Electro-Reactivity of Resorcinol on Pt(111) Single-Crystal Plane and Its Influence on the Kinetics of Underpotentially Deposited Hydrogen and Hydrogen Evolution Reaction Processes in 0.1 M NaOH Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
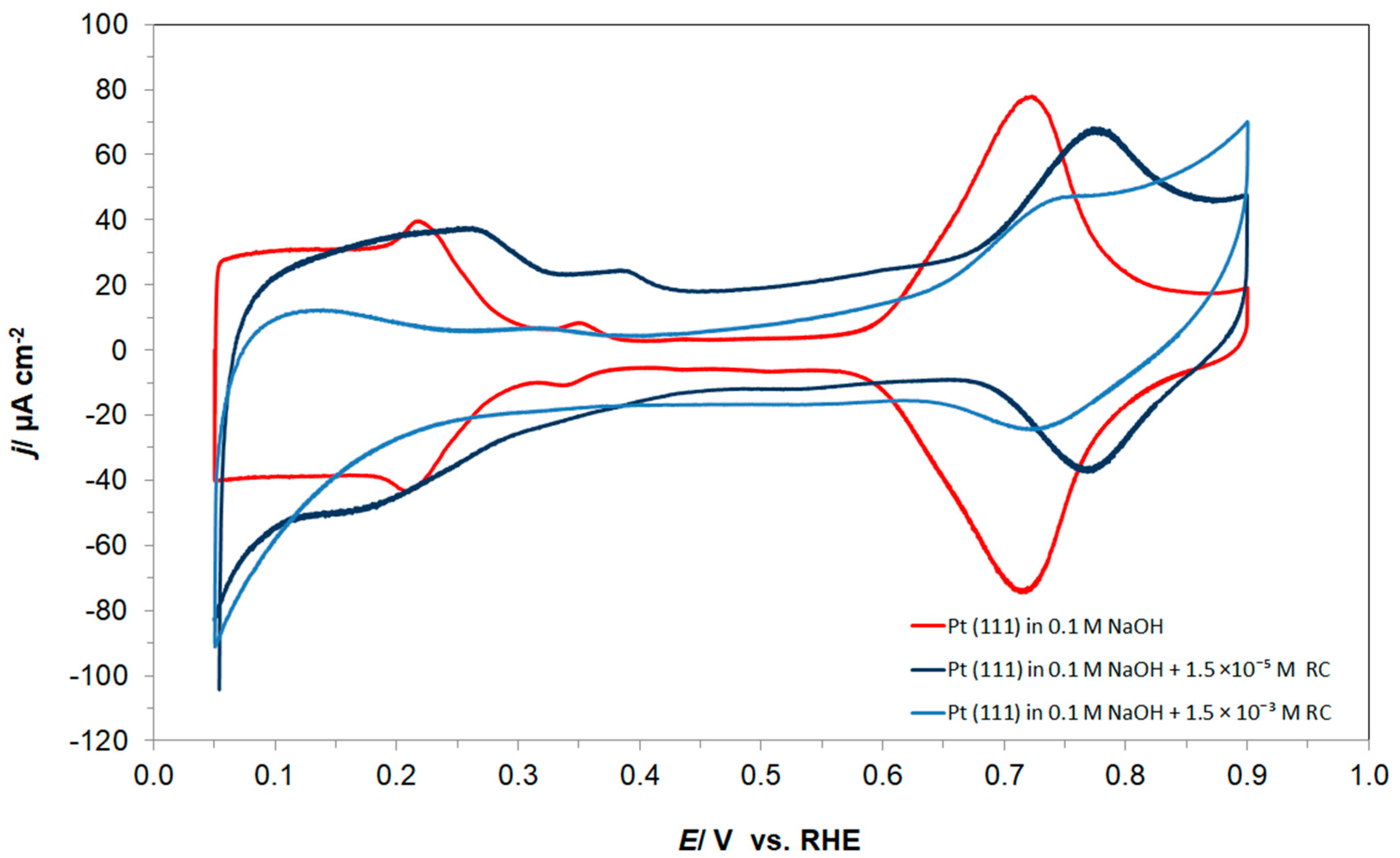
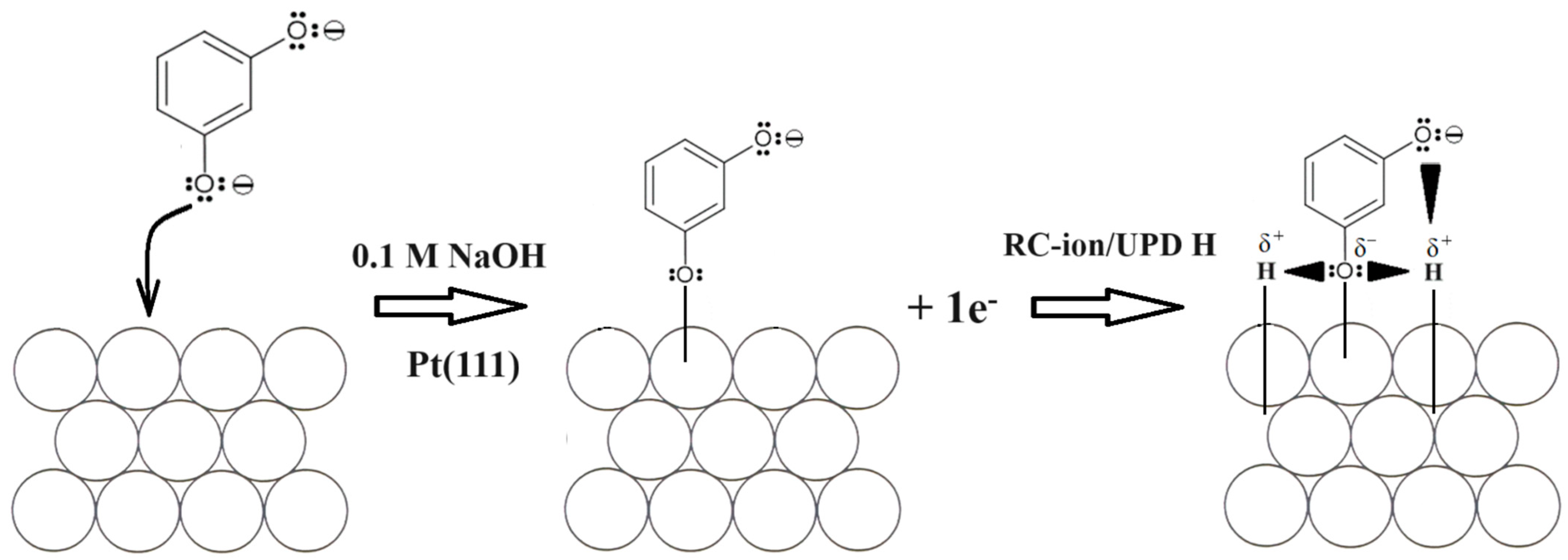
3.1. Cyclic Voltammetry, Linear Sweep Voltammetry and Tafel Polarization Behaviour of Pt(111) in 0.1 M NaOH, in the Absence and Presence of Resorcinol
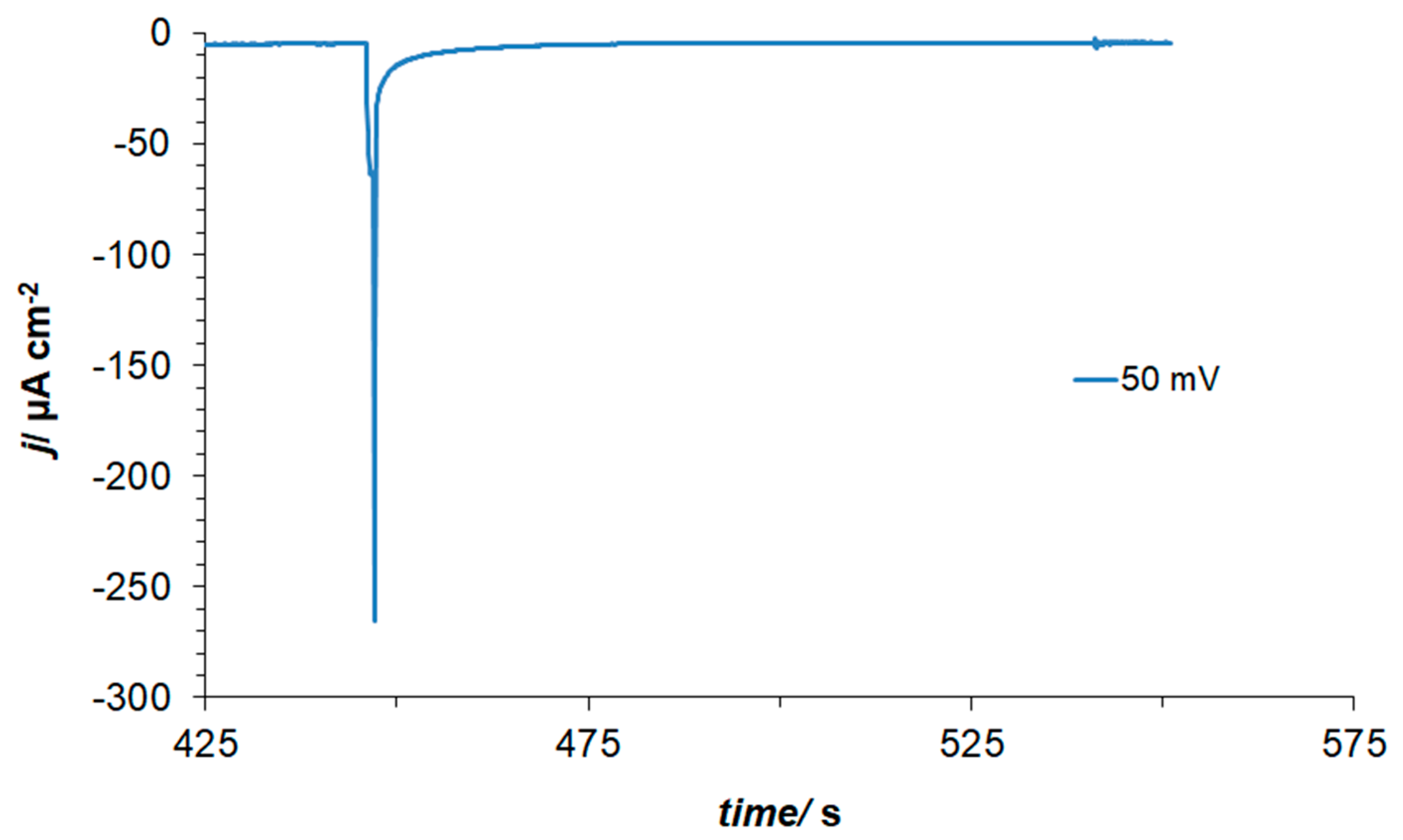
3.2. Ac. Impedance Behaviour of Pt(111) in 0.1 M NaOH, in the Absence and Presence of Resorcinol
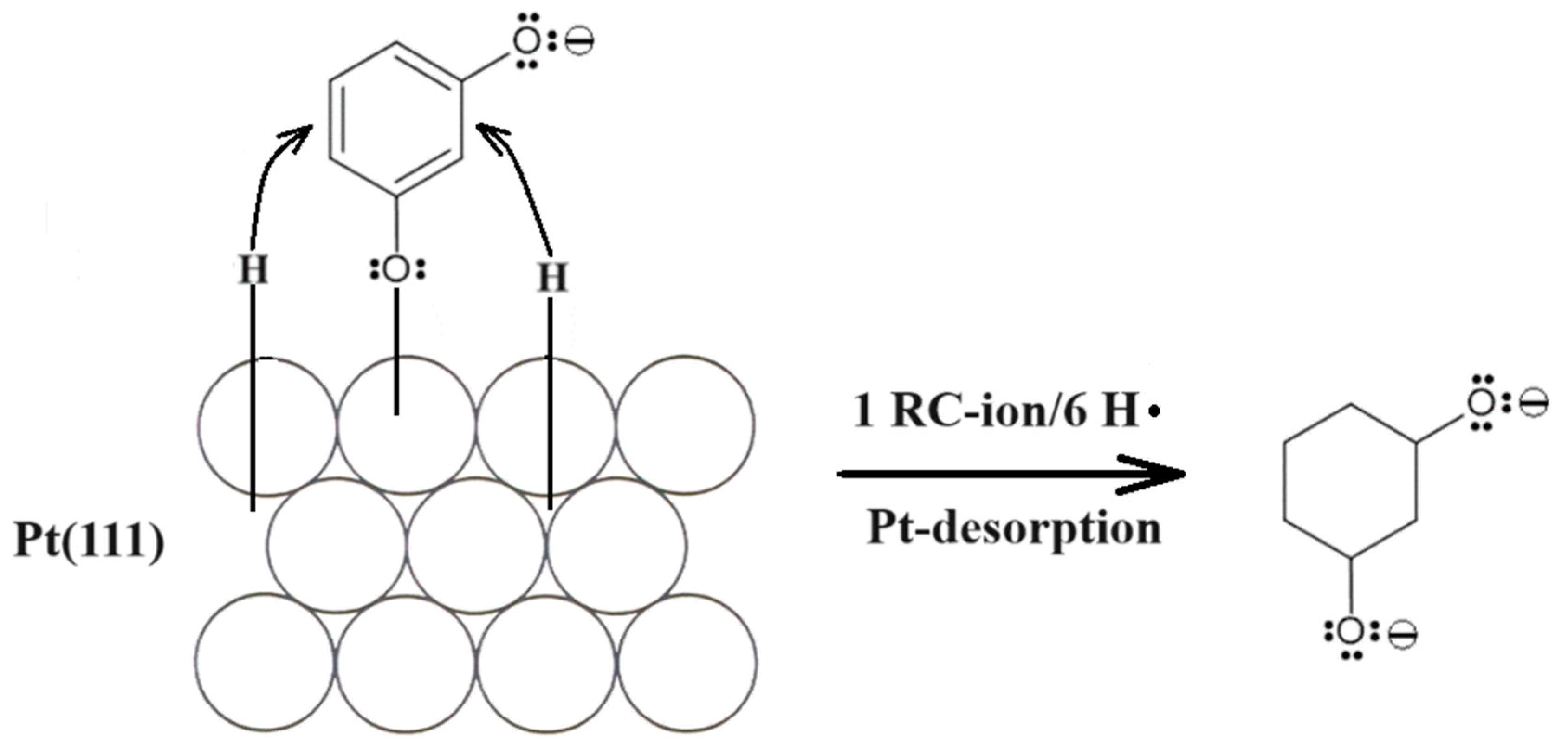
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, N.; Sanyal, U.; Fulton, J.L.; Gutiérrez, O.Y.; Lercher, J.A.; Campbell, C.T. Quantifying adsorption of organic molecules on platinum in aqueous phase by hydrogen site blocking and in situ X-ray absorption spectroscopy. ACS Catal. 2019, 9, 6869–6881. [Google Scholar] [CrossRef]
- Sui, C.; Ma, X.Y.; Fu, W.H.; Zeng, S.P.; Xie, R.R.; Zhang, Z.P. Regulating Pt-based noble metal catalysts for the catalytic oxidation of volatile organic compounds: A mini review. Rev. Inorg. Chem. 2023, 43, 561–570. [Google Scholar] [CrossRef]
- Conway, B.E.; Pierozynski, B. Ac impedance behaviour of processes involving adsorption and reactivity of guanidonium-type cations at Pt(100) surface. J. Electroanal. Chem. 2008, 622, 10–14. [Google Scholar] [CrossRef]
- Conway, B.E.; Pierozynski, B. Influence of acetamidine on the electrosorption of UPD H at Pt single-crystal surfaces. J. Electroanal. Chem. 2008, 623, 102–108. [Google Scholar] [CrossRef]
- Pierozynski, B.; Kowalski, I.M. Electrochemical reactivity of formamidoxime on Pt(111) and (100) single-crystal surfaces in 0.1MNaOH solution. J. Electroanal. Chem. 2011, 662, 432–436. [Google Scholar] [CrossRef]
- Pierozynski, B. Electrochemical Behaviour of Urea at Pt(111) Single-Crystal Surface in 0.1 M NaOH. Electrocatalysis 2012, 4, 37–41. [Google Scholar] [CrossRef]
- Pierozynski, B. Electrochemical reactivity of urea at Pt(100) surface in 0.5 M H2SO4 by AC impedance spectroscopy. J. Solid State Electrochem. 2012, 17, 889–893. [Google Scholar] [CrossRef]
- Kuczyński, M.; Łuba, M.; Mikołajczyk, T.; Pierożyński, B.T. Effect of resorcinol on the kinetics of underpotentially deposited hydrogen and the oxygen evolution reaction, studied on polycrystalline Pt in a 0.5 M H2SO4 solution. Energies 2022, 15, 1092. [Google Scholar] [CrossRef]
- Kuczyński, M.; Łuba, M.; Mikołajczyk, T.; Pierożyński, B. The influence of resorcinol on the kinetics of underpotentially deposited hydrogen, cathodic hydrogen and anodic oxygen evolution reactions, examined at polycrystalline Pt electrode in 0.1 M NaOH solution. Int. J. Hydrogen Energy 2023, 48, 10755–10764. [Google Scholar] [CrossRef]
- Clavilier, J.; Rodes, A.; El Achi, K.; Zamakhchari, M. Electrochemistry at platinum single crystal surfaces in acidic media: Hydrogen and oxygen adsorption. J. Chim. Phys. 1991, 88, 1291–1337. [Google Scholar] [CrossRef]
- Morin, S.; Dumont, H.; Conway, B. Evaluation of the effect of two-dimensional geometry of Pt single-crystal faces on the kinetics of upd of H using impedance spectroscopy. J. Electroanal. Chem. 1996, 412, 39–52. [Google Scholar] [CrossRef]
- Jerkiewicz, G. Rlectrochemical hydrogen adsorption and absorption. Part 1: Under-potential deposition of hydrogen. Electrocatalysis 2010, 1, 179–199. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Jurczakowski, R.; Lasia, A. Kinetics of hydrogen underpotential deposition at iridium in sulfuric and perchloric acids. Electrochim. Acta 2017, 225, 160–167. [Google Scholar] [CrossRef]
- Lynch, B.S.; Delzell, E.S.; Bechtel, D.H. Toxicology review and risk assessment of resorcinol: Thyroid effects. Regul. Toxicol. Pharmacol. 2002, 36, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, D.; Palanivelu, K.; Mohan, N. Electrochemical oxidation of resorcinol for wastewater treatment—A kinetic study. Indian J. Chem. Technol. 2003, 10, 396–401. [Google Scholar]
- Nady, H.; El-Rabiei, M.; El-Hafez, G.A. Electrochemical oxidation behavior of some hazardous phenolic compounds in acidic solution. Egypt. J. Pet. 2017, 26, 669–678. [Google Scholar] [CrossRef]
- Yang, J.; Williams, C.L.; Ramasubramaniam, A.; Dauenhauer, P.J. Aqueous-phase hydrodeoxygenation of highly oxygenated aromatics on platinum. Green Chem. 2013, 16, 675–682. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, J.; Chen, L. Selective hydrogenation of phenol and related derivatives. Catal. Sci. Technol. 2014, 4, 3555–3569. [Google Scholar] [CrossRef]
- Wei, Z.; Pan, R.; Hou, Y.; Yang, Y.; Liu, Y. Graphene-supported Pd catalyst for highly selective hydrogenation of resorcinol to 1, 3-cyclohexanedione through giant π-conjugate interactions. Sci. Rep. 2015, 5, 15664. [Google Scholar] [CrossRef]
- Chen, C.; Liu, P.; Zhou, M.; Sharma, B.K.; Jiang, J. Selective hydrogenation of phenol to cyclohexanol over Ni/CNT in the absence of external hydrogen. Energies 2020, 13, 846. [Google Scholar] [CrossRef]
- Hamelin, A. Modern Aspects of Electrochemistry; Conway, B.E., Bockris, J.O.M., White, R.E., Eds.; Plenum Press: New York, NY, USA, 1985; Chapter 1; p. 16. [Google Scholar]
- Macdonald, J.R. Impedance Spectroscopy, Emphasizing Solid Materials and Systems; John Wiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Schouten, K.J.P.; van der Niet, M.J.T.C.; Koper, M.T.M. Impedance spectroscopy of H and OH adsorption on stepped single-crystal platinum electrodes in alkaline and acidic media. Phys. Chem. Chem. Phys. 2010, 12, 15217–15224. [Google Scholar] [CrossRef] [PubMed]
- Morallón, E.; Vázquez, J.L.; Aldaz, A. Electrochemical behaviour of Pt(111) in alkaline media. Effect of specific adsorption of anions. J. Electroanal. Chem. 1992, 334, 323–338. [Google Scholar] [CrossRef]
- Ngamchuea, K.; Tharat, B.; Hirunsit, P.; Suthirakun, S. Electrochemical oxidation of resorcinol: Mechanistic insights from experimental and computational studies. RSC Adv. 2020, 10, 28454–28463. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.; Gasteiger, H.A.; Horn, Y.S. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536. [Google Scholar] [CrossRef]
- Raveendran, A.; Chandran, M.; Dhanusuraman, R. A comprehensive review on the electrochemical parameters and recent material development of electrochemical water splitting electrocatalysts. RSC Adv. 2023, 13, 3843–3876. [Google Scholar] [CrossRef]
- Pajkossy, T. Impedance of rough capacitive electrodes. J. Electroanal. Chem. 1994, 364, 111–125. [Google Scholar] [CrossRef]
- Botello, L.E.; Feliu, J.M.; Climent, V. Activation energy of hydrogen adsorption on Pt(111) in alkaline media: An impedance spectroscopy study at variable temperatures. ACS Appl. Mater. Interfaces 2020, 12, 42911–42917. [Google Scholar] [CrossRef]
- Conway, B.; Barber, J.; Morin, S. Comparative evaluation of surface structure specificity of kinetics of UPD and OPD of H at single-crystal Pt electrodes. Electrochim. Acta 1998, 44, 1109–1125. [Google Scholar] [CrossRef]
- Barber, J.; Conway, B. Structural specificity of the kinetics of the hydrogen evolution reaction on the low-index surfaces of Pt single-crystal electrodes in 0.5 M dm−3 NaOH. J. Electroanal. Chem. 1999, 461, 80–89. [Google Scholar] [CrossRef]
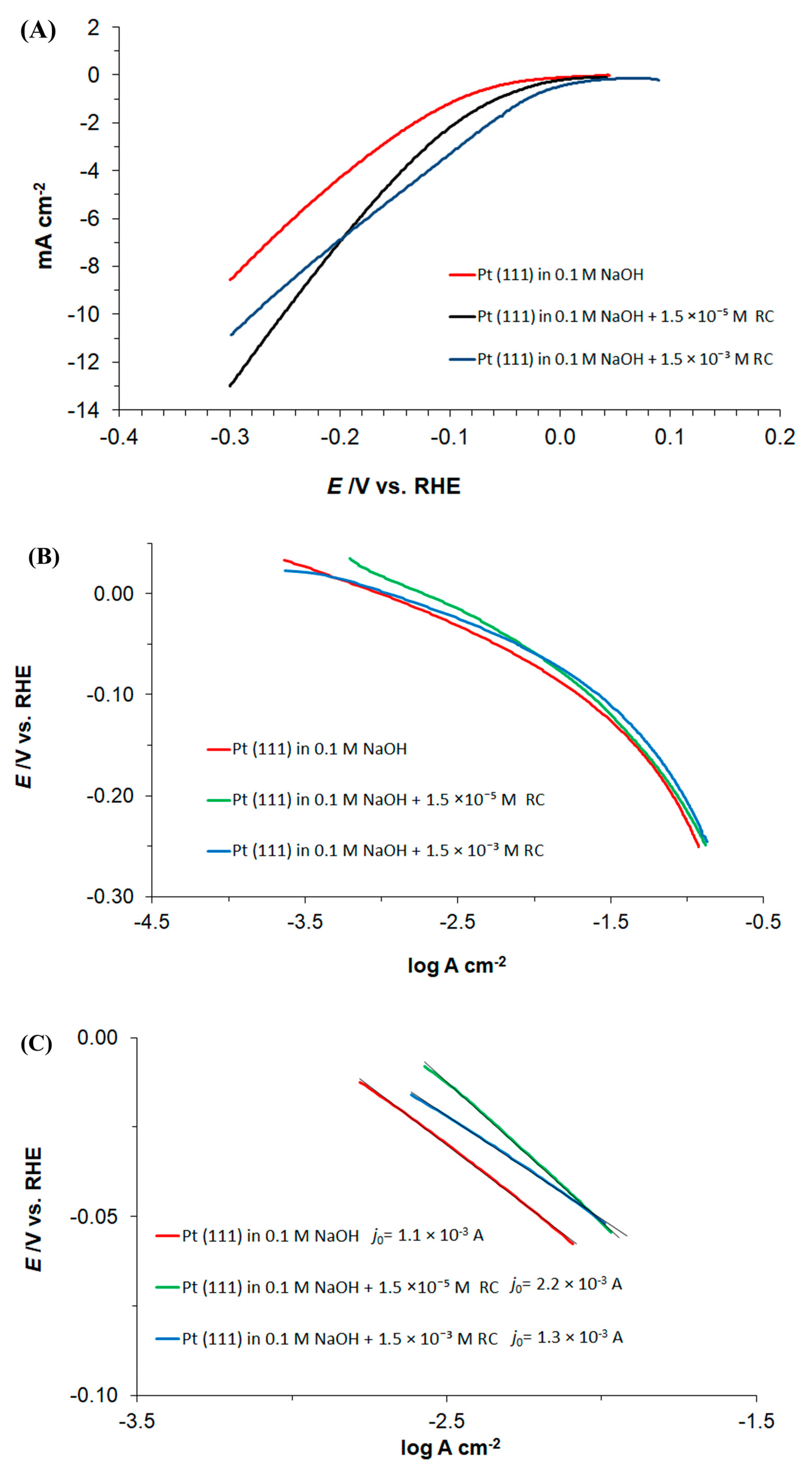

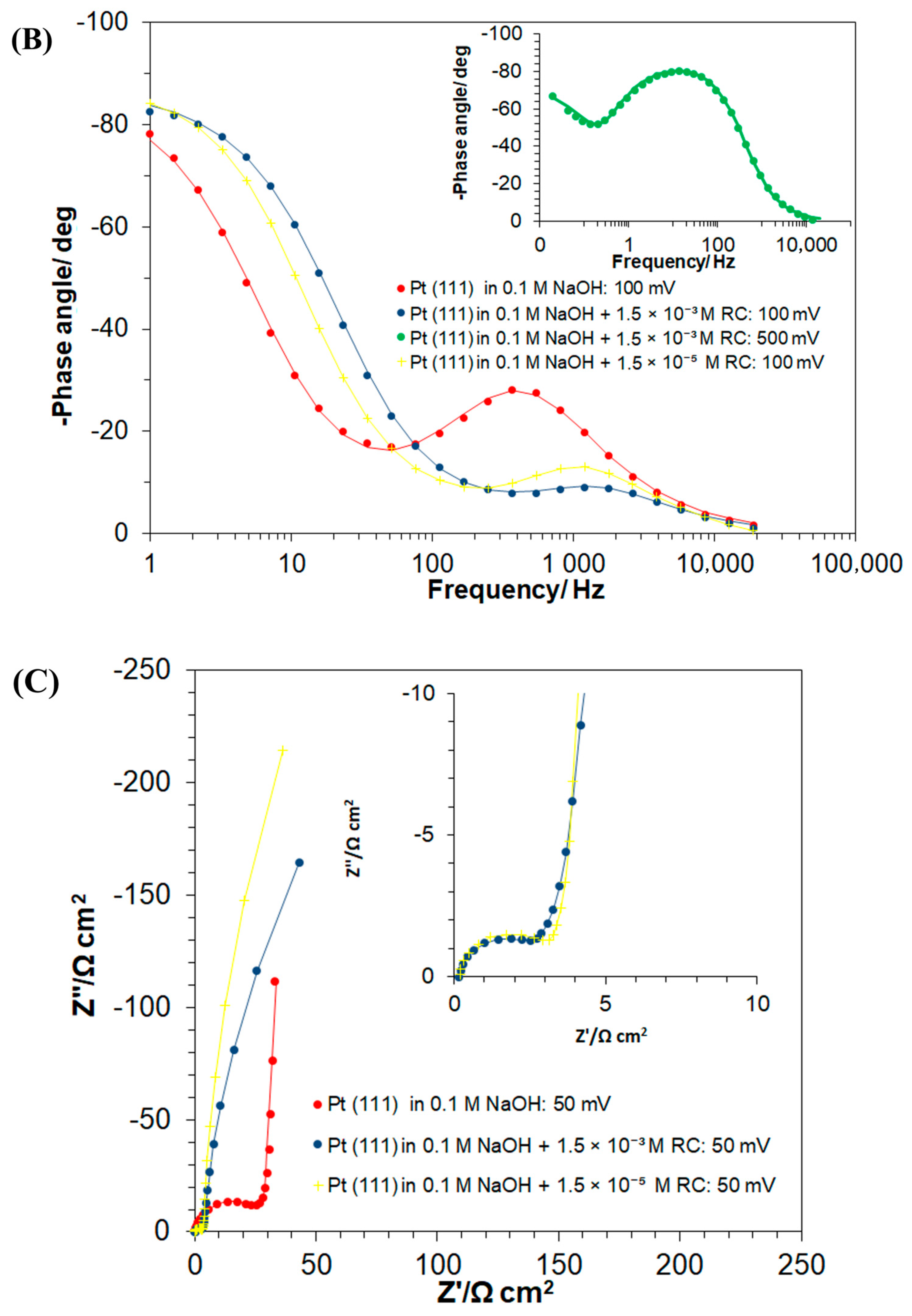
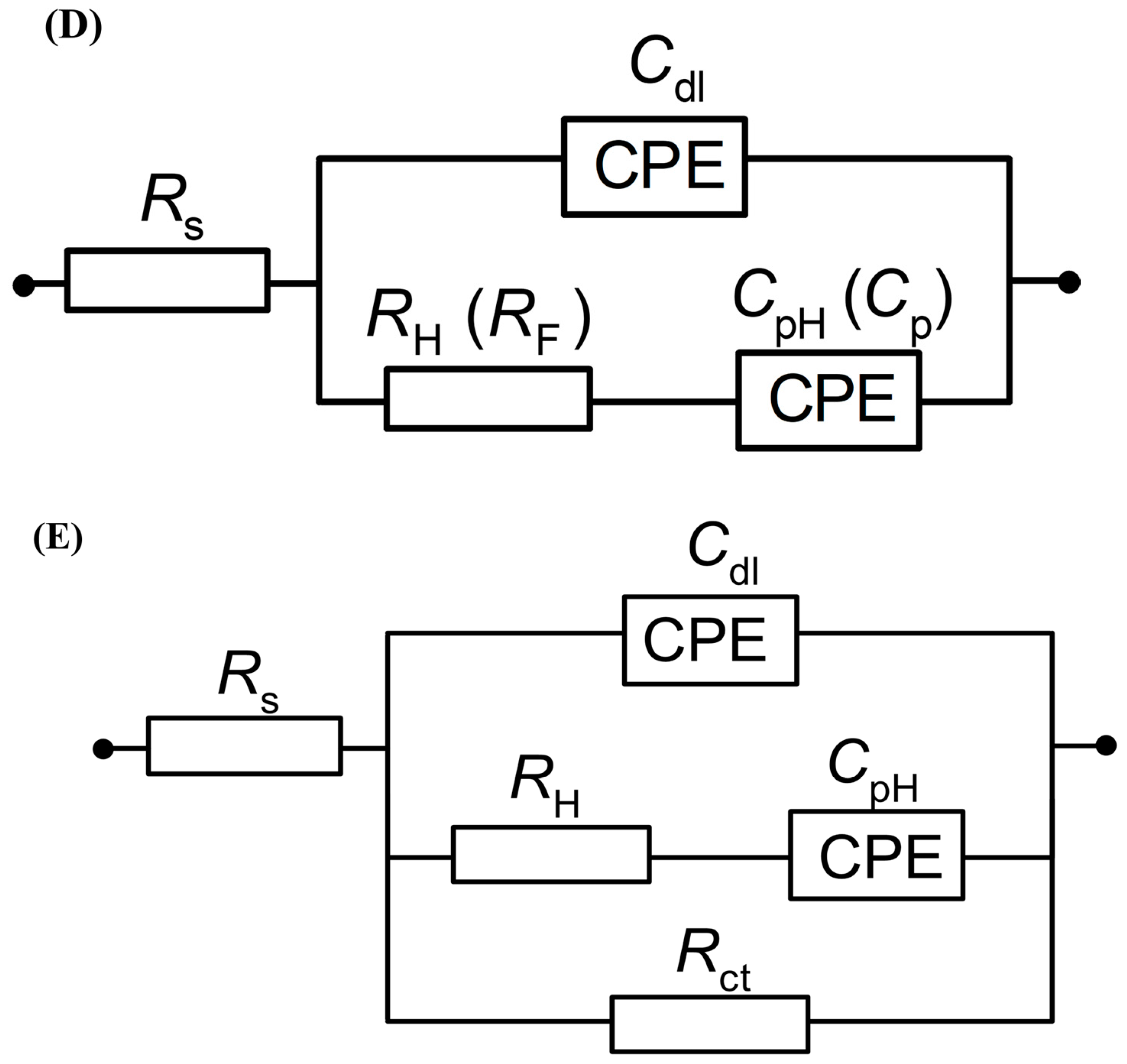
| E/mV | RH/Ω cm2 | Cdl/µF cm−2 | Rct/Ω cm2 | CpH/µF cm−2 |
|---|---|---|---|---|
| 0.1 M NaOH | ||||
| 50 | 17.6 ± 0.2 | 45.7 ± 3.8 | - | 664 ± 7 |
| 100 | 31.2 ± 0.3 | 46.5 ± 2.7 | - | 662 ± 8 |
| 150 | 53.4 ± 0.5 | 43.7 ± 1.9 | - | 657 ± 6 |
| 200 | 80.2 ± 0.7 | 48.3 ± 1.0 | - | 610 ± 7 |
| 300 | 311.6 ± 3.7 | 47.4 ± 0.7 | - | 116 ± 1 |
| 500 | - | 59.3 ± 0.7 | - | - |
| 0.1 M NaOH + 1.5 × 10−3 M RC | ||||
| 50 | 3.3 ± 0.2 | 53.2 ± 5.3 | 1005 ± 13 | 533 ± 25 |
| 100 | 4.5 ± 0.1 | 53.6 ± 10.1 | - | 556 ± 11 |
| 150 | 7.0 ± 0.2 | 54.7 ± 8.8 | - | 425 ± 8 |
| 200 | 11.1 ± 0.3 | 45.9 ± 4.9 | - | 232 ± 5 |
| RF/Ω cm2 | Cp/µF cm−2 | |||
| 300 | - | 57.9 ± 0.6 | 91,366 ± 3820 | 128 ± 15 |
| 400 | - | 90.7± 0.7 | 10,906 ± 784 | 99 ± 5 |
| 500 | - | 56.4 ± 0.9 | 8880 ± 488 | 122 ± 4 |
| 0.1 M NaOH + 1.5 × 10−5 M RC | ||||
| Rct/Ω cm2 | CpH/µF cm−2 | |||
| 50 | 3.1 ± 0.2 | 57.8 ± 5.5 | 1716 ± 23 | 698 ± 33 |
| 100 | 7.0 ± 0.5 | 29.4 ± 2.9 | - | 672 ± 31 |
| 150 | 11.3 ± 0.7 | 35.6 ± 3.2 | - | 523 ± 29 |
| 200 | 20.3 ± 1.3 | 37.2 ± 2.4 | - | 458 ± 26 |
| RF/Ω cm2 | Cp/µF cm−2 | |||
| 300 | - | 55.9 ± 0.5 | 12,240 ± 178 | 242 ± 5 |
| 400 | - | 60.7± 0.6 | 68,072 ± 4120 | 72 ± 3 |
| 500 | - | 53.4 ± 0.8 | 69,479 ± 1589 | 66 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierożyński, B.; Kuczyński, M.; Mikołajczyk, T. Electro-Reactivity of Resorcinol on Pt(111) Single-Crystal Plane and Its Influence on the Kinetics of Underpotentially Deposited Hydrogen and Hydrogen Evolution Reaction Processes in 0.1 M NaOH Solution. Crystals 2024, 14, 545. https://doi.org/10.3390/cryst14060545
Pierożyński B, Kuczyński M, Mikołajczyk T. Electro-Reactivity of Resorcinol on Pt(111) Single-Crystal Plane and Its Influence on the Kinetics of Underpotentially Deposited Hydrogen and Hydrogen Evolution Reaction Processes in 0.1 M NaOH Solution. Crystals. 2024; 14(6):545. https://doi.org/10.3390/cryst14060545
Chicago/Turabian StylePierożyński, Bogusław, Mateusz Kuczyński, and Tomasz Mikołajczyk. 2024. "Electro-Reactivity of Resorcinol on Pt(111) Single-Crystal Plane and Its Influence on the Kinetics of Underpotentially Deposited Hydrogen and Hydrogen Evolution Reaction Processes in 0.1 M NaOH Solution" Crystals 14, no. 6: 545. https://doi.org/10.3390/cryst14060545
APA StylePierożyński, B., Kuczyński, M., & Mikołajczyk, T. (2024). Electro-Reactivity of Resorcinol on Pt(111) Single-Crystal Plane and Its Influence on the Kinetics of Underpotentially Deposited Hydrogen and Hydrogen Evolution Reaction Processes in 0.1 M NaOH Solution. Crystals, 14(6), 545. https://doi.org/10.3390/cryst14060545








