase | 0.0 ± 0.0% | 0.0 ± 0.0% | 1.0 ± 0.6% | 8.1 ± 1.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-H.; Kim, D.; Yoon, J.-H. Crystallization of Secondary Phase on Super-Duplex Stainless Steel SAF2507: Advanced Li-Ion Battery Case Materials. Crystals 2024, 14, 378. https://doi.org/10.3390/cryst14040378
Shin B-H, Kim D, Yoon J-H. Crystallization of Secondary Phase on Super-Duplex Stainless Steel SAF2507: Advanced Li-Ion Battery Case Materials. Crystals. 2024; 14(4):378. https://doi.org/10.3390/cryst14040378
Chicago/Turabian StyleShin, Byung-Hyun, Dohyung Kim, and Jang-Hee Yoon. 2024. "Crystallization of Secondary Phase on Super-Duplex Stainless Steel SAF2507: Advanced Li-Ion Battery Case Materials" Crystals 14, no. 4: 378. https://doi.org/10.3390/cryst14040378
APA StyleShin, B.-H., Kim, D., & Yoon, J.-H. (2024). Crystallization of Secondary Phase on Super-Duplex Stainless Steel SAF2507: Advanced Li-Ion Battery Case Materials. Crystals, 14(4), 378. https://doi.org/10.3390/cryst14040378






