Abstract
This article primarily presents cyclic voltammetry, Tafel polarization and ac. impedance spectroscopy electrochemical examinations of resorcinol (RC) electro-reactivity on the Pt(111) surface and its influence on the kinetics of UPD H (underpotentially deposited hydrogen) and the HER (hydrogen evolution reaction) in a 0.1 M NaOH supporting solution. The collected data provided evidence of the RC-ion’s surface adsorption and its further electroreduction in the presence of surface-adsorbed H radicals along with their primary beneficial role on the kinetics of the UPD H process. The above was elucidated through an evaluation of the associated charge-transfer resistance and capacitance parameters, and was carried out on the platinum (111) electrode plane, comparatively, for the RC-free and resorcinol-modified NaOH electrolyte. In addition, the recorded cathodic charge transients (obtained by injecting small amounts of RC-based 0.1 M NaOH solution to initially resorcinol-free electrolyte, carried out at the constant electrode potential characteristic to the UPD H potential zone) provided evidence that the RC species undergoes electrocatalytic reduction through the involvement of the Pt(111)-chemisorbed hydrogen radicals.
1. Introduction
The exceptional catalytic properties of platinum catalysts have led to wide-ranging research into the electrochemical performance of different organic molecules and well-ordered single-crystal Pt planes [1,2]. The current research can be traced back to some earlier works published from this laboratory on the electrochemical reactivity of small organic molecules, including guanidine [3], acetamidine [4], formamidoxime [5], urea [6,7] and single Pt crystals, as well as recently published articles on resorcinol (RC) [8,9] on polycrystalline Pt electrodes, examined in both H2SO4 and NaOH solutions.
The new work reported in the present article refers in particular to the process of the electrosorption of resorcinol ions onto the surface of a Pt(111) plane in a 0.1 M NaOH solution, which then, in the adsorbed state, become reduced by H radicals and influenced the kinetics of underpotentially deposited hydrogen (UPD H). The importance of this surface choice relates to the fact that under alkaline conditions the (111) is the only Pt plane that provides a wide potential separation between the H and OH species adsorption regions. The above not only allows for the quantification of the effect of RC adsorption on the extent of H or/and OH co-adsorption, but also for an examination of the Pt surface-based RC’s reactivity over the potential range of double-layer charging.
The UPD H appears at potentials positive to the H2’s reversible potential, when the Gibbs energy of the hydrogen atoms’ binding with the metal surface (observed at e.g., Pd, Rh, Ir and Pt) is numerically greater than half of the Gibbs energy needed to bond to a hydrogen molecule [10,11,12,13] (see Equation (1) for the UPD of H on Pt in a neutral/alkaline medium with H atoms being extracted directly from water molecules instead of H3O+ in acidic media).
On the other hand, resorcinol (phenol’s hydroxy derivative) is extensively used in industry in the production of rubber and many organic chemicals. The presence of water-soluble phenolic species such as resorcinol could have a significant impact on the performance of fuel cells and water electrolyser systems. This is because RC molecules under suitable external conditions could become susceptible to catalytic (particularly significant for semi-noble and noble metals, and their alloys) surface adsorption and electrooxidation processes. Then, under such circumstances, the RC-modified catalyst’s reactivity (e.g., Pt for hydrogen oxidation or H2 evolution reaction) might significantly change [14,15,16]. Also, phenolic species (including phenol and resorcinol) are known in the literature to be prone to selective catalytic hydrogenation in the presence and absence of external hydrogen [17,18,19,20].
2. Materials and Methods
A 0.1 M NaOH supporting electrolyte was made from sodium hydroxide monohydrate pellets (MERCK, Darmstadt, Germany, 99.99%) and ultra-pure water produced by Millipore Direct-Q3 UV water purification system (MERCK, Darmstadt, Germany) with 18.2 MΩ cm water resistivity. The resorcinol concentration (Sigma-Aldrich, St. Louis, MO, USA, >99.0%) in the working solution was in the order of 1.5 × 10−3 (and comparatively 1.5 × 10−5) M.
All electrochemical experiments were performed with a typical, three-compartment Pyrex glass electrochemical cell. The cell contained three electrodes, including the Pt(111) working electrode, aligned to the chosen crystallographic orientation by means of the back reflection von Laue X-ray diffraction method [21] with SA ≅ 0.067 cm2, Pd RHE: reversible hydrogen electrode as reference (1.0 mm diameter, Sigma-Aldrich, 99.99%), and a counter electrode (CE) made from a coiled Pt wire (1.0 mm diameter, 99.9998% purity, Johnson Matthey Inc., London, UK). Before conducting experiments, solutions were de-aerated with high-purity argon (6.0, Linde, Woking, UK), the flow of which was also maintained above the solution’s during the measurements.
Furthermore, the charge-transient experiments were carried out at the constant electrode potential mode by injecting 100 µL amounts (Hamilton 710 N micro-syringe model, Hamilton Company, Reno, NV, USA) of 1.2 M RC into the 0.1 M NaOH (previously de-aerated by bubbling with high-purity Ar) to create an initially resorcinol-free (also de-aerated) unmodified sodium hydroxide solution. The experiments were performed (six independent trials) at the electrode potential characteristic of the process of the UPD H.
Biologic SP-240 Electrochemical System was used to carry out all electrochemistry tests. Cyclic voltammetry (performed at 50 mV s−1), Tafel polarization (carried-out at the scan-rate of 0.5 mV s−1) and ac. impedance spectroscopy experiments were conducted in this work. For the impedance measurements, the generator provided an output signal of 5 mV, whereas the frequency range was swept between 1.0 × 105 and 1.0 Hz (or 20 mHz). The instrument was controlled by EC-Lab® V11.36 software for Windows. Three impedance measurements were carried out at each electrode potential with the duplicability of such obtained results being in the order of 10% or below. Data analysis was performed with ZView 4.0 software package for Windows, where the impedance spectra were fitted by means of LEVM 6—a complex, non-linear, least-squares immittance fitting program, written by J.R. Macdonald in Ref. [22].
3. Results and Discussion
3.1. Cyclic Voltammetry, Linear Sweep Voltammetry and Tafel Polarization Behaviour of Pt(111) in 0.1 M NaOH, in the Absence and Presence of Resorcinol
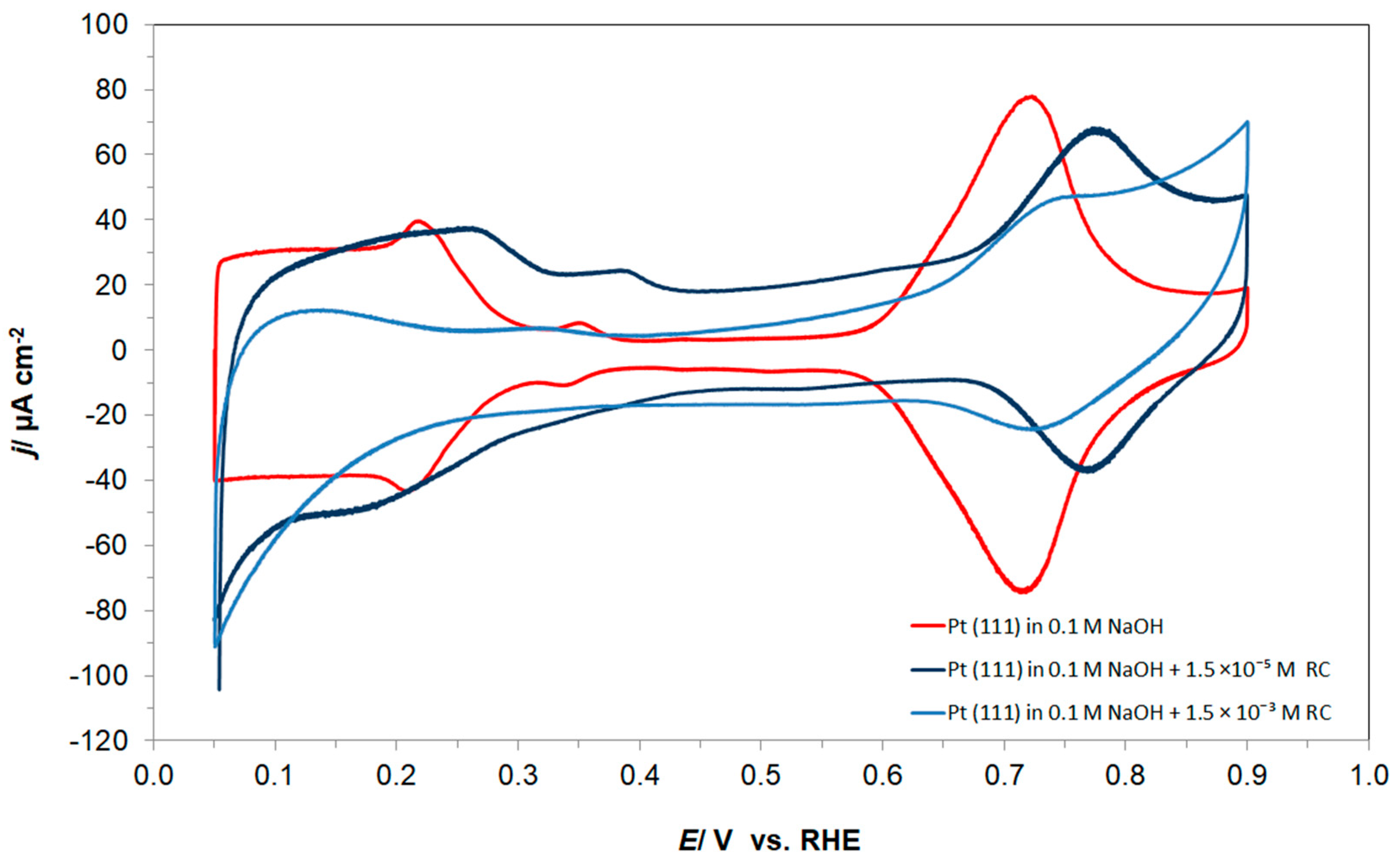
The cyclic voltammetric behaviour of the Pt(111) electrode in contact with 0.1 M NaOH supporting solution, in the absence and presence of resorcinol (at 1.5 × 10−3 M), is shown in Figure 1 below. Hence, in the absence of resorcinol, the CV profile of the single-crystal Pt(111) plane in an alkaline medium is characterized by the presence of two well-separated adsorption regions over the potential ranges 0.05–0.40 V and 0.60–0.90 V vs. RHE, isolated by a double-layer charging region. The former corresponds to the reversible adsorption of H atoms (underpotential deposition of hydrogen), whereas the latter, so-called “butterfly”, region refers to the reversible electrosorption of OH− anions [6,23] (see Figure 1). Hence, the mean electric charge measured between 0.05 and 0.90 V, after subtracting the double-layer charge contribution, came to 283 µC cm−2, being a sum of 106 and 177 µC cm−2, corresponding to the first low potential zone, and to the high potential OH adsorption “butterfly” region, respectively. These charges translate to a maximum coverage of H by UPD (θH) of a ca. 0.44 hydrogen monolayer and to an OH species (θOH) of about 0.73 of an equivalent monolayer of H (compare with e.g., Ref. [24]).
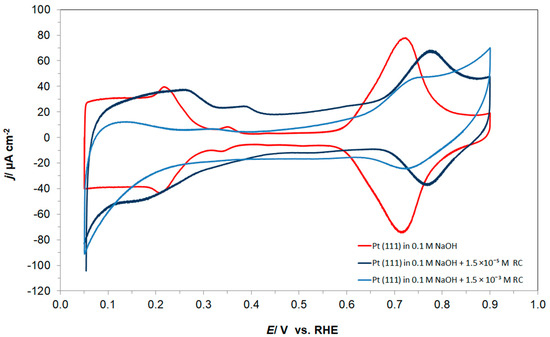
Figure 1.
Cyclic voltammograms for Pt(111) electrode surface, recorded over the potential range characteristic to UPD H and OH reversible adsorption in 0.1 M NaOH supporting solution (on the 2nd cycle), at a sweep-rate of 50 mV s−1, in the absence and presence of resorcinol, at the concentrations indicated.
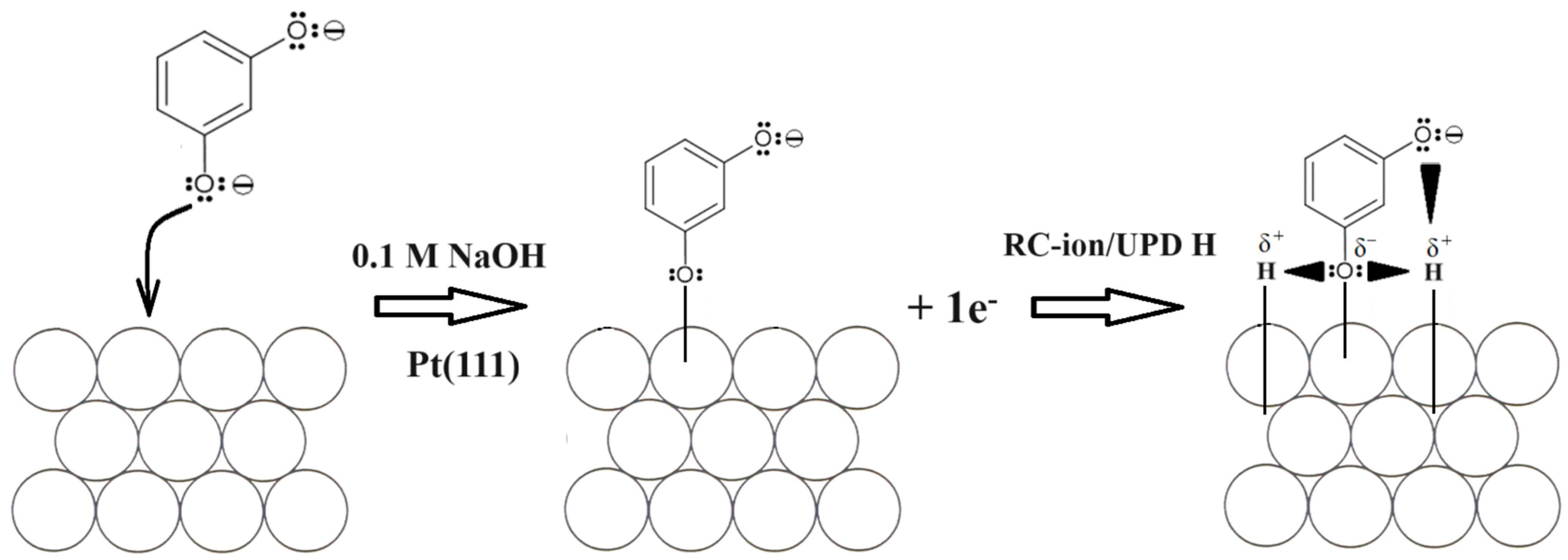
Having introduced resorcinol at the concentration of 1.5 × 10−3 M into the supporting NaOH solution, the total voltammetric charge (blue curve in Figure 1) radically depleted to reach 154 µC cm−2, which split to 56 and 98 µC cm−2, correspondingly, for the UPD H (θH/RC ≈ 0.23) and OH (θOH/RC ≈ 0.40) adsorption regions. The above is strongly believed to be a result of simultaneous Pt surface co-adsorption and the interaction of large RC ions (formed after complete resorcinol ionization in sodium hydroxide [25]) with hydrogen atoms and also OH groups (the latter is also evidenced through a significant thickening of the double-layer charging zone). The proposed mechanism for the resorcinol oxidative Pt electrosorption and the RC ion-to-H attractive interaction, i.e., via partial or complete negative charge on the oxygen atom vs. partial positive charge on the hydrogen atom, is illustrated in Scheme 1 below. The above is also confirmed through the recorded ac. impedance behaviour described in detail in Section 3.2.
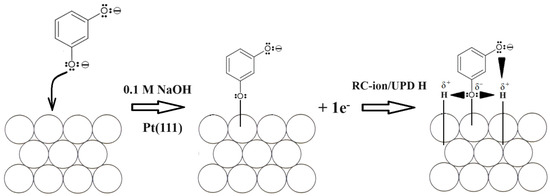
Scheme 1.
The proposed mechanism of RC-ion anodic electrosorption on the surface of Pt(111) electrode in 0.1 M NaOH solution along with consecutive RC-ion-to-H attractive interaction in the adsorbed state over the potential range characteristic to UPD of H.
On the other hand, both the UPD of H and the OH reversible adsorption regions are much more pronounced (in reference to the resorcinol free cyclic voltammogram) in the voltammetric profile of the radically reduced RC-ion concentration at the level of 1.5 × 10−5 M (see a dark blue line in Figure 1).
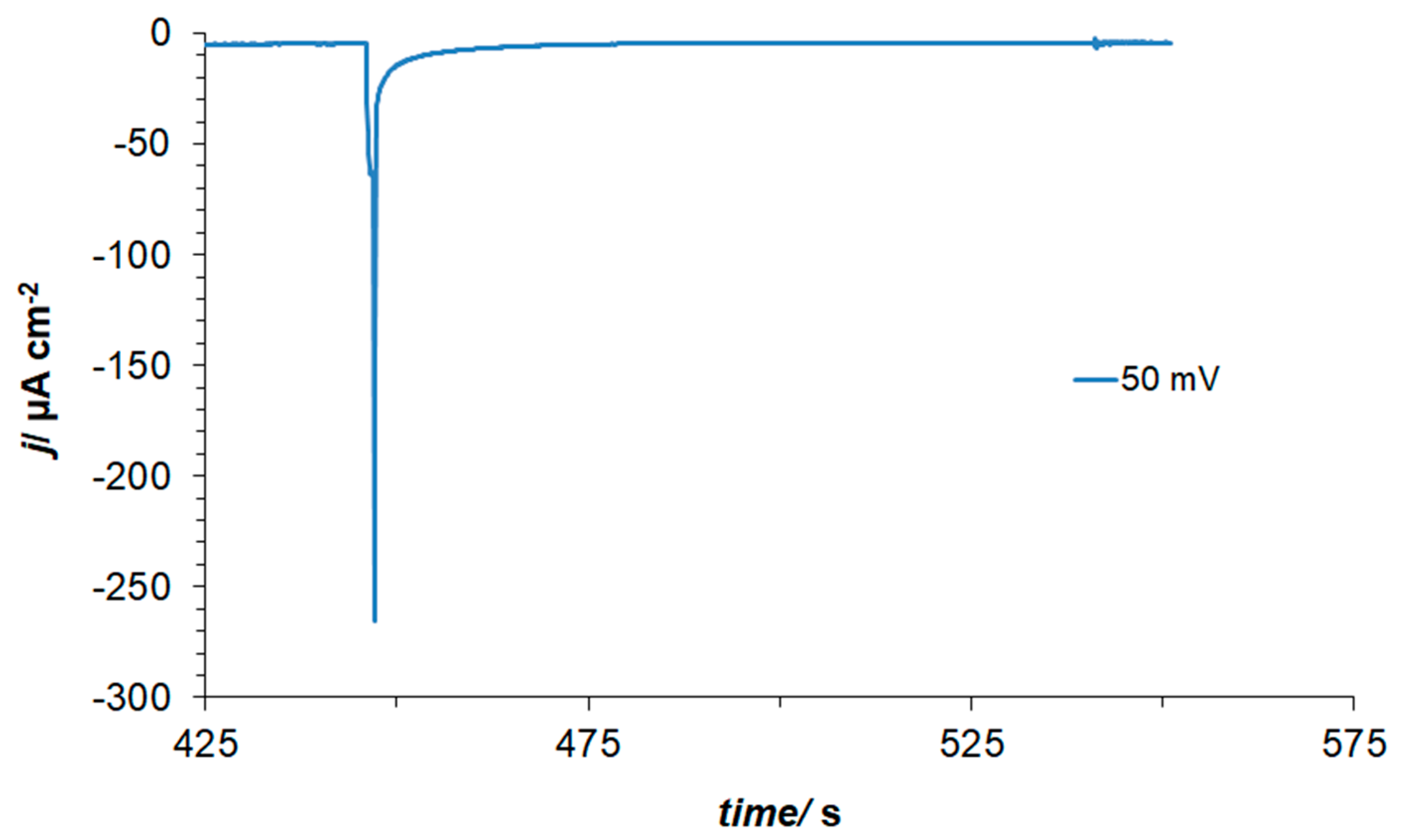
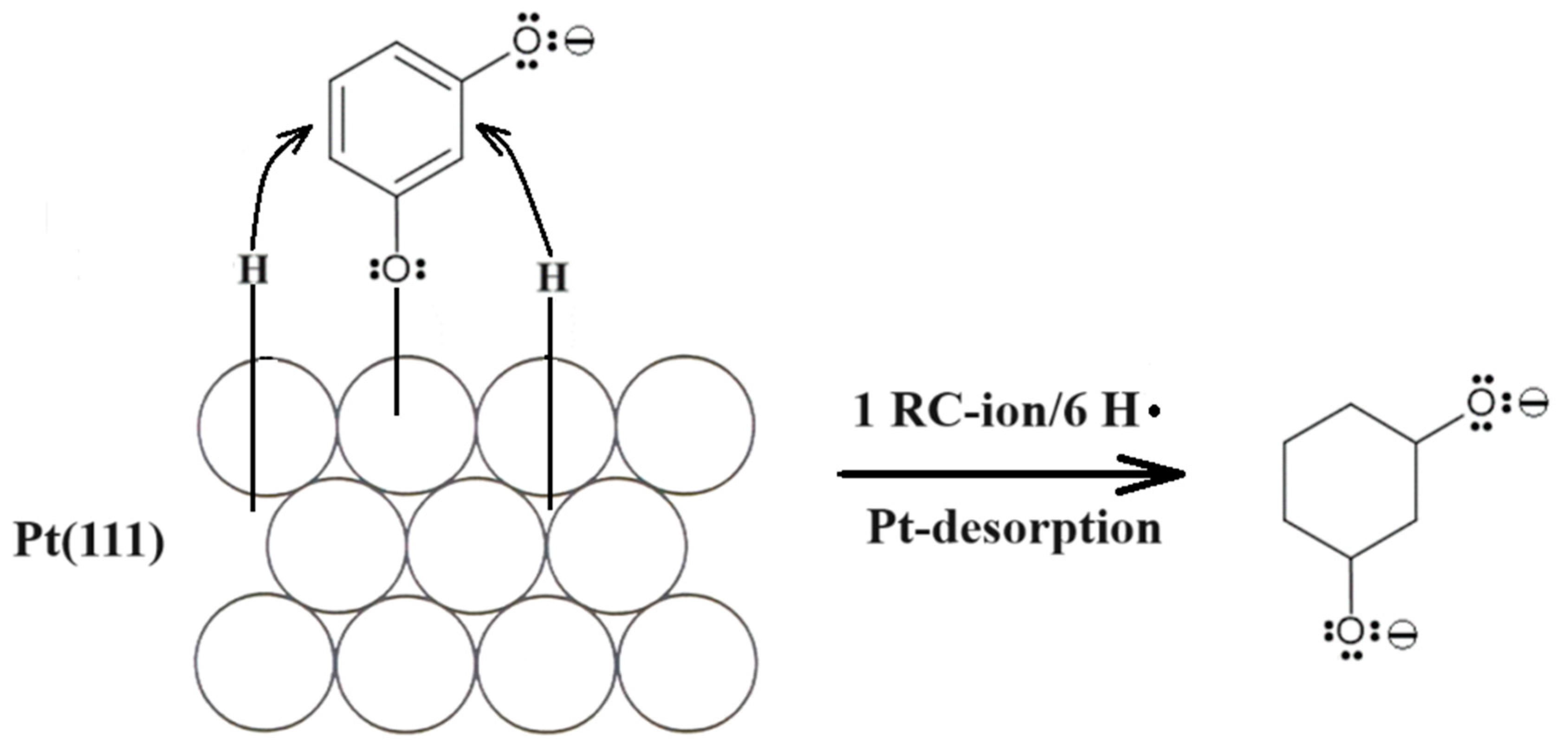
Furthermore, in order to explain the presence of a non-negligible negative current flowing between 0.05 and 0.20 V in the presence of resorcinol (see Figure 1 again), charge-transient experiments were performed at the electrode potential of 0.05 V. As a result, a large, average cathodic charge-transient density of ca. −220 µC cm−2 was recorded (Figure 2) after subtracting the O2 reduction contribution (about −45 µC cm−2). Hence, no charge-transient behaviour (beyond that involving inevitable oxygen reduction, as small amounts of air are always being sucked in along with solution’s sample) was observed for injections of resorcinol-free 0.1 M NaOH solution samples. In conclusion, under the experimental conditions, the observed cathodic charge-transients recorded at the potential value of 50 mV could only correspond to the process of resorcinol ion electroreduction at the Pt(111) single-crystal plane, realized via the underpotentially deposited hydrogen radicals. The resorcinol ion reduction process proposed here to form a 1,3-cyclohexanediolate ion [17,18,19] with six UPD H atoms per single RC entity is illustrated in Scheme 2 below.
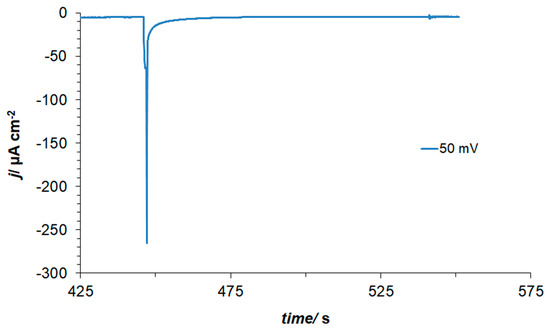
Figure 2.
Current–density vs. time transient recorded for the potential of 50 mV vs. RHE.
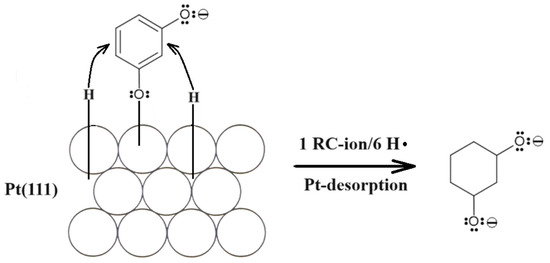
Scheme 2.
The proposed mechanism of RC-ion reduction to 1,3-cyclohexanediolate ion by UPD H on the surface of Pt(111) electrode in 0.1 M NaOH solution.
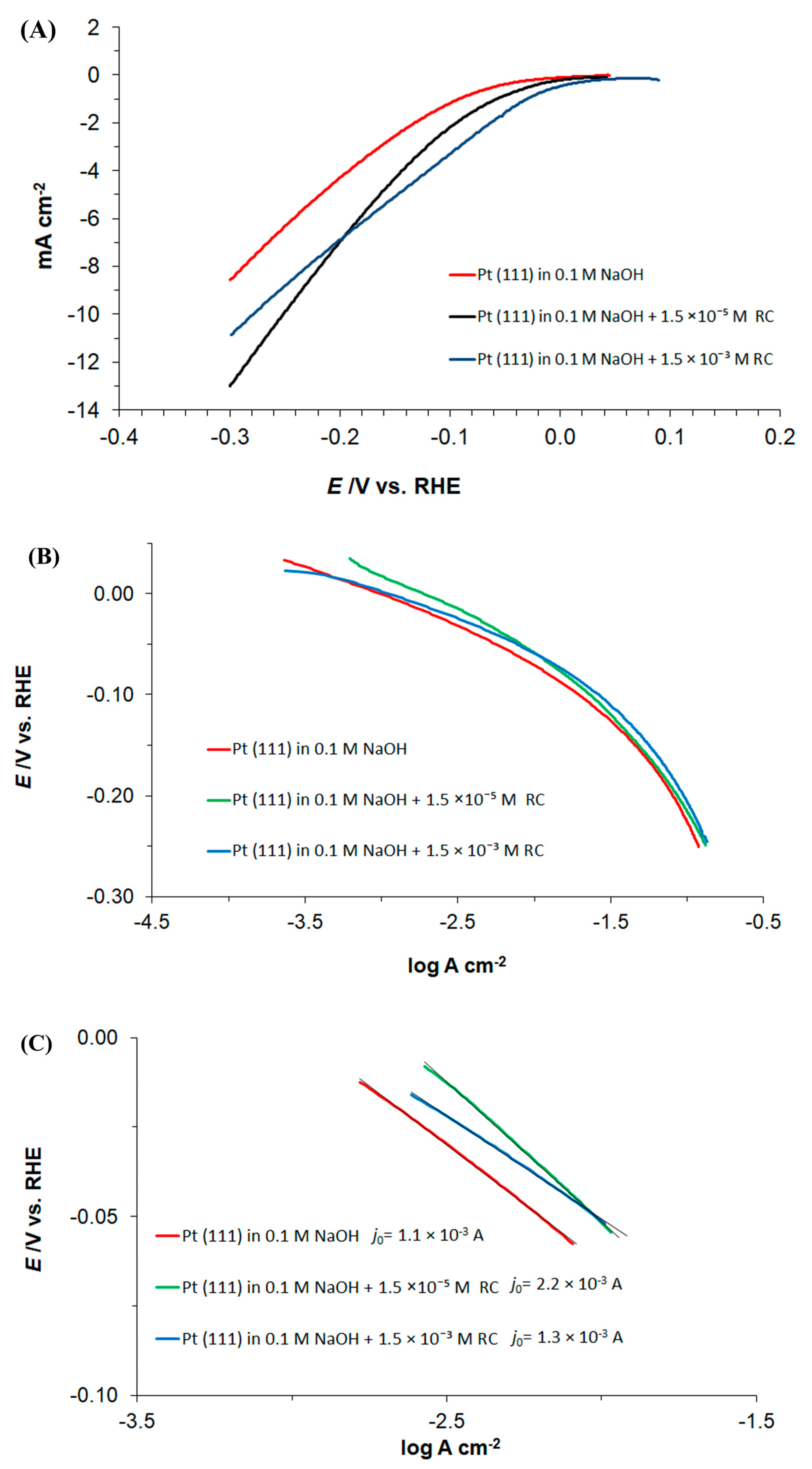
Interestingly, additional linear-sweep voltammetry experiments, extended to the potential range negative to the hydrogen reversible potential (0.00 to −0.30 V/RHE), showed that the Pt surface-adsorbed resorcinol species (or its electroreduction product/s) exhibited a significant positive influence on the HER rates, which can clearly be seen in Figure 3A. Hence, for the cathodic overpotential of 0.3 V, the recorded current densities were 1.3× and 1.5× greater for the RC-ion concentrations of 1.5 × 10−3 and 1.5 × 10−5 M, respectively, than those recorded in the absence of resorcinol in the solution (contrast to the behaviour at low overpotentials, where higher concentration of the RC-ion provided superior facilitation of the HER kinetics).
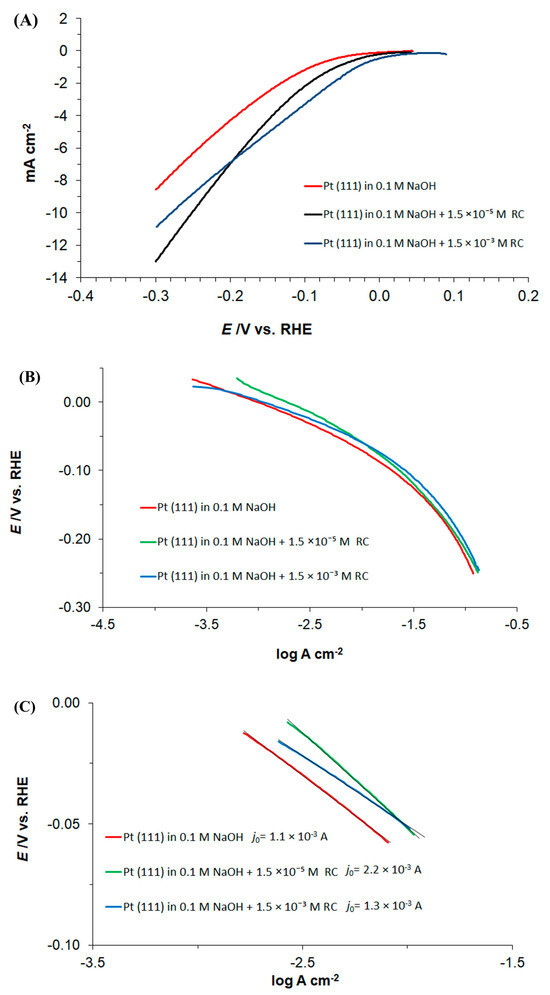
Figure 3.
(A) Linear sweep voltammetry measurements for Pt(111) electrode surface, recorded over the potential range characteristic to HER (0.0 to −0.3 V vs. RHE) in 0.1 M NaOH supporting solution, at a sweep-rate of 50 mV s−1, in the absence and presence of resorcinol, at the concentrations indicated; (B) as above, but quasi-potentiostatic cathodic Tafel polarization curves for the HER (recorded at a rate of 0.5 mV s−1) with appropriate iR corrections made, based on the solution resistance derived from the impedance measurements; (C) as in (B), but showing low overpotential linear region with fitting lines along with calculated Tafel slopes.
In addition, a significant enhancement in the the HER characteristics upon introduction of resorcinol into the supporting electrolyte could also unambiguously be observed over the kinetically controlled, low overpotential region of the cathodic polarization curves in Figure 3B,C. The recorded cathodic Tafel slopes (parameter b) approached 66, 78 and 57 mV dec−1 for the unmodified NaOH and the RC concentrations of 1.5 × 10−5, and 1.5 × 10−3 M, respectively (Figure 3C). Also, the presence of resorcinol somewhat facilitated Tafel-estimated values of an exchange current-density (j0) parameter, which came to 1.1 mA cm−2 (0.1 M NaOH), 2.2 mA cm−2 (0.1 M NaOH + 1.5×10−5 M RC) and 1.3 mA cm−2 (0.1 M NaOH + 1.5×10−3 M RC). The latter observation is in line with the literature-based j0 values recorded for platinum or Pt-based HER catalysts [26,27]. Finally, as the HER rate begins to be restricted by the mass transfer rate (for overpotentials beyond ca. 200 mV, see Figure 3B again), all polarization curves begin to converge.
3.2. Ac. Impedance Behaviour of Pt(111) in 0.1 M NaOH, in the Absence and Presence of Resorcinol
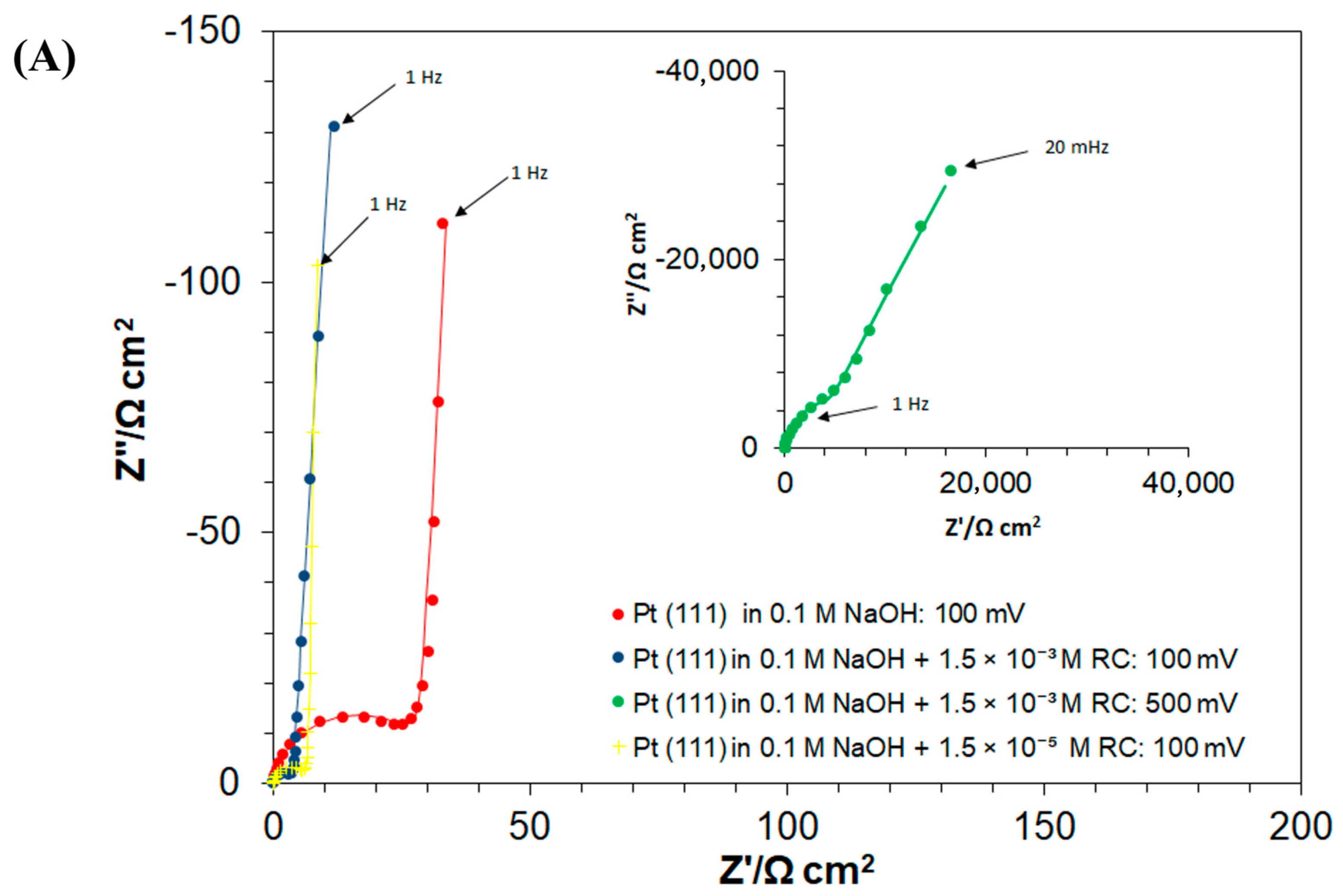
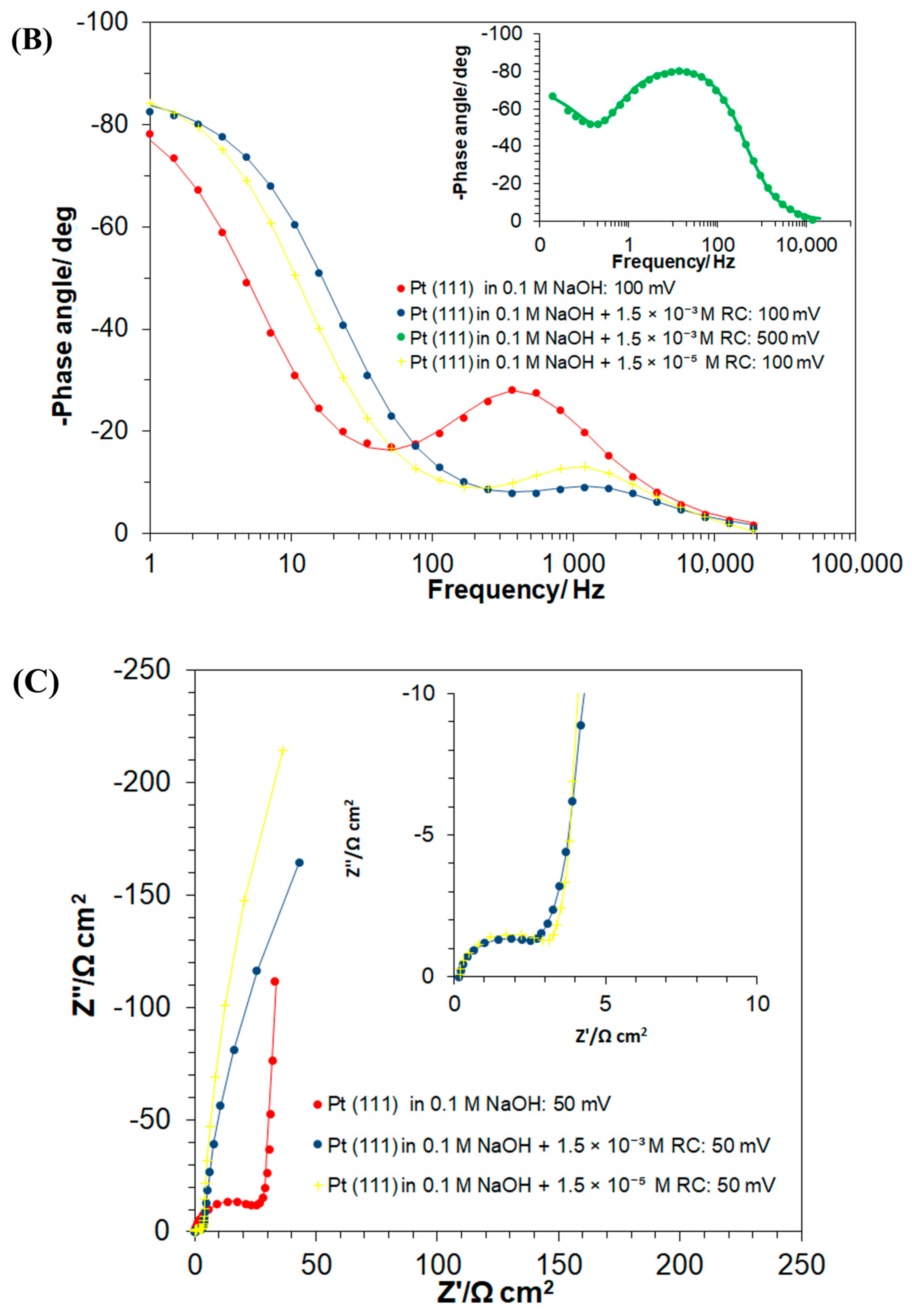
The Ac. impedance electrochemical behaviour of the Pt(111) electrode in unmodified 0.1 M NaOH solution and in the presence of 1.5 × 10−3 M RC, examined over the potential range characteristic to the processes of UPD of H and the RC-ion electrosorption, is shown in Figure 4A–C and Table 1. The process of the UPD of H generates a semicircle present over the high and intermediate frequency range in the Nyquist impedance plot along with a vertical line (Figure 4A), which characterizes purely capacitive behaviour at relatively low frequencies. The latter often deviates from a 90° angle, due to the so-called capacitance dispersion effect [3,28], related to surface roughness and heterogeneity.
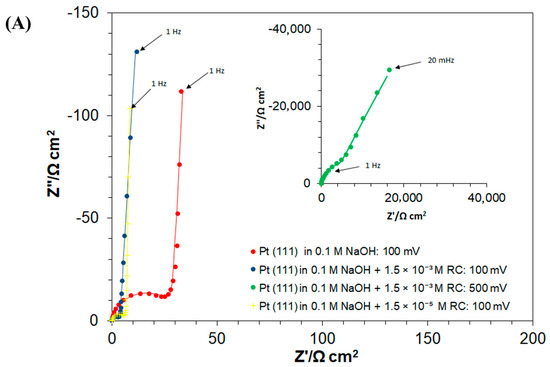
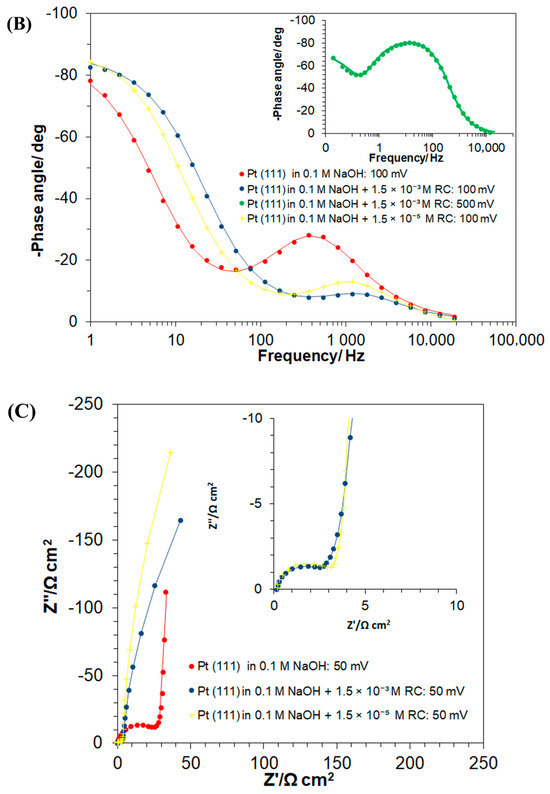
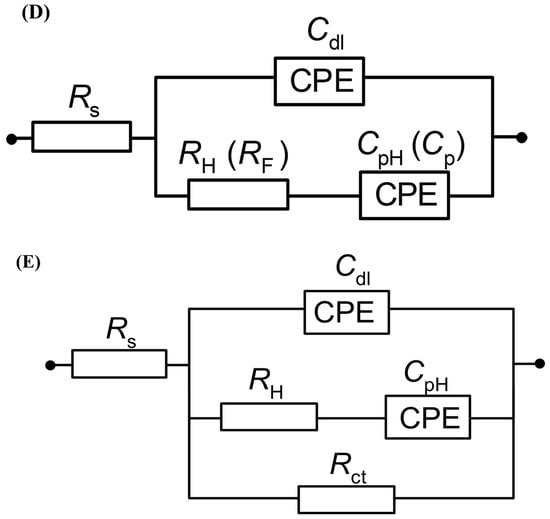
Figure 4.
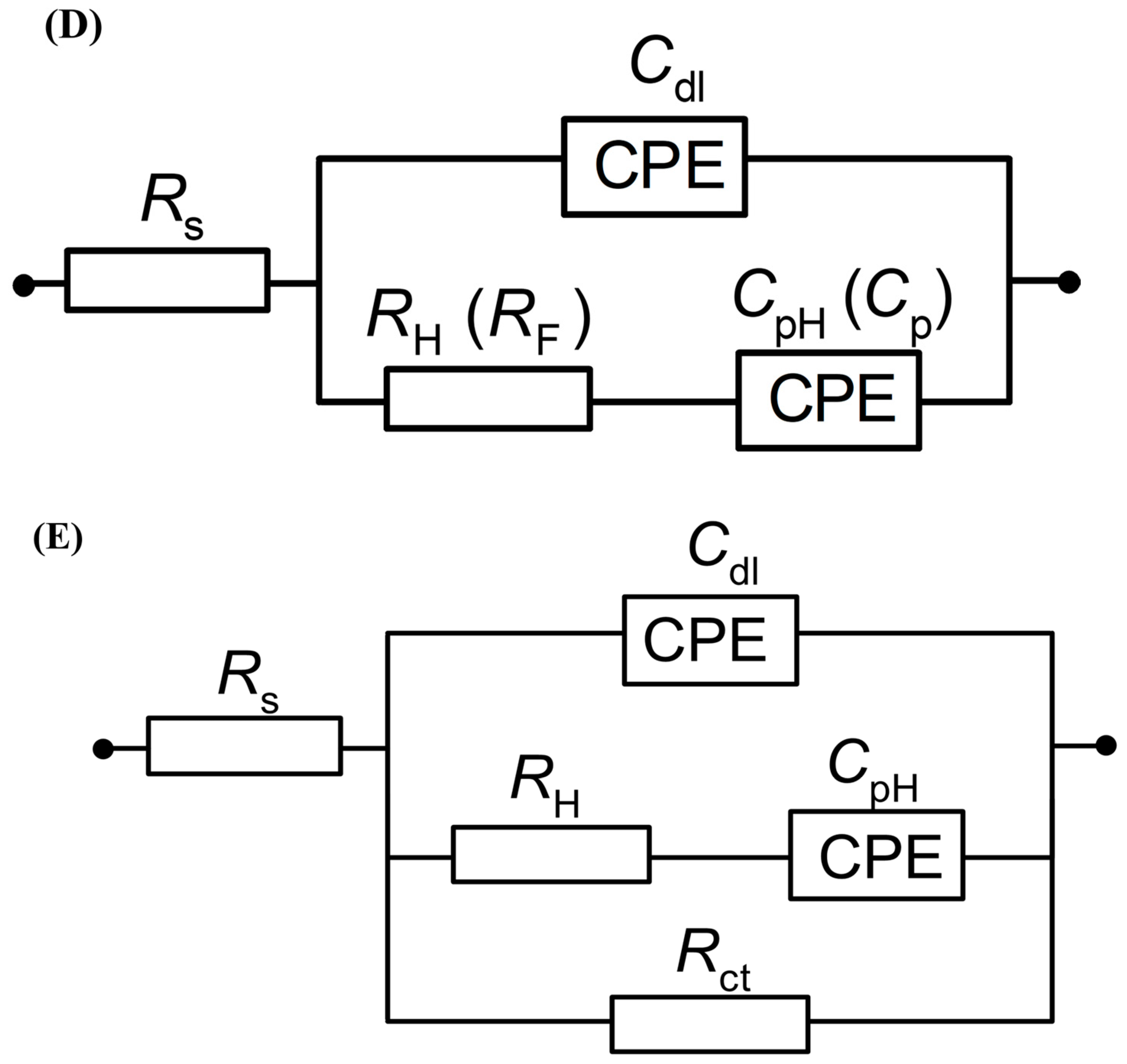
(A) Complex-plane Nyquist impedance plots for the processes of underpotential deposition of hydrogen (UPD of H) and electrosorption of resorcinol ion on Pt(111) electrode surface in contact with 0.1 M NaOH and in the presence of RC for the concentrations of 1.5 × 10−3 and 1.5 × 10−5 M, recorded at 293 K for the potentials indicated. The solid lines correspond to representation of the data according to the equivalent circuit shown in (D); (B) as above, but Bode phase-angle plots; (C) complex-plane Nyquist impedance plots for the process of underpotential deposition of hydrogen (UPD of H) and electroreduction of resorcinol species on Pt(111) electrode surface in contact with 0.1 M NaOH, for the RC-ion concentrations of 1.5 × 10−3 and 1.5 × 10−5 M, recorded at 293 K for the electrode potential of 50 mV. The solid lines correspond to representation of the data according to the equivalent circuits shown in (D,E); (D) equivalent circuit for UPD of H adsorption (or resorcinol ion adsorption) process, exhibiting Faradaic pseudocapacitance, CpH (or Cp), charged via a Faradaic resistance, RH (or RF), in a parallel combination with the double-layer capacitance, Cdl (both capacitance parameters are CPE: constant phase element-modified), jointly in series with an uncompensated solution resistance, RS; (E) equivalent circuit for UPD of H upon parallel Pt surface electroreduction of resorcinol ion (Rct); other details as above.

Table 1.
Resistance and capacitance parameters for UPD of H, electrosorption and electroreduction of resorcinol ion on Pt(111) electrode surface in contact with 0.1 M NaOH and in the presence of RC-ion (1.5 × 10−3 and 1.5 × 10−5 M, and 293 K), obtained by fitting the equivalent circuits shown in Figure 4D,E to the acquired impedance data (values of dimensionless φ parameter for the CPE circuits fluctuated between 0.73 and 0.98; impedance reproducibility typically remained below 10%; χ2 = 3 × 10−5 to 5 × 10−3).
Hence, the corresponding charge-transfer resistance, the RH parameter (proportional to the inverse of the exchange rate for the process of UPD of H), considerably increased from 17.6 (at 50 mV) to reach 311.6 Ω cm2 at 300 mV (where H adsorption became radically depleted). Then, the double-layer capacitance, Cdl, values fluctuated between 44 and 48 μF cm−2 for the corresponding electrode potential range. On the other hand, the H adsorption pseudocapacitance, CpH, parameter stayed relatively constant (above 600 μF cm−2) over the potential range of 50–200 mV, which then dramatically dropped to 116 μF cm−2 at the mentioned electrode potential of 300 mV vs. RHE (see Table 1 for details and a comparison of these results with analogous data on the kinetics of the UPD of H published in refs. [6,23,29]).
Then, the introduction of resorcinol (at the concentration of 1.5 × 10−3 M) into the supporting electrolyte caused the RH parameter to become radically reduced, i.e., to 3.3 Ω cm2 at 50 mV (5.3 times) and to 11.1 Ω cm2 at 200 mV (7.2 times), as compared to the RC-free electrolyte. Then, the recorded Cdl and CpH capacitance values largely resembled those of the RC-unmodified sodium hydroxide solution. Interestingly, the occurrence of another partial semicircle over intermediate and low frequencies, at the potential of 50 mV (see Figure 4C,E, and the respective Rct value of 1005 Ω cm2 in Table 1) coincides with the voltammetric cathodic feature observed in Figure 1, over the potential range of 0.05–0.20 V. The latter is strongly believed to be associated with the Faradaic process of RC-ion reduction, realized via surface-chemisorbed UPD H atoms (see Scheme 2 again) and is in line with the results obtained through the above-discussed charge-transient experiments in Figure 2 above.
However, in contrast to the RC-free electrolyte, in the presence of resorcinol, another partial semicircle (seen over high and intermediate frequencies) with a low frequency capacitive line, at an inclination to the Z′ axis significantly lower than 90°, can be observed in the Nyquist impedance spectra. The latter was identified over the potential range of 300–500 mV/RHE, characteristic of the double-layer charging zone (see as examples a Nyquist impedance spectrum and a Bode phase-angle plot recorded at 500 mV in insets to Figure 4A, and Figure 4B, respectively). This behaviour is strongly believed to be associated with the Faradaic process of the RC-ion electrosorption on the Pt(111) surface, where its kinetics are several orders in magnitude slower than those of the process of UPD of H (see the respective charge-transfer resistance, RF, Cp and Cdl capacitance parameter values, recorded in Table 1 and Scheme 1 above). Then, while in the adsorbed state, the RC ions and chemisorbed H atoms (now with significantly limited surface coverage though) undergo attractive interaction (see Scheme 1 again), which as a result considerably facilitates the kinetics of the process of underpotential deposition of H (again, compare the recorded values of the RH parameter for the RC-free and resorcinol-modified NaOH solution in Table 1). Furthermore, as for platinum single-crystals’ electrodes, the UPD H kinetics play “a prerequisite role” in the Faradaic process of bulk hydrogen evolution [30,31], the surface-electrosorbed resorcinol entity (or its electroreduction product/s) might potentially be considered as an efficient, HER (hydrogen evolution reaction) catalytic Pt surface modifier.
Furthermore, comparative ac. impedance experiments carried-out at a radically lower RC-ion concentration in the working solution (1.5 × 10−5 M) provided kinetic UPD H data that are generally in-line with those obtained for a higher resorcinol concentration, although the corresponding charge transfer resistance, Rct parameter value (recorded at 50 mV vs. RHE, Table 1) is about 1.7 times greater. The above implies that a higher RC-ion concentration in the electrolyte significantly facilitates its Pt surface reduction process. Moreover, contrary to the behaviour observed at higher RC concentration, at the resorcinol concentration of 1.5 × 10−5 M, the rate of RC electrosorption on the Pt surface seems to be inversely proportional to the electrode potential. The latter, we believe, might be related to RC-to-OH species surface competitive interactions, which could become significant at high anodic potentials. However, this effect requires further study and detailed elucidation.
Interestingly, in recent work from this laboratory [8], resorcinol has been found to have a detrimental effect on the kinetics of the process of UPD of H on polycrystalline Pt electrode in 0.5 M H2SO4, already at the concentration of 1.0 × 10−5 M RC (with θH/RC ≈ 0.29). The latter most likely results from the fact that under alkaline conditions (in contrast to the behaviour in acidic solution) surface electrosorption of resorcinol species is significantly limited, due to the existing repulsive interactions, exhibited between the adsorbed RC anions (Pt–RC–-to-–RC–Pt).
4. Conclusions
The employment of cyclic voltammetry, cathodic Tafel polarization, ac. impedance spectroscopy along with a chronoamperometric charge-transient electrochemical techniques to study the influence of resorcinol (a valuable industrial chemical and possible contaminant of electrolyser/fuel cell systems) on the kinetics of the UPD of H and HER processes, studied on a Pt(111) single-crystal electrode under alkaline conditions, provided confirmation of significant positive role of platinum-electrosorbed resorcinol ions (which simultaneously undergo a Faradaic reduction process, most likely to form 1,3-cyclohexanediol derivative in the presence of UPD H atoms) on the rates of underpotential deposition of hydrogen, which is a precursor of the hydrogen evolution reaction with platinum series catalysts. Furthermore, a constructive role (e.g., through facilitation of final H2 surface desorption step) of the RC species adsorbed onto the Pt(111) surface (and/or its electroreduction derivatives) on the kinetics of bulk hydrogen evolution process was also illustrated through additional linear-sweep voltammetry and Tafel polarization experiments, conducted over the potential range of ca. 0.00 to −0.30 V vs. RHE.
However, further laboratory work (perhaps with the involvement of additional, in-situ infrared experiments) would be required in order to provide detailed understanding of the process of RC-ion adsorption on the surface of Pt(111) plane and to provide more insights into competitive RC-ion-to-UPD of H adsorption, especially on how it might affect the kinetics and mechanisms of processes of UPD and OPD of H, and HER Faradaic reaction, also in relation to the behaviour recently reported in Ref. [8] for 0.5 M H2SO4.
Author Contributions
B.P. was a scientific initiator and work coordinator, and prepared a final version of the manuscript; M.K. ran the experiments and carried-out treatment of all the results; T.M. supervised laboratory work and helped with preparation of the draft manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by the internal research grant no. 30.610.001-110, provided by The University of Warmia and Mazury in Olsztyn.
Data Availability Statement
Data supporting reported results will be available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, N.; Sanyal, U.; Fulton, J.L.; Gutiérrez, O.Y.; Lercher, J.A.; Campbell, C.T. Quantifying adsorption of organic molecules on platinum in aqueous phase by hydrogen site blocking and in situ X-ray absorption spectroscopy. ACS Catal. 2019, 9, 6869–6881. [Google Scholar] [CrossRef]
- Sui, C.; Ma, X.Y.; Fu, W.H.; Zeng, S.P.; Xie, R.R.; Zhang, Z.P. Regulating Pt-based noble metal catalysts for the catalytic oxidation of volatile organic compounds: A mini review. Rev. Inorg. Chem. 2023, 43, 561–570. [Google Scholar] [CrossRef]
- Conway, B.E.; Pierozynski, B. Ac impedance behaviour of processes involving adsorption and reactivity of guanidonium-type cations at Pt(100) surface. J. Electroanal. Chem. 2008, 622, 10–14. [Google Scholar] [CrossRef]
- Conway, B.E.; Pierozynski, B. Influence of acetamidine on the electrosorption of UPD H at Pt single-crystal surfaces. J. Electroanal. Chem. 2008, 623, 102–108. [Google Scholar] [CrossRef]
- Pierozynski, B.; Kowalski, I.M. Electrochemical reactivity of formamidoxime on Pt(111) and (100) single-crystal surfaces in 0.1MNaOH solution. J. Electroanal. Chem. 2011, 662, 432–436. [Google Scholar] [CrossRef]
- Pierozynski, B. Electrochemical Behaviour of Urea at Pt(111) Single-Crystal Surface in 0.1 M NaOH. Electrocatalysis 2012, 4, 37–41. [Google Scholar] [CrossRef]
- Pierozynski, B. Electrochemical reactivity of urea at Pt(100) surface in 0.5 M H2SO4 by AC impedance spectroscopy. J. Solid State Electrochem. 2012, 17, 889–893. [Google Scholar] [CrossRef]
- Kuczyński, M.; Łuba, M.; Mikołajczyk, T.; Pierożyński, B.T. Effect of resorcinol on the kinetics of underpotentially deposited hydrogen and the oxygen evolution reaction, studied on polycrystalline Pt in a 0.5 M H2SO4 solution. Energies 2022, 15, 1092. [Google Scholar] [CrossRef]
- Kuczyński, M.; Łuba, M.; Mikołajczyk, T.; Pierożyński, B. The influence of resorcinol on the kinetics of underpotentially deposited hydrogen, cathodic hydrogen and anodic oxygen evolution reactions, examined at polycrystalline Pt electrode in 0.1 M NaOH solution. Int. J. Hydrogen Energy 2023, 48, 10755–10764. [Google Scholar] [CrossRef]
- Clavilier, J.; Rodes, A.; El Achi, K.; Zamakhchari, M. Electrochemistry at platinum single crystal surfaces in acidic media: Hydrogen and oxygen adsorption. J. Chim. Phys. 1991, 88, 1291–1337. [Google Scholar] [CrossRef]
- Morin, S.; Dumont, H.; Conway, B. Evaluation of the effect of two-dimensional geometry of Pt single-crystal faces on the kinetics of upd of H using impedance spectroscopy. J. Electroanal. Chem. 1996, 412, 39–52. [Google Scholar] [CrossRef]
- Jerkiewicz, G. Rlectrochemical hydrogen adsorption and absorption. Part 1: Under-potential deposition of hydrogen. Electrocatalysis 2010, 1, 179–199. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Jurczakowski, R.; Lasia, A. Kinetics of hydrogen underpotential deposition at iridium in sulfuric and perchloric acids. Electrochim. Acta 2017, 225, 160–167. [Google Scholar] [CrossRef]
- Lynch, B.S.; Delzell, E.S.; Bechtel, D.H. Toxicology review and risk assessment of resorcinol: Thyroid effects. Regul. Toxicol. Pharmacol. 2002, 36, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, D.; Palanivelu, K.; Mohan, N. Electrochemical oxidation of resorcinol for wastewater treatment—A kinetic study. Indian J. Chem. Technol. 2003, 10, 396–401. [Google Scholar]
- Nady, H.; El-Rabiei, M.; El-Hafez, G.A. Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef]
- Yang, J.; Williams, C.L.; Ramasubramaniam, A.; Dauenhauer, P.J. Aqueous-phase hydrodeoxygenation of highly oxygenated aromatics on platinum. Green Chem. 2013, 16, 675–682. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, J.; Chen, L. Selective hydrogenation of phenol and related derivatives. Catal. Sci. Technol. 2014, 4, 3555–3569. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, R.; Hou, Y.; Yang, Y.; Liu, Y. Graphene-supported Pd catalyst for highly selective hydrogenation of resorcinol to 1, 3-cyclohexanedione through giant π-conjugate interactions. Sci. Rep. 2015, 5, 15664. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Zhou, M.; Sharma, B.K.; Jiang, J. Selective hydrogenation of phenol to cyclohexanol over Ni/CNT in the absence of external hydrogen. Energies 2020, 13, 846. [Google Scholar] [CrossRef]
- Hamelin, A. Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O.M., White, R.E., Eds.; Plenum Press: New York, NY, USA, 1985; Chapter 1; p. 16. [Google Scholar]
- Macdonald, J.R. Impedance Spectroscopy, Emphasizing Solid Materials and Systems; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Schouten, K.J.P.; van der Niet, M.J.T.C.; Koper, M.T.M. Impedance spectroscopy of H and OH adsorption on stepped single-crystal platinum electrodes in alkaline and acidic media. Phys. Chem. Chem. Phys. 2010, 12, 15217–15224. [Google Scholar] [CrossRef] [PubMed]
- Morallón, E.; Vázquez, J.L.; Aldaz, A. Electrochemical behaviour of Pt(111) in alkaline media. Effect of specific adsorption of anions. J. Electroanal. Chem. 1992, 334, 323–338. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Tharat, B.; Hirunsit, P.; Suthirakun, S. Electrochemical oxidation of resorcinol: Mechanistic insights from experimental and computational studies. RSC Adv. 2020, 10, 28454–28463. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Gasteiger, H.A.; Horn, Y.S. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Pajkossy, T. Impedance of rough capacitive electrodes. J. Electroanal. Chem. 1994, 364, 111–125. [Google Scholar] [CrossRef]
- Botello, L.E.; Feliu, J.M.; Climent, V. Activation energy of hydrogen adsorption on Pt(111) in alkaline media: An impedance spectroscopy study at variable temperatures. ACS Appl. Mater. Interfaces 2020, 12, 42911–42917. [Google Scholar] [CrossRef]
- Conway, B.; Barber, J.; Morin, S. Comparative evaluation of surface structure specificity of kinetics of UPD and OPD of H at single-crystal Pt electrodes. Electrochim. Acta 1998, 44, 1109–1125. [Google Scholar] [CrossRef]
- Barber, J.; Conway, B. Structural specificity of the kinetics of the hydrogen evolution reaction on the low-index surfaces of Pt single-crystal electrodes in 0.5 M dm−3 NaOH. J. Electroanal. Chem. 1999, 461, 80–89. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).