The Inability of Metal Coordination to Control the Regioselectivity of Dimerization of Trans-Cinnamic Acid Derivatives
Abstract
:1. Introduction

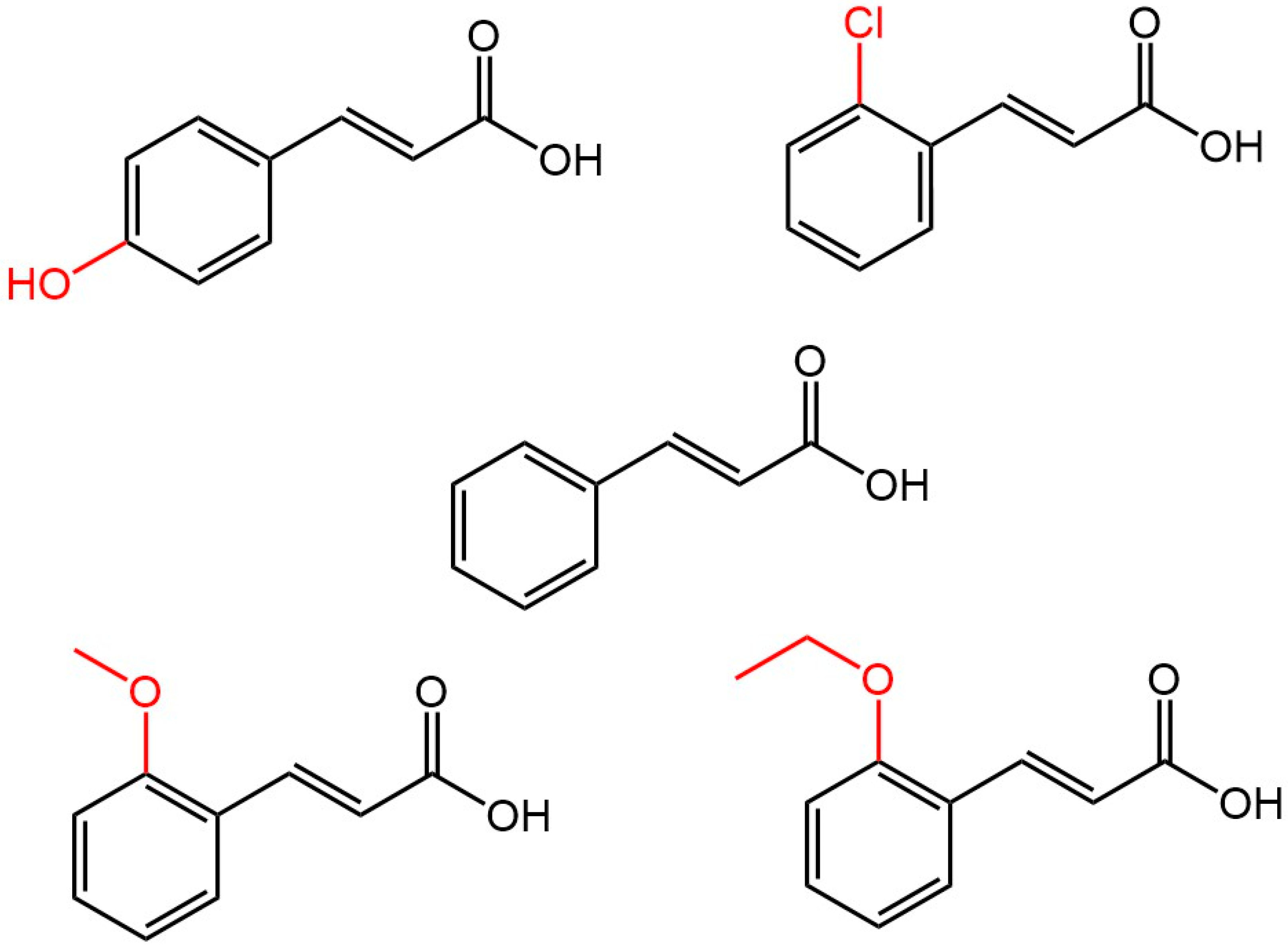
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
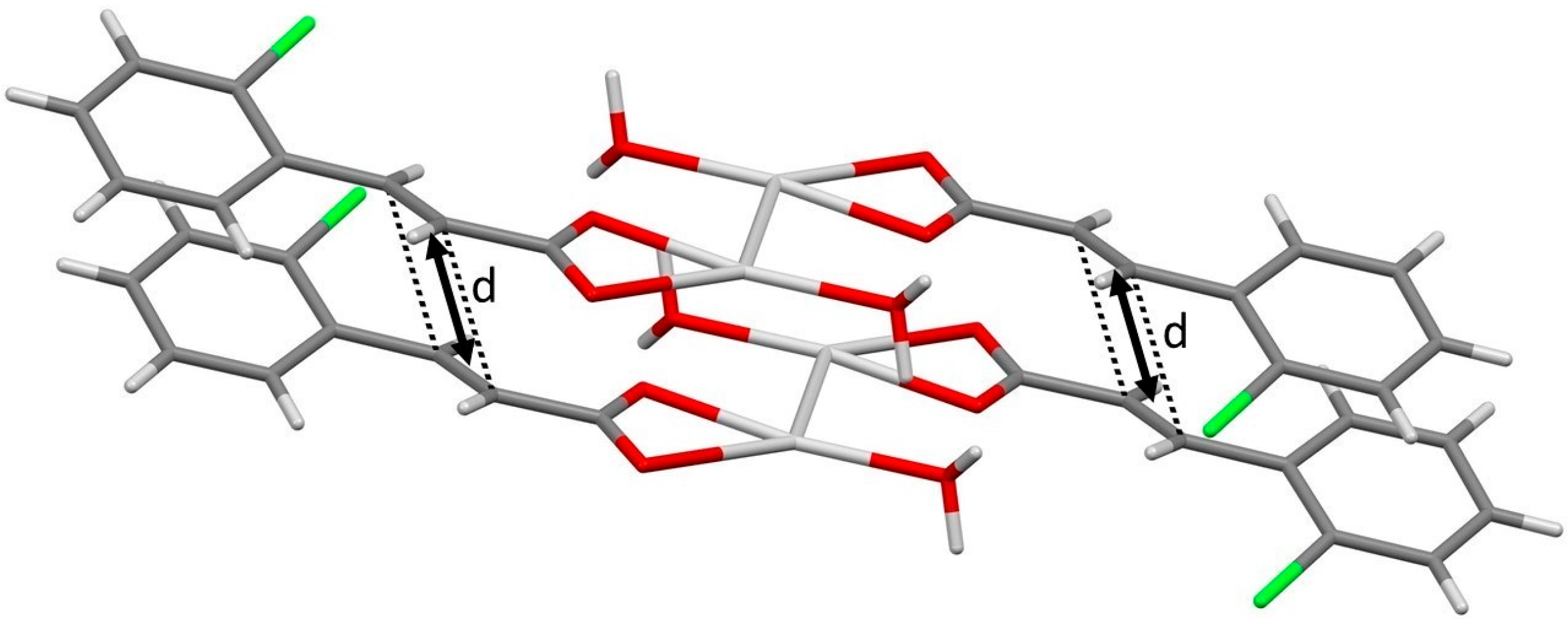
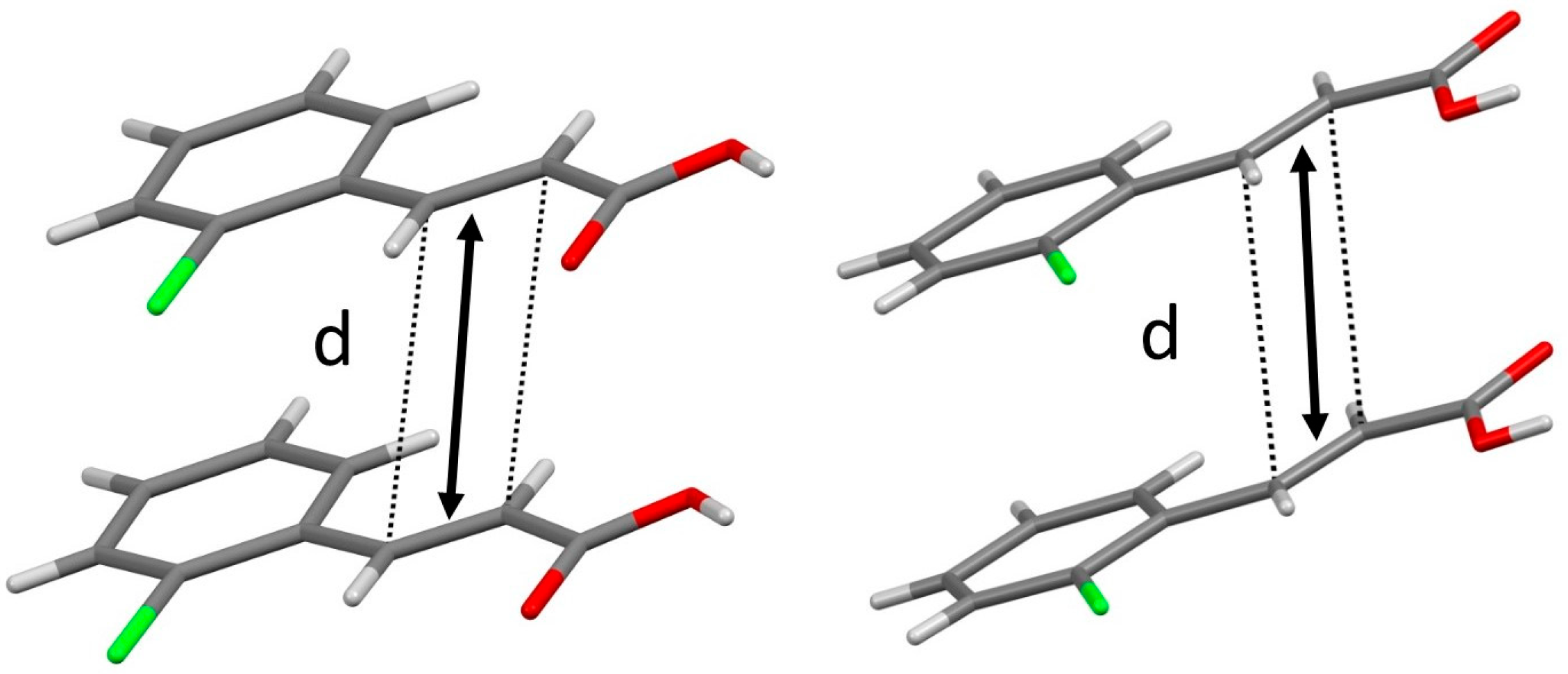
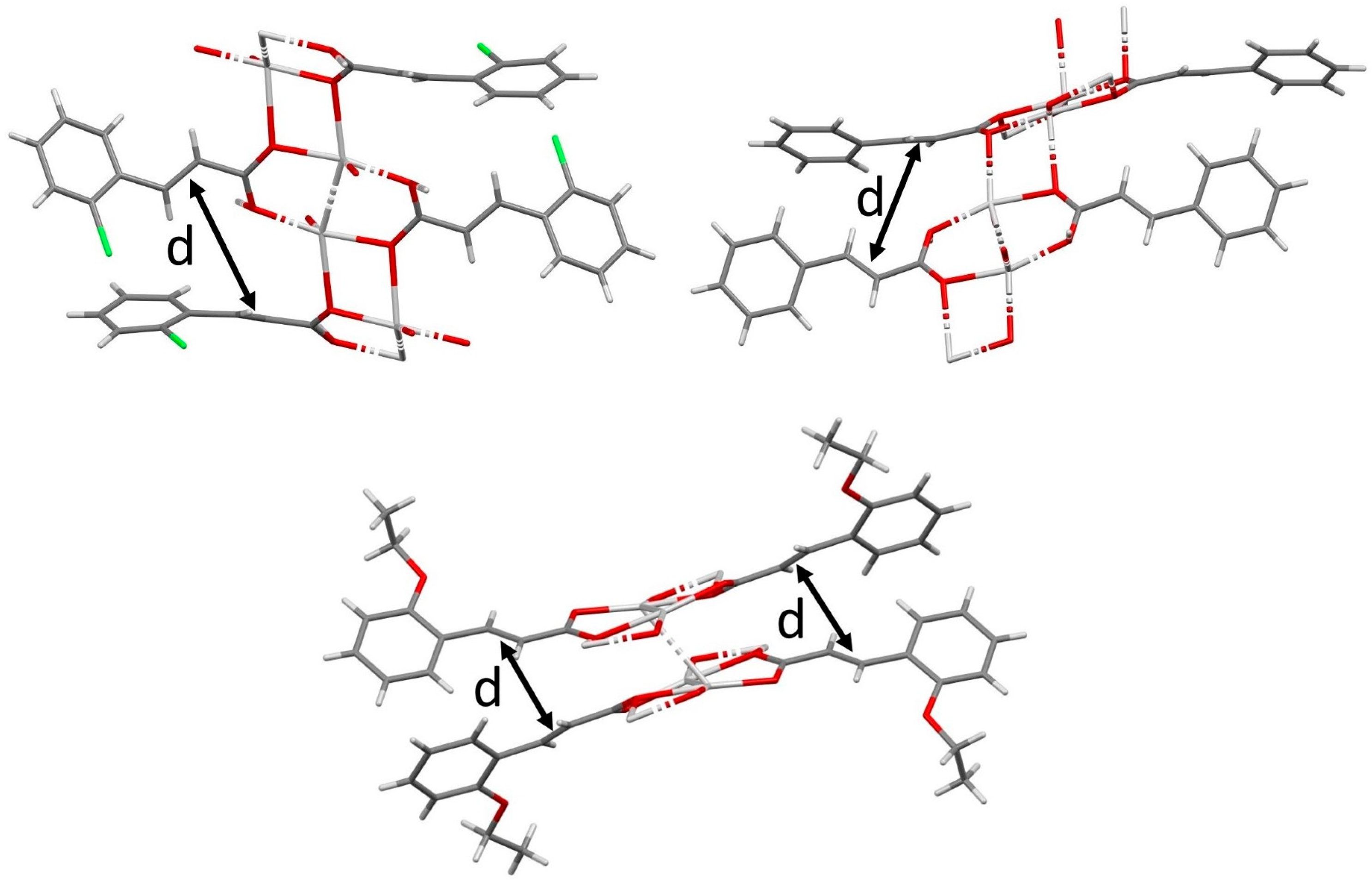
3.1. Single Crystal Structures of o-Cl-TCA
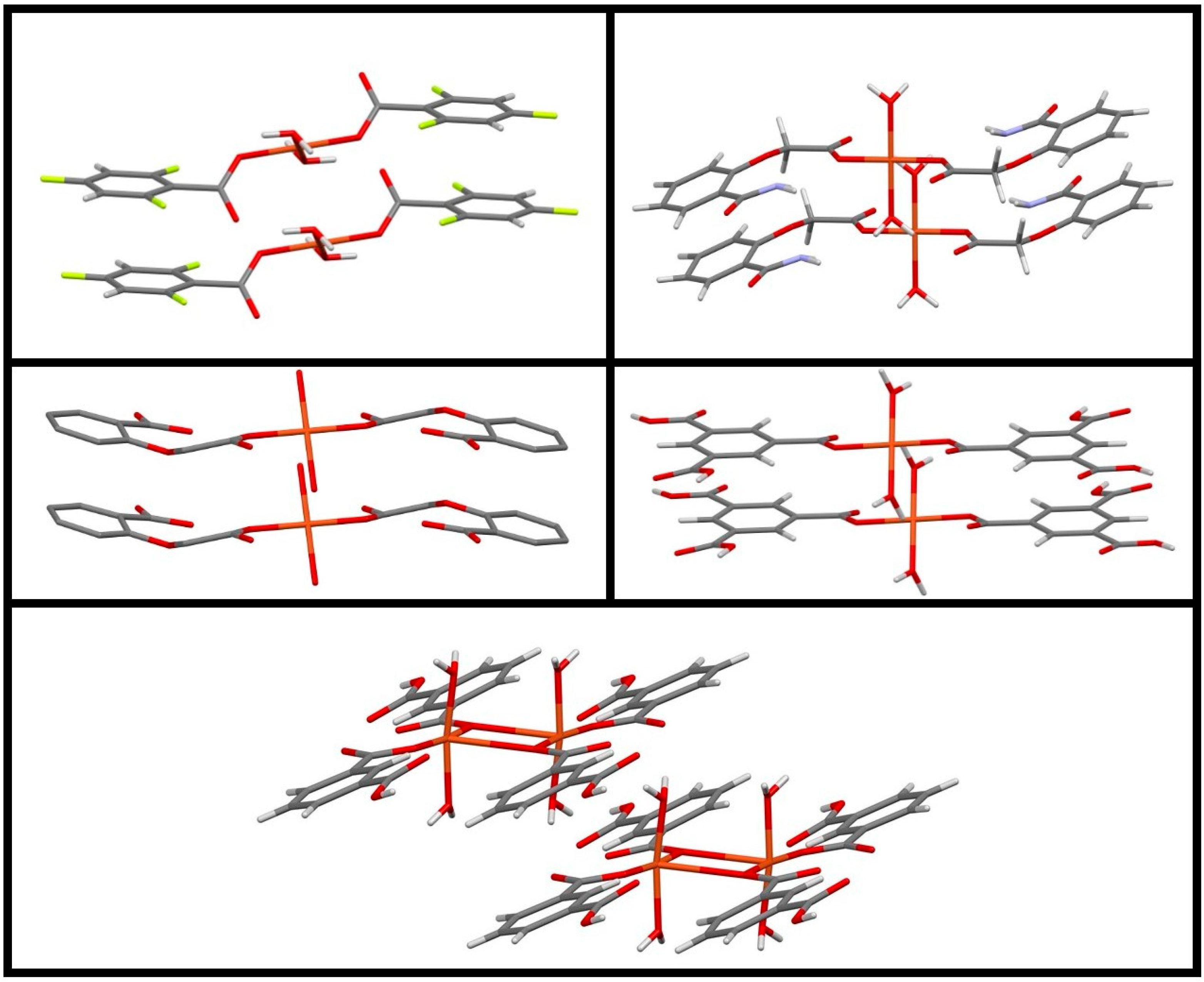
3.2. Metallic Complexes
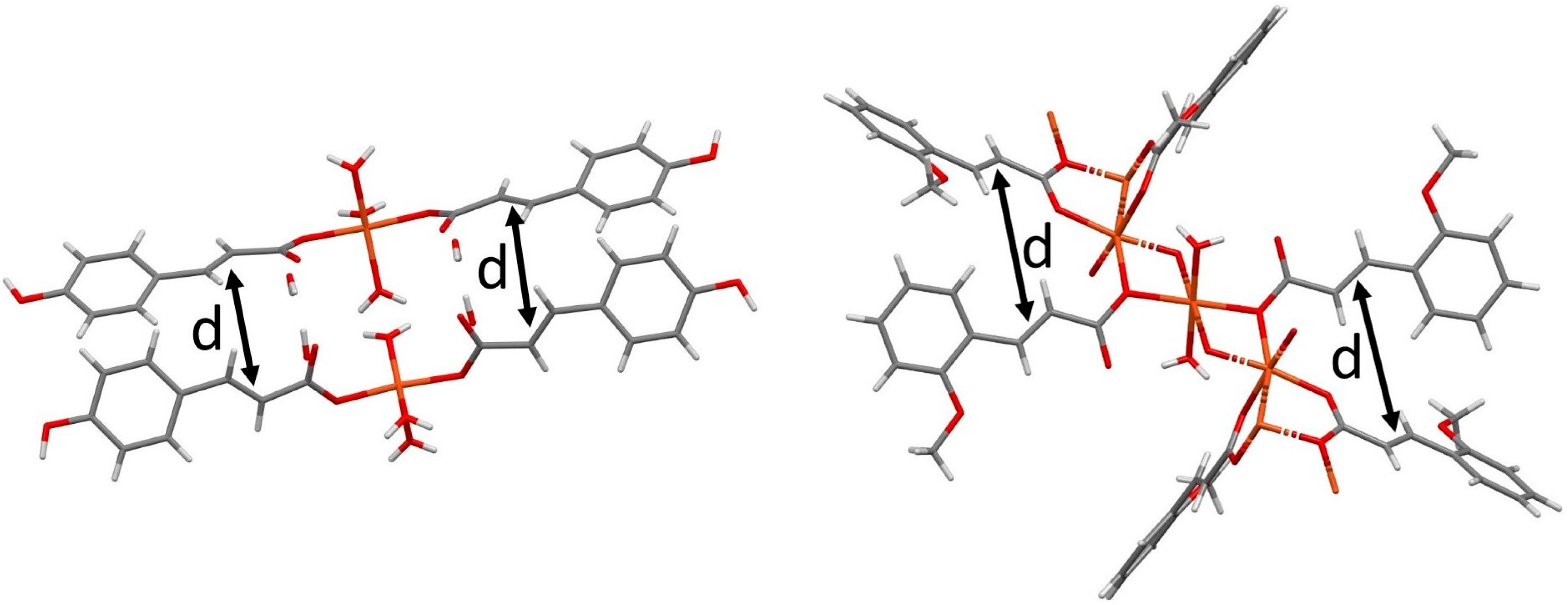
3.2.1. Silver
3.2.2. Copper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jessop, P.G.; Trakhtenberg, S.; Warner, J. The Twelve Principles of Green Chemistry. In Innovations in Industrial and Engineering Chemistry; American Chemical Society: Washington, DC, USA, 2009; pp. 401–436. ISBN 9780841269637. [Google Scholar]
- Bruice, P.Y. Pericyclic Reactions. In Organic Chemistry; Prentice Hall: London, UK, 2011; pp. 1209–1225. [Google Scholar]
- Cohen, M.D.; Schmidt, G.M.J. Topochemistry. Part I. A Survey. J. Chem. Soc. 1964, 1996–2000. [Google Scholar] [CrossRef]
- Cohen, M.D. Solid-State Photochemical Reactions. Tetrahedron 1987, 43, 1211–1224. [Google Scholar] [CrossRef]
- Ramamurthy, V.; Sivaguru, J. Supramolecular Photochemistry as a Potential Synthetic Tool: Photocycloaddition. Chem. Rev. 2016, 116, 9914–9993. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.M.J. Photodimerization in the Solid State. Pure Appl. Chem. 1971, 27, 647–678. [Google Scholar] [CrossRef]
- Amjaour, H.; Wang, Z.; Mabin, M.; Puttkammer, J.; Busch, S.; Chu, Q.R. Scalable Preparation and Property Investigation of a Cis-Cyclobutane-1,2-Dicarboxylic Acid from β-Trans-Cinnamic Acid. Chem. Commun. 2019, 55, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.D.; Schmidt, G.M.J.; Sonntag, F.I. Topochemistry. Part II. The Photochemistry of Trans-Cinnamic Acids. J. Chem. Soc. 1964, 2000–2013. [Google Scholar] [CrossRef]
- Schmidt, G.M.J. Topochemistry. Part III. The Crystal Chemistry of Some Trans-Cinnamic Acids. J. Chem. Soc. 1964, 2014–2021. [Google Scholar] [CrossRef]
- Fernandes, M.A.; Levendis, D.C.; Schoening, F.R.L. A New Polymorph of Ortho-Ethoxy-Trans-Cinnamic Acid: Single-to-Single- Crystal Phase Transformation and Mechanism. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.A.; Levendis, D.C.; De Koning, C.B. Solvate and Polymorphs of Ortho-Ethoxy-Trans-Cinnamic Acid: The Crystal and Molecular Structures. Cryst. Eng. 2001, 4, 215–231. [Google Scholar] [CrossRef]
- PubChem SID 274614092. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/274614092 (accessed on 15 January 2024).
- PubChem SID 273537784. Available online: https://pubchem.ncbi.nlm.nih.gov/substance/273537784 (accessed on 15 January 2024).
- Bryan, R.F.; Freyberg, D.P. Crystal Structures of α-Trans- and p-Methoxy-Cinnamic Acids and Their Relation to Thermal Mesomorphism. J. Chem. Soc. Perkin Trans. 2 1975, 181, 1835–1840. [Google Scholar] [CrossRef]
- Bryan, R.F.; Forcier, P.G. Crystal Structure Basis for the Absence of Thermal Mesomorphism in P-Hydroxy-Trans-Cinnamic Acid. Mol. Cryst. Liq. Cryst. 1980, 60, 157–165. [Google Scholar] [CrossRef]
- Atkinson, S.D.M.; Almond, M.J.; Bowmaker, G.A.; Drew, M.G.B.; Feltham, E.J.; Hollins, P.; Jenkins, S.L.; Wiltshire, K.S. The Photodimerisation of the Chloro-, Methoxy- and Nitro-Derivatives of Trans-Cinnamic Acid: A Study of Single Crystals by Vibrational Microspectroscopy. J. Chem. Soc. Perkin Trans. 2 2002, 1533–1537. [Google Scholar] [CrossRef]
- Abdelmoty, I.; Buchholz, V.; Di, L.; Guo, C.; Kowitz, K.; Enkelmann, V.; Wegner, G.; Foxman, B.M. Polymorphism of Cinnamic and α-Truxillic Acids: New Additions to an Old Story. Cryst. Growth Des. 2005, 5, 2210–2217. [Google Scholar] [CrossRef]
- Desiraju, G.R. Crystal Engineering: A Brief Overview. J. Chem. Sci. 2010, 122, 667–675. [Google Scholar] [CrossRef]
- Body, C.; Wery, G.; Gubbels, L.; Robeyns, K.; Leyssens, T. Control of Regioselectivity in the Dimerization of Trans-Cinnamic Acid and Its Derivatives Using Cocrystal Engineering. Cryst. Growth Des. 2024, 24, 2117–2125. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, Y.; Gou, Y.; Wang, Q. Synthesis, Characterization and Biological Evaluation of Two Silver(I) Trans-Cinnamate Complexes as Urease Inhibitors. Z. Anorg. Allg. Chem. 2014, 640, 423–428. [Google Scholar] [CrossRef]
- Lamann, R.; Hülsen, M.; Dolg, M.; Ruschewitz, U. Syntheses, Crystal Structures and Thermal Behavior of Five New Complexes Containing 2, 4, 6-Trifluorobenzoate as Ligand. Z. Anorg. Allg. Chem. 2012, 638, 1424–1431. [Google Scholar] [CrossRef]
- Mak, T.C.W.; Yip, W.-H.; Smith, G.; O’Reilly, E.J.; Kennard, C.H.L. Metal-Phenoxyalkanoic Acid Interactions. Part20. The Crystal Structures of Diaquabis(2-Carbamoylphenoxyacetato)-Copper(II) and Tetraaquabis(2-Carbamoylphenoxyacetato)Nickel(II). Inorganica Chim. Acta 1986, 112, 53–57. [Google Scholar] [CrossRef]
- Kennard, C.H.L.; Smith, G.; O’Reilly, E.J. Metal-Phenoxyalkanoic Acid Interactions. Part 19. The Divalent Metal Complexes of 2-Carboxyphenoxyacetic Acid and the Crystal Structures of Catena-µ[(2-Carboxylato-O)Phenoxyacetatoaquacopper(II)] and Diaquabis(2-Carboxyphenoxyacetato)Copper(II). Inorganica Chim. Acta 1986, 112, 47–51. [Google Scholar] [CrossRef]
- Stolar, T.; Batzdorf, L.; Lukin, S.; Žilić, D.; Motillo, C.; Friščić, T.; Emmerling, F.; Halasz, I.; Užarević, K. In Situ Monitoring of the Mechanosynthesis of the Archetypal Metal-Organic Framework HKUST-1: Effect of Liquid Additives on the Milling Reactivity. Inorg. Chem. 2017, 56, 6599–6608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Z.J.; Qin, Y.Y.; Zhang, J.; Cheng, J.K.; Yin, P.X.; Yao, Y.G. PH-Controlled Assembly of Two Supramolecular Architectures Based on Cu(II)-Metallacycle Building Blocks. J. Mol. Struct. 2008, 891, 138–142. [Google Scholar] [CrossRef]
- Basato, M.; Vettori, U.; Veronese, A.C.; Grassi, A.; Valle, G. Synthesis of β-Imino Carbonyl Enolato Complexes by Reaction of Nickel(II), Palladium(II), and Copper(II) Acetates with the Enaminodiones (MeOCO)(RCO)CC(R’)NH2 (R = Me, OMe; R’ = Et, EtOCO). Inorg. Chem. 1998, 37, 6737–6745. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction. CrysalisPro Software System, Version 1.171.38.41l; Rigaku Oxford Diffraction Ltd.: Oxford, UK, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure Validation in Chemical Crystallography. Acta Crystallogr. Sect. D 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- MacRae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wery, G.; Pastucha, K.; Robeyns, K.; Leyssens, T. The Inability of Metal Coordination to Control the Regioselectivity of Dimerization of Trans-Cinnamic Acid Derivatives. Crystals 2024, 14, 530. https://doi.org/10.3390/cryst14060530
Wery G, Pastucha K, Robeyns K, Leyssens T. The Inability of Metal Coordination to Control the Regioselectivity of Dimerization of Trans-Cinnamic Acid Derivatives. Crystals. 2024; 14(6):530. https://doi.org/10.3390/cryst14060530
Chicago/Turabian StyleWery, Guillaume, Karol Pastucha, Koen Robeyns, and Tom Leyssens. 2024. "The Inability of Metal Coordination to Control the Regioselectivity of Dimerization of Trans-Cinnamic Acid Derivatives" Crystals 14, no. 6: 530. https://doi.org/10.3390/cryst14060530







