Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
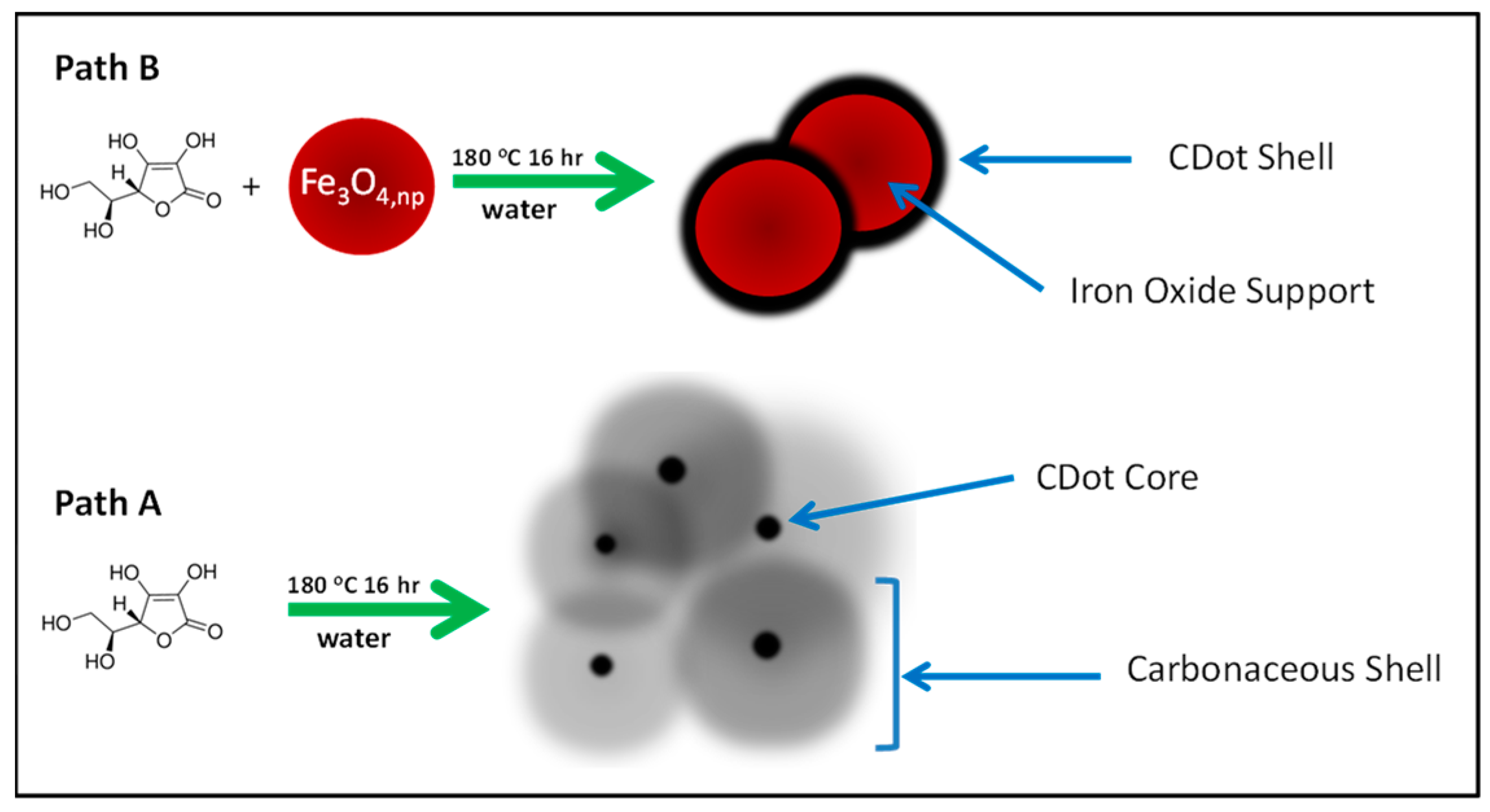
2.3. Preparation
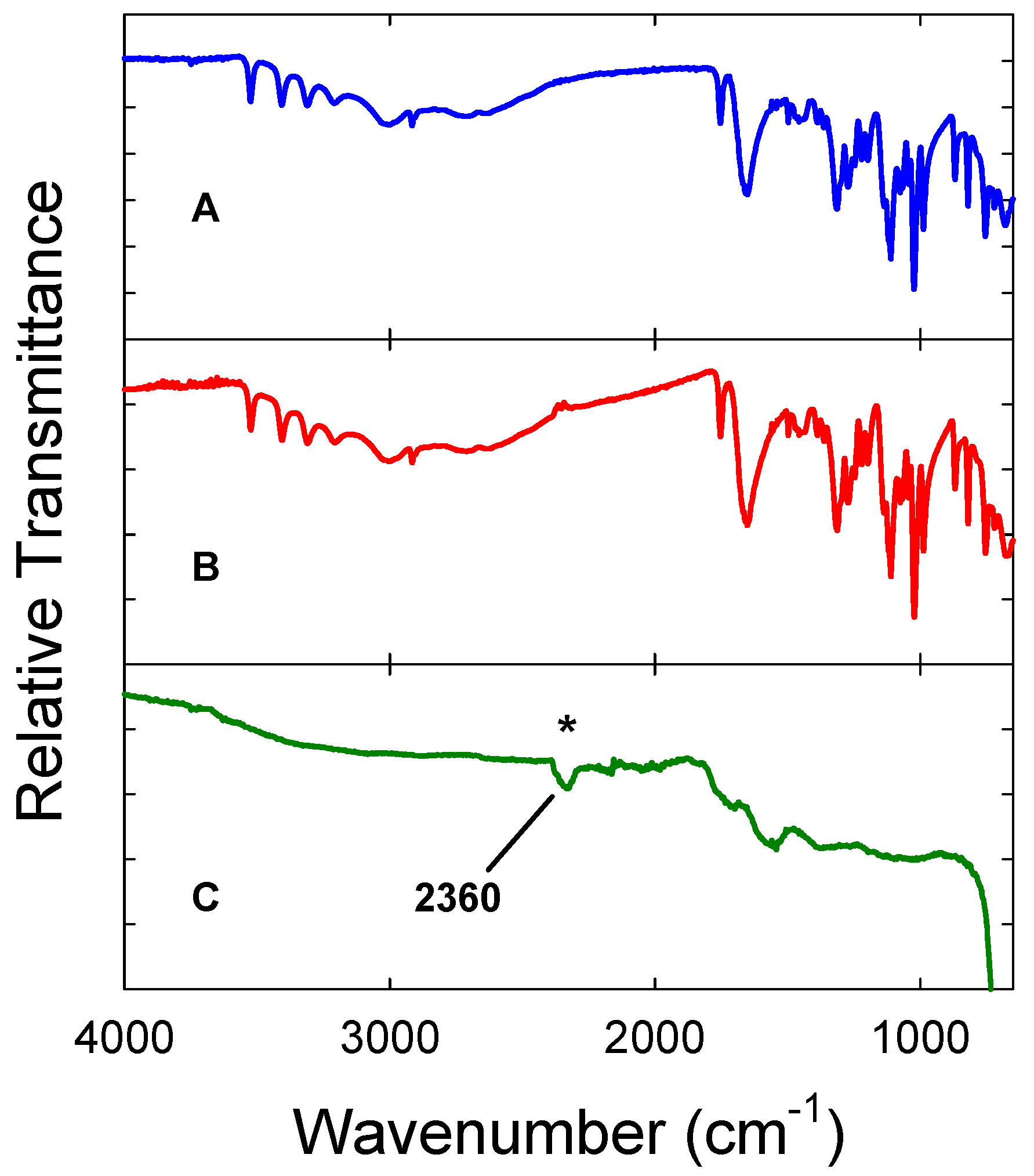
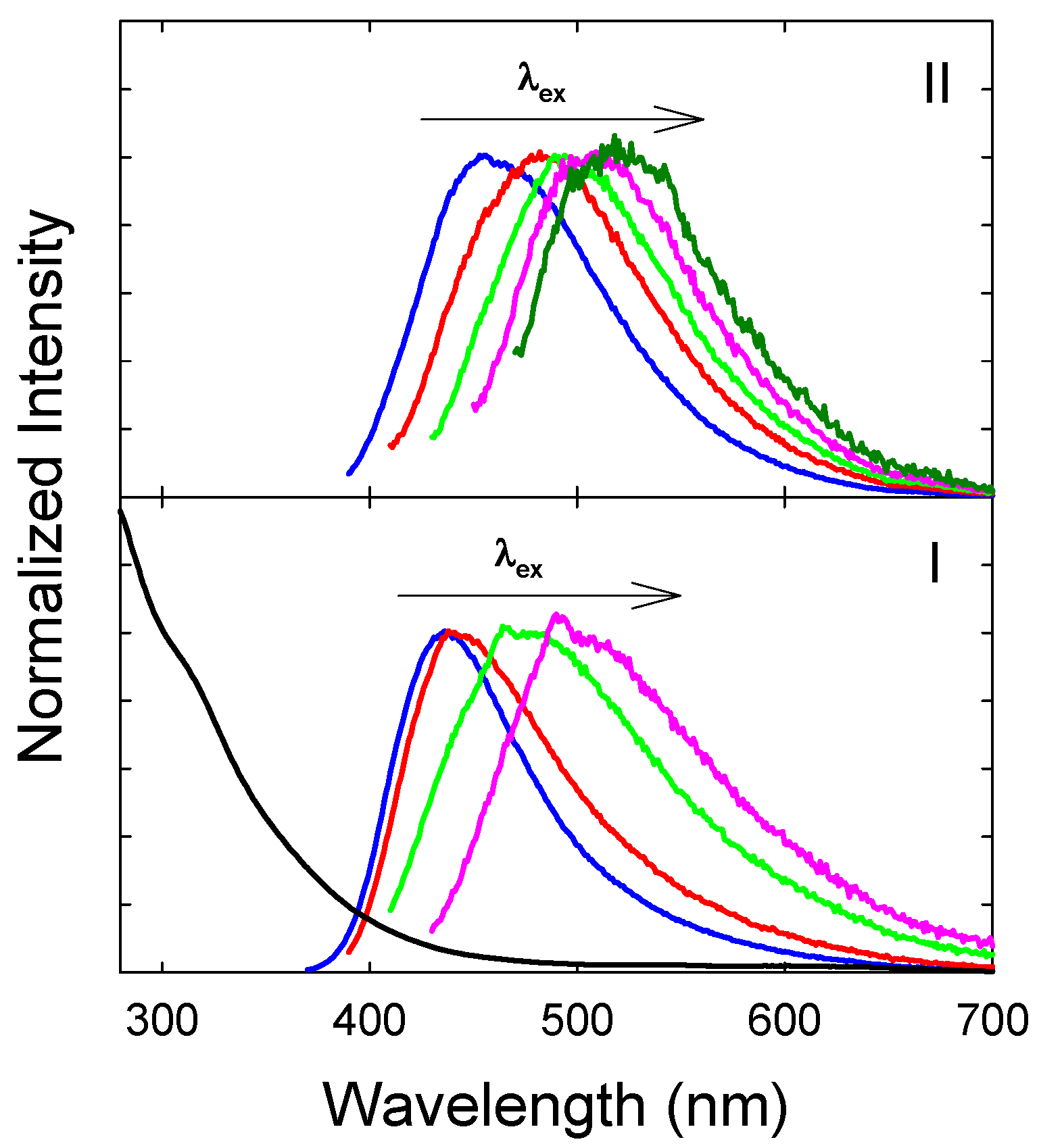
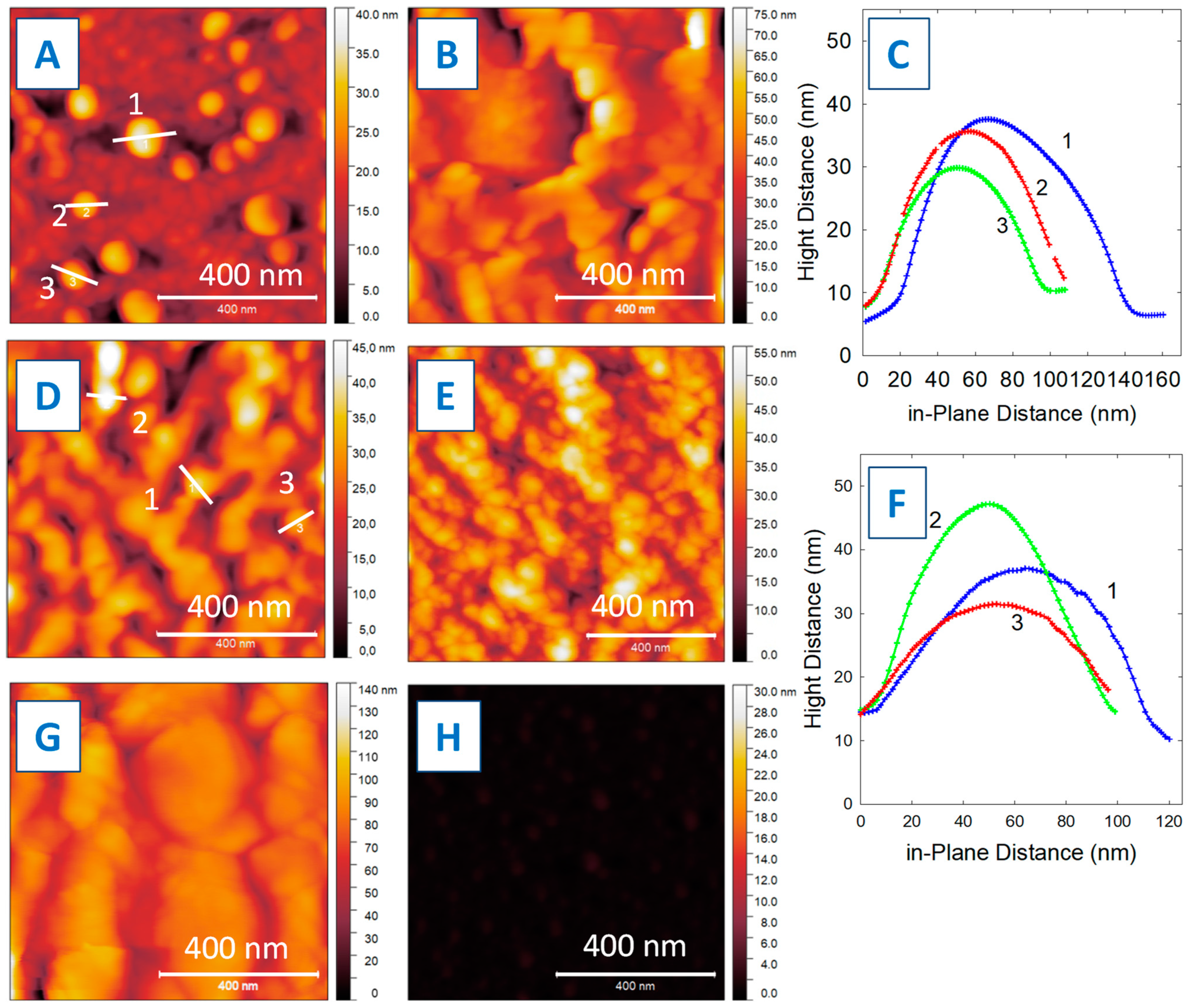
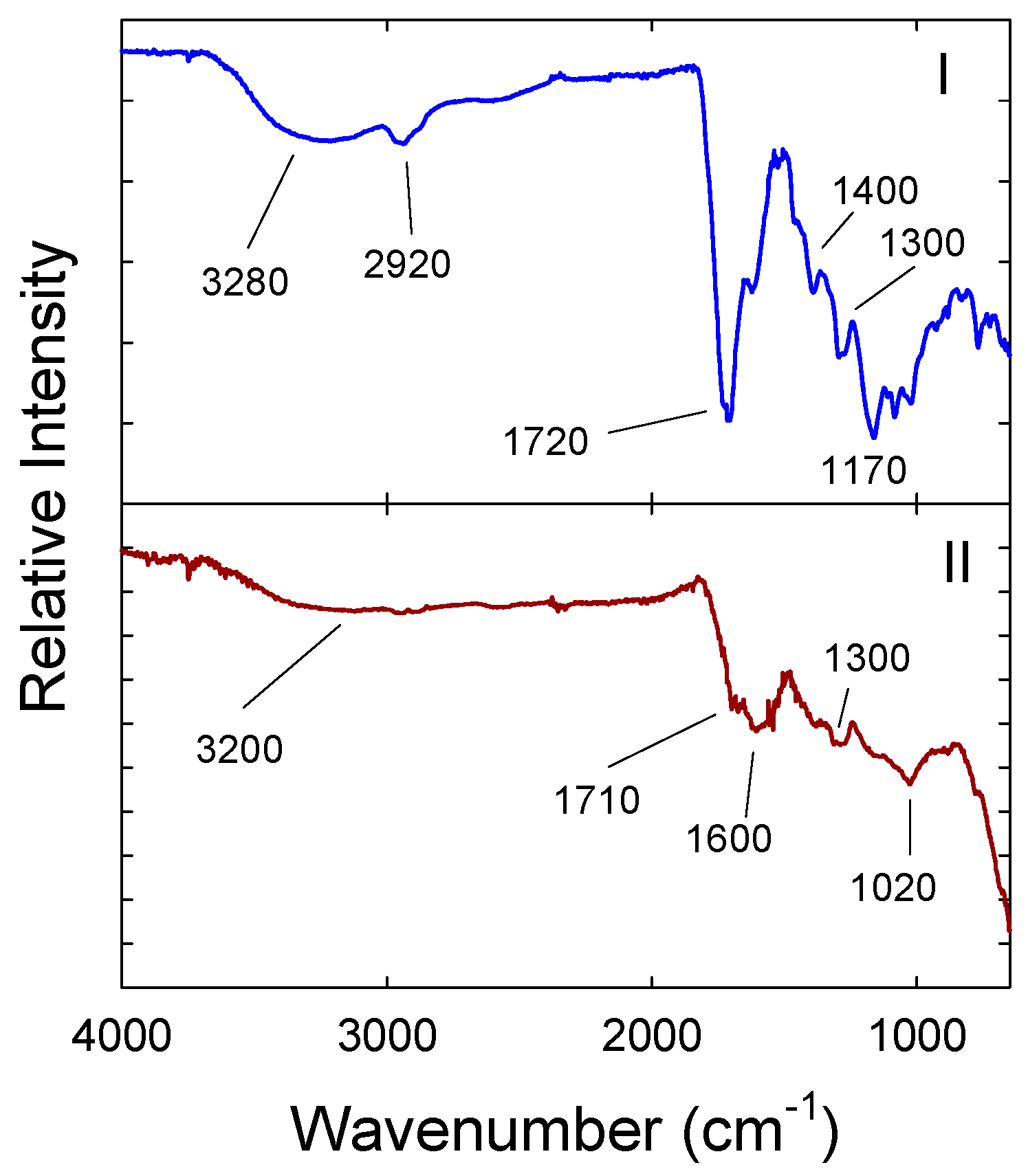
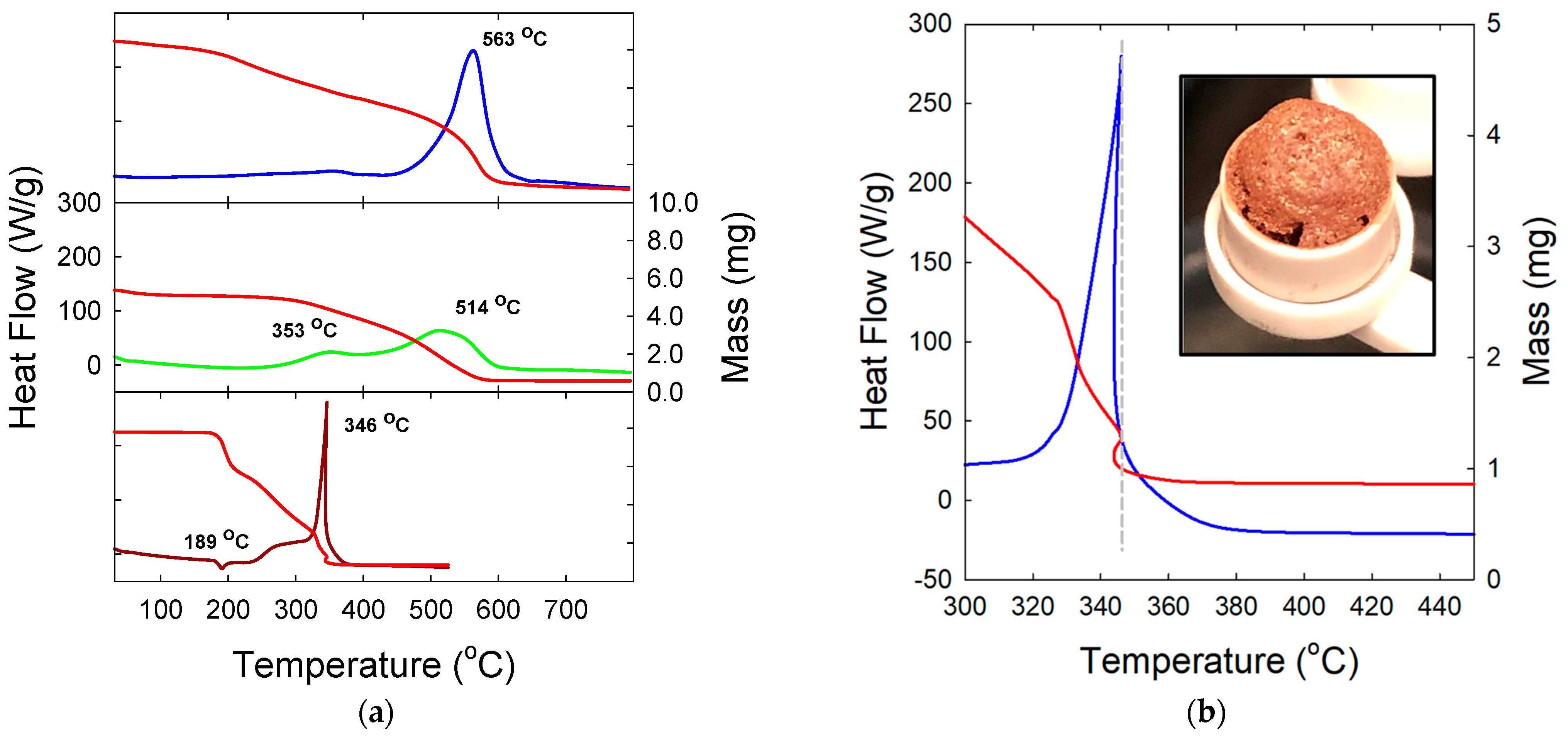
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wand, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Cao, L.; Fernando, K.A.S.; Liang, W.; Seilkop, A.; Veca, L.M.; Sun, Y.-P.; Bunker, C.E. Carbon dots for energy conversion applications. J. Appl. Phys. 2019, 125, 220903. [Google Scholar] [CrossRef]
- Wang, P.; Meziani, M.J.; Fu, Y.; Bunker, C.E.; Hou, X.; Yang, L.; Msellek, H.; Zaharias, M.; Darby, J.P.; Sun, Y.-P. Carbon dots versus nano-carbon/organic hybrids—Dramatically different behaviors in fluorescence sensing of metal cations with structural and mechanistic implications. Nanoscale Adv. 2021, 3, 2316–2324. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Bunker, C.E.; Sun, Y.-P. Carbon Dots: Zero-Dimensional Carbon Allotrope with Unique Photoinduced Redox Characteristics. ACS Omega 2020, 5, 965–971. [Google Scholar] [CrossRef]
- LeCroy, G.E.; Sonkar, S.K.; Yang, F.; Veca, L.M.; Wang, P.; Tackett, K.N., II; Yu, J.-J.; Vasile, E.; Qian, H.; Liu, Y.; et al. Toward Structurally Defined Carbon Dots as Ultracompact Fluorescent Probes. ACS Nano 2014, 8, 4522–4529. [Google Scholar] [CrossRef] [PubMed]
- Ullal, N.; Muthamma, K.; Sunil, D. Carbon dots from eco-friendly precursors for optical sensing application: An up-to-date review. Chem. Pap. 2022, 76, 6097–6127. [Google Scholar] [CrossRef]
- Kumar, P.; Dua, S.; Kaur, R.; Kumar, M.; Bhatt, G. A review on advancements in carbon quantum dots and their application in photovoltaics. RSC Adv. 2022, 12, 4714–4759. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Wang, S.; Zhang, Y.; Li, Y.; Liu, Y.; Li, W.; Wang, Y.; Yan, X.; Luo, D. Carbon Dots in Biomedicine: A Review. ACS Appl. Bio Mater. 2022, 5, 2031–2045. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, D.; Kobaisi, M.A.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Irmawati, R.; Hashim, H.S.; Ramdzan, N.S.M.; Fauzi, N.I.M. A Review on Carbon Dots: Synthesis, Characterization and Its Application in Optical Sensor for Environmental Monitoring. Nanomaterials 2022, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.K.K.; Tan, H.L.; Lim, Y.P.; So’aid, M.S.; Bakar, N.F.A. A Review on Multifunctional Carbon-Dots Synthesized From Biomass Waste: Design/Fabrication, Characterization and Applications. Front. Energy Res. 2021, 9, 686549. [Google Scholar]
- Zhou, Y.; Zhang, W.; Leblanc, R.M. Structure−Property−Activity Relationships in Carbon Dots. J. Phys. Chem. B 2022, 126, 10777–10796. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, L.; Yu, S.; Yang, Y.; Liu, X. Advances in Magnetic Carbon Dots: A Theranostics Platform for Fluorescence/Magnetic Resonance Bimodal Imaging and Therapy for Tumors. ACS Biomater. Sci. Eng. 2023, 9, 6548–6566. [Google Scholar] [CrossRef] [PubMed]
- Kailasa, S.K.; Koduru, J.R. Perspectives of magnetic nature carbon dots in analytical chemistry: From separation to detection and bioimaging. Trends Environ. Anal. Chem. 2022, 33, e00153. [Google Scholar] [CrossRef]
- Klekotka, U.; Zambrzycka-Szelewa, E.; Satula, D.; Kalska-Szostko, B. Stability Studies of Magnetite Nanoparticles in Environmental Solutions. Materials 2021, 14, 5069. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, P.; Bunker, C.E.; Teisl, L.R.; Reibold, M.; Yan, S.; Qian, H.; He, D.; Sun, Y.-P. Preparation and optical properties of magnetic carbon/iron oxide hybrid dots. RSC Adv. 2017, 7, 41304–41310. [Google Scholar] [CrossRef]
- Stachowska, J.D.; Gamza, M.B.; Mellor, C.; Gibbons, E.N.; Krysmann, M.J.; Kelarakis, A.; Gumieniczek-Chlopek, E.; Straczek, T.; Kapusta, C.; Szwajca, A. Carbon dots/iron oxide nanoparticles with tuneable composition and properties. Nanomaterials 2022, 12, 674. [Google Scholar] [CrossRef]
- Xie, R.; Qu, Y.; Tang, M.; Zhao, J.; Chua, S.; Li, T.; Zhang, F.; Wheatley, A.E.H.; Chai, F. Carbon dots-magnetic nanocomposites for the detection and removal of Hg2+. Food Chem. 2021, 364, 130366. [Google Scholar] [CrossRef]
- Perelshtein, I.; Perkas, N.; Rahimipour, S.; Gedanken, A. Bifunctional Carbon Dots—Magnetic and Fluorescent Hybrid Nanoparticles for Diagnostic Applications. Nanomaterials 2020, 10, 1384. [Google Scholar] [CrossRef]
- Stepanova, M.; Dubavik, A.; Efimova, A.; Konovalova, M.; Svirshchevskaya, E.; Zakharov, V.; Orlova, A. Magneto-Luminescent Nanocomposites Based on Carbon Dots and Ferrite with Potential for Bioapplication. Nanomaterials 2022, 12, 1396. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.-Y.; Gedda, G.; Girma, W.M.; Yen, C.-L.; Ling, Y.-C.; Chang, J.-Y. Magnetofluorescent Carbon Dots Derived from Crab Shell for Targeted Dual-Modality Bioimaging and Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 13887–13899. [Google Scholar] [CrossRef]
- Wang, H.; Shen, J.; Li, Y.; Wei, Z.; Cao, G.; Gai, Z.; Hong, K.; Banerjee, P.; Zhou, S. Magnetic iron oxide–fluorescent carbon dots integrated nanoparticles for dual-modal imaging, near-infrared light-responsive drug carrier and photothermal therapy. Biomater. Sci. 2014, 2, 915–923. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, H.; Ye, J.; Zhao, C.; Gao, S.; Wu, C.; Li, C.; Li, J.; Wang, X. Nitrogen-Doped Carbon Quantum Dot Stabilized Magnetic Iron Oxide Nanoprobe for Fluorescence, Magnetic Resonance, and Computed Tomography Triple-Modal In Vivo Bioimaging. Adv. Funct. Mater. 2016, 26, 8694–8706. [Google Scholar] [CrossRef]
- Cao, L.; Anilkumar, P.; Wang, X.; Liu, J.H.; Sahu, S.; Meziani, M.J.; Myers, E.; Sun, Y.-P. Reverse Stern–Volmer Behavior for Luminescence Quenching in Carbon Nanoparticles. Can. J. Chem. 2011, 89, 104–109. [Google Scholar] [CrossRef]
- Jessup, R.S. Heats of Combustion of Diamond and of Graphite. J. Res. Nat. Bur. Stand. 1938, 21, 475. [Google Scholar] [CrossRef]
- Farivar, F.; Yap, P.L.; Karunagaran, R.U.; Losic, D. Thermogravimetric Analysis (TGA) of Graphene Materials: Effect of Particle Size of Graphene, Graphene Oxide and Graphite on Thermal Parameter. C 2021, 7, 41. [Google Scholar] [CrossRef]
- He, H.; Zhong, Y.; Liang, X.; Tan, W.; Zhu, J.; Wang, C.Y. Natural Magnetite: An efficient catalyst for the degradation of organic contaminant. Sci. Rep. 2015, 5, 10139. [Google Scholar] [CrossRef] [PubMed]
- Trimm, D.L. Catalytic Combustion (Review). Appl. Catal. 1983, 7, 249–282. [Google Scholar] [CrossRef]
- Jones, R.L. Catalytic Combustion Effects in Internal Combustion Engines. Combust. Sci. Tech. 1997, 129, 185–195. [Google Scholar] [CrossRef]
- Forzatti, P.; Groppi, G. Catalytic combustion for the production of energy. Catal. Today 1999, 54, 165–180. [Google Scholar] [CrossRef]
- Dey, S.; Sun, S.; Mehta, N.S. Carbon monoxide catalytic oxidation over various iron-based nanoparticles at ambient conditions: A Review. Carbon Capture Sci. Technol. 2021, 1, 100013. [Google Scholar] [CrossRef]
- Monazam, E.R.; Breault, R.W.; Siriwardane, R. Kinetics of Magnetite (Fe3O4) Oxidation to Hematite (Fe2O3) in Air for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2014, 53, 13320. [Google Scholar] [CrossRef]
- Smiltens, J. The Standard Free Energy of Oxidation of Magnetite to Hematite at Temperatures above 1000°. J. Am. Chem. Soc. 1957, 79, 4877–4880. [Google Scholar] [CrossRef]
- Zheng, H.; Schenk, J.; Spreitzer, D.; Wolfinger, T.; Daghagheleh, O. Review on the Oxidation Behaviors and Kinetics of Magnetite in Particle Scale. Steel Res. Int. 2021, 92, 2000687. [Google Scholar] [CrossRef]
- Jingyan, S.; Yuwen, L.; Zhiyong, W.; Cunxin, W. Investigation of thermal decomposition of ascorbic acid by TG-FTIR and thermal kinetics analysis. J. Pharm. Biomed. Anal. 2013, 77, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Vernin, G.; Chakib, S.; Rogacheva, S.M.; Obretenov, T.D.; Parkanyi, C. Thermal decomposition of ascorbic acid. Carbohydr. Res. 1997, 305, 1–15. [Google Scholar] [CrossRef]
- Rahmawati, S.; Bundjali, B. Kinetics of the Oxidation of Vitamin C. Indo. J. Chem. 2012, 12, 291. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Lerdkanchanaporn, S.; Dollimore, D.; Alexander, K.S. A Thermal Analysis Study of Ascorbic Acid and Its Pharmaceutical Formulations, A Consideration of Time-Temperature Plots. J. Therm. Anal. 1997, 49, 887–896. [Google Scholar] [CrossRef]
- Yadav, R.A.; Rani, P.; Kumar, M.; Singh, R.; Singh, P.; Singh, N.P. Experimental IR and Raman spectra and quantum chemical studies of molecular structures, conformers and vibrational characteristics of L-ascorbic acid and its anion and cation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 84, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guihong, X.; He, D.; Zhang, L. Ascorbic acid promoted magnetite Fenton degradation of alachlor: Mechanistic insights and kinetic modeling. Appl. Catal. B Environ. 2020, 267, 118383. [Google Scholar] [CrossRef]
- Usman, M.; Monfort, O.; Haderlein, S.; Hanna, K. Enhancement of Pentachlorophenol Removal in a Historically Contaminated Soil by Adding Ascorbic Acid to H2O2/Magnetite System. Catalysts 2021, 11, 331. [Google Scholar] [CrossRef]
- Hou, X.; Huang, X.; Li, M.; Zhang, Y.; Yuan, S.; Ai, Z.; Zhao, J.; Zhang, L. Fenton oxidation of organic contaminants with aquifer sediment activated by ascorbic acid. Chem. Eng. J. 2018, 348, 255–262. [Google Scholar] [CrossRef]
- Hakim, A.; Marliza, T.S.; Tahari, N.M.A.; Isahak, R.W.N.W.; Yusop, T.M.; Hisham, W.M.M.; Yarmo, A.M. Studies on CO2 Adsorption and Desorption Properties from Various Types of Iron Oxides (FeO, Fe2O3, and Fe3O4). Ind. Eng. Chem. Res. 2016, 55, 7888. [Google Scholar] [CrossRef]
- Kumar, S.; Drozd, V.; Durygin, A.; Saxena, S.K. Capturing CO2 Emissions in the Iron Industries using a Magnetite–Iron Mixture. Energy Technol. 2016, 4, 560. [Google Scholar] [CrossRef]
- Mendoza, E.Y.M.; Santos, A.S.; Lopez, E.V.; Drozd, V.; Durygin, A.; Chen, J.; Saxena, S.K. Iron oxides as efficient sorbents for CO2 capture. J. Mater. Res. Technol. 2019, 8, 2944–2956. [Google Scholar] [CrossRef]
- Mirabella, F.; Zaki, E.; Ivars-Barcelo, F.; Schauermann, S.; Shaikhutdinov, S. CO2 Adsorption on Magnetite Fe3O4(111). J. Phys. Chem. C 2018, 122, 27433. [Google Scholar] [CrossRef]
- Santos-Carballal, D.; Roldan, A.; Dzade, N.Y.; de Leeuw, N.H. Reactivity of CO2 on the surfaces of magnetite (Fe3O4), greigite (Fe3S4) and mackinawite (FeS). Phil. Trans. R. Soc. A 2018, 376, 20170065. [Google Scholar] [CrossRef] [PubMed]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corr. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A review of iron carbonate (FeCO3) formation in the oil and gas industry. Corr. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Lewis, W.K.; Rumchik, C.G.; Smith, M.J.; Fernando, K.S.; Crouse, C.A.; Spowart, J.E.; Guliants, E.A.; Bunker, C.E. Comparison of post-detonation combustion in explosives incorporating aluminum nanoparticles: Influence of the passivation layer. J. Appl. Phys. 2013, 113, 044907. [Google Scholar] [CrossRef]
- Fernando, K.S.; Smith, M.J.; Harruff, B.A.; Lewis, W.K.; Guliants, E.A.; Bunker, C.E. Sonochemically assisted thermal decomposition of alane N, N-dimethylethylamine with titanium (IV) isopropoxide in the presence of oleic acid to yield air-stable and size-selective aluminum core−shell nanoparticles. J. Phys. Chem. C 2009, 113, 500. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouhri, K.; Snyder, L.J.; McFarland, M.; Laubie, P.O.; Fernando, K.A.S.; Bunker, C.E. Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate. Crystals 2024, 14, 520. https://doi.org/10.3390/cryst14060520
Zouhri K, Snyder LJ, McFarland M, Laubie PO, Fernando KAS, Bunker CE. Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate. Crystals. 2024; 14(6):520. https://doi.org/10.3390/cryst14060520
Chicago/Turabian StyleZouhri, Khalid, Luke J. Snyder, Michael McFarland, Parker O. Laubie, K. A. Shiral Fernando, and Christopher E. Bunker. 2024. "Combustion-Induced Endothermic Process in Carbon Dots Synthesized on Magnetite Nanoparticle Substrate" Crystals 14, no. 6: 520. https://doi.org/10.3390/cryst14060520




