Deposition and Structural Characterization of Mg-Zn Co-Doped GaN Films by Radio-Frequency Magnetron Sputtering in a N2-Ar2 Environment
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Raw Material to Prepare the Mg-Zn Co-Doped GaN Target
2.2. Preparation of the Mg-Zn Co-Doped GaN Target for Film Deposition
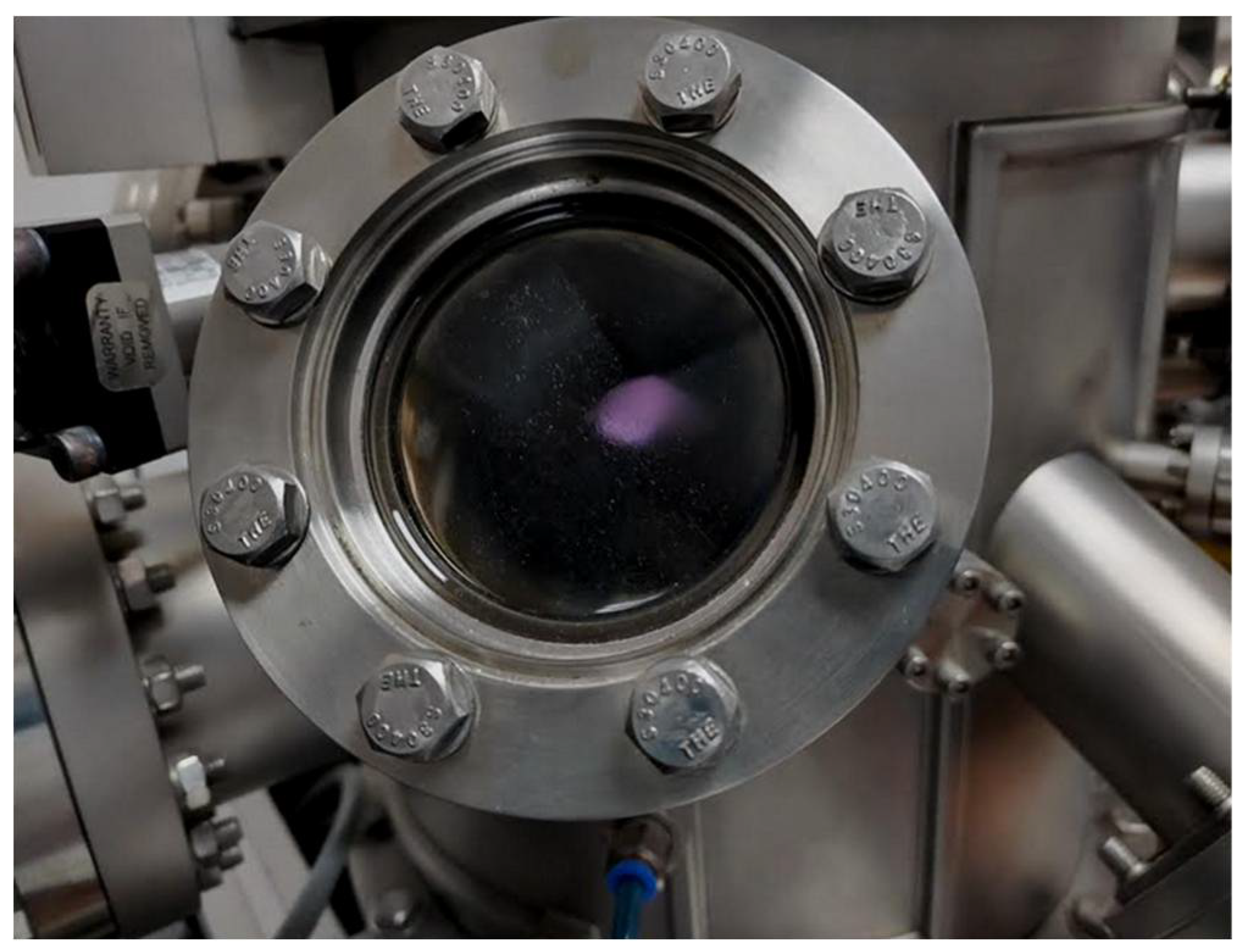
2.3. Deposition of the Mg-Zn Co-Doped GaN Films in a N2-Ar2 Environment
2.4. Characterizations
3. Results and Discussion
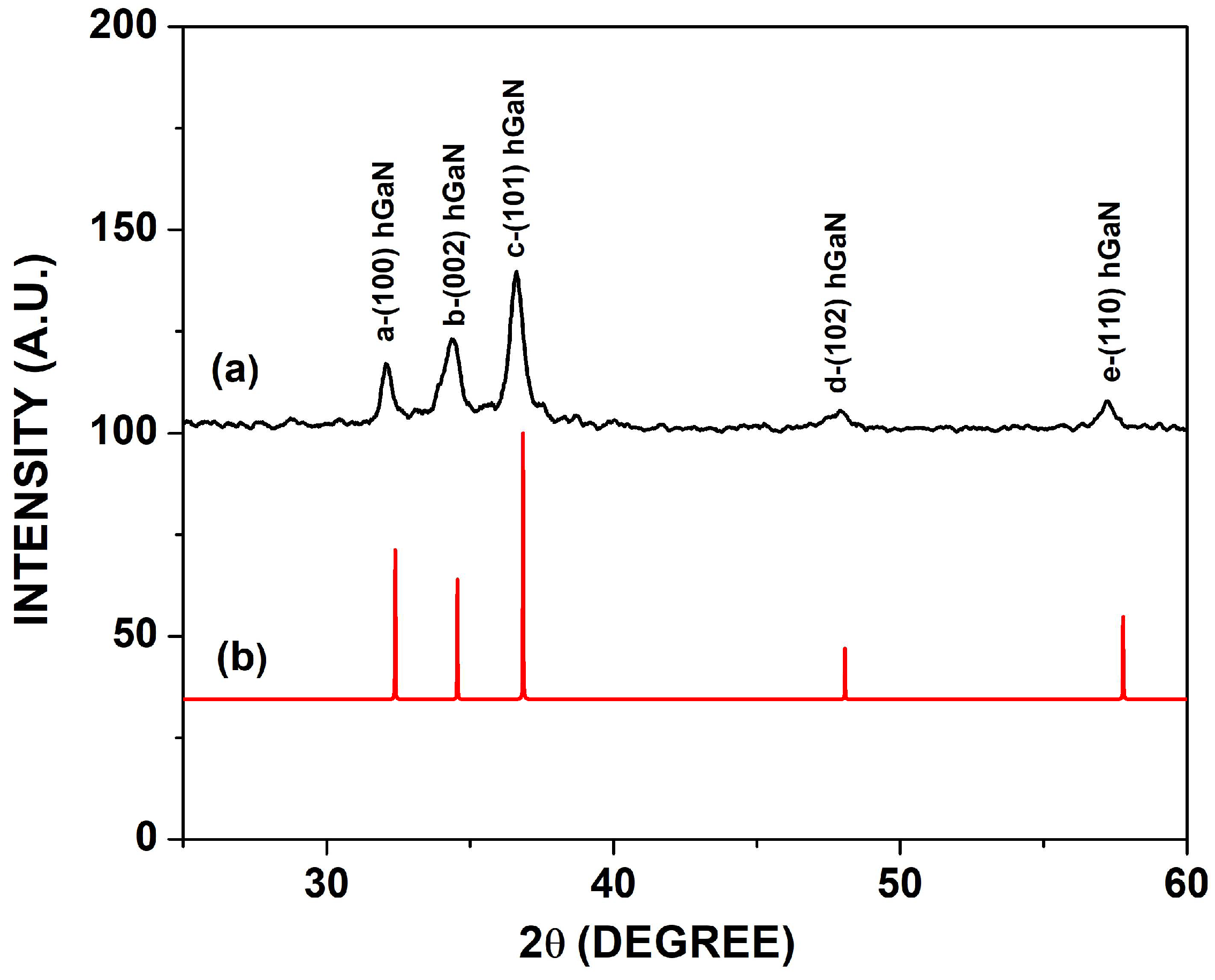
3.1. Structural Analysis
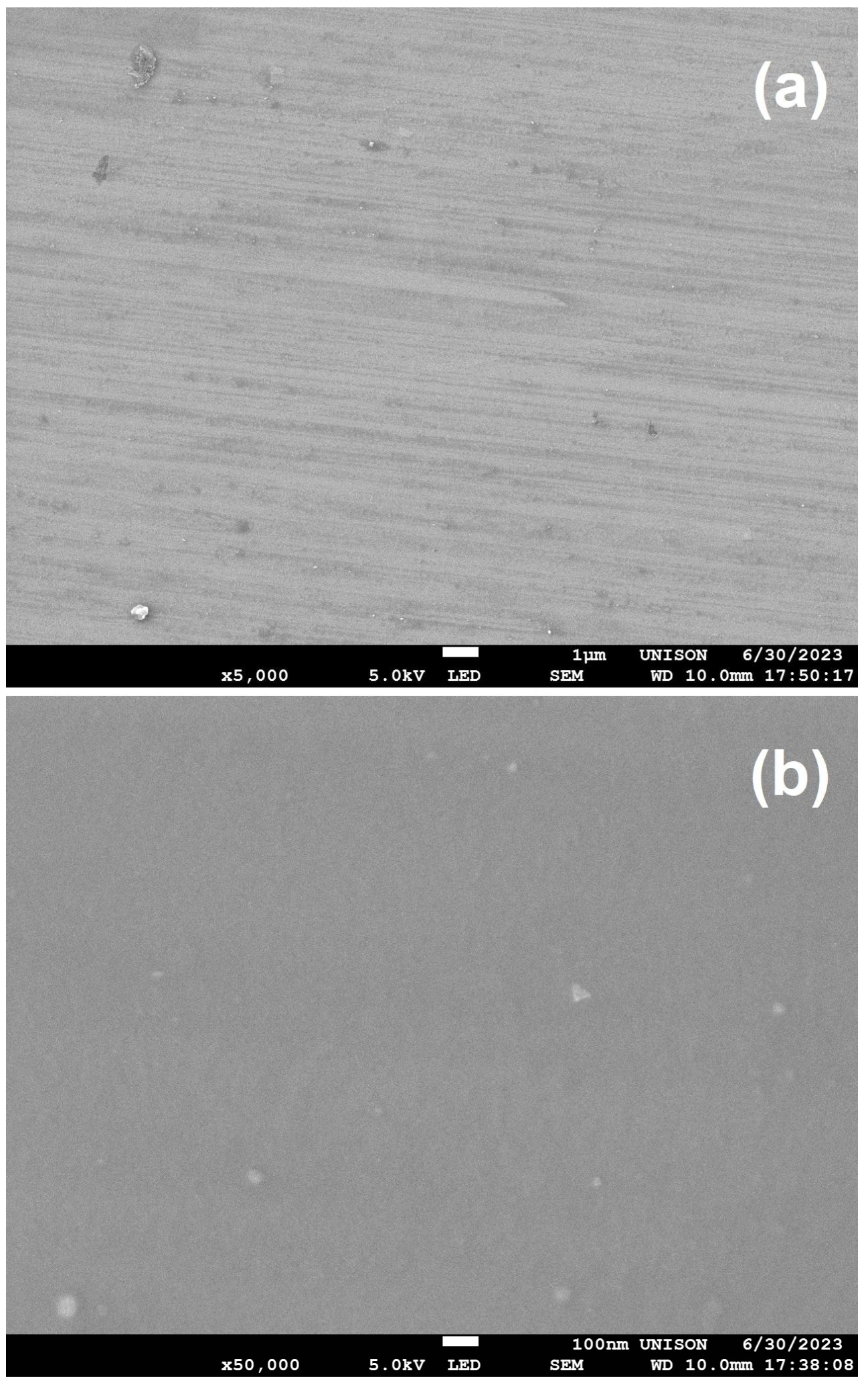
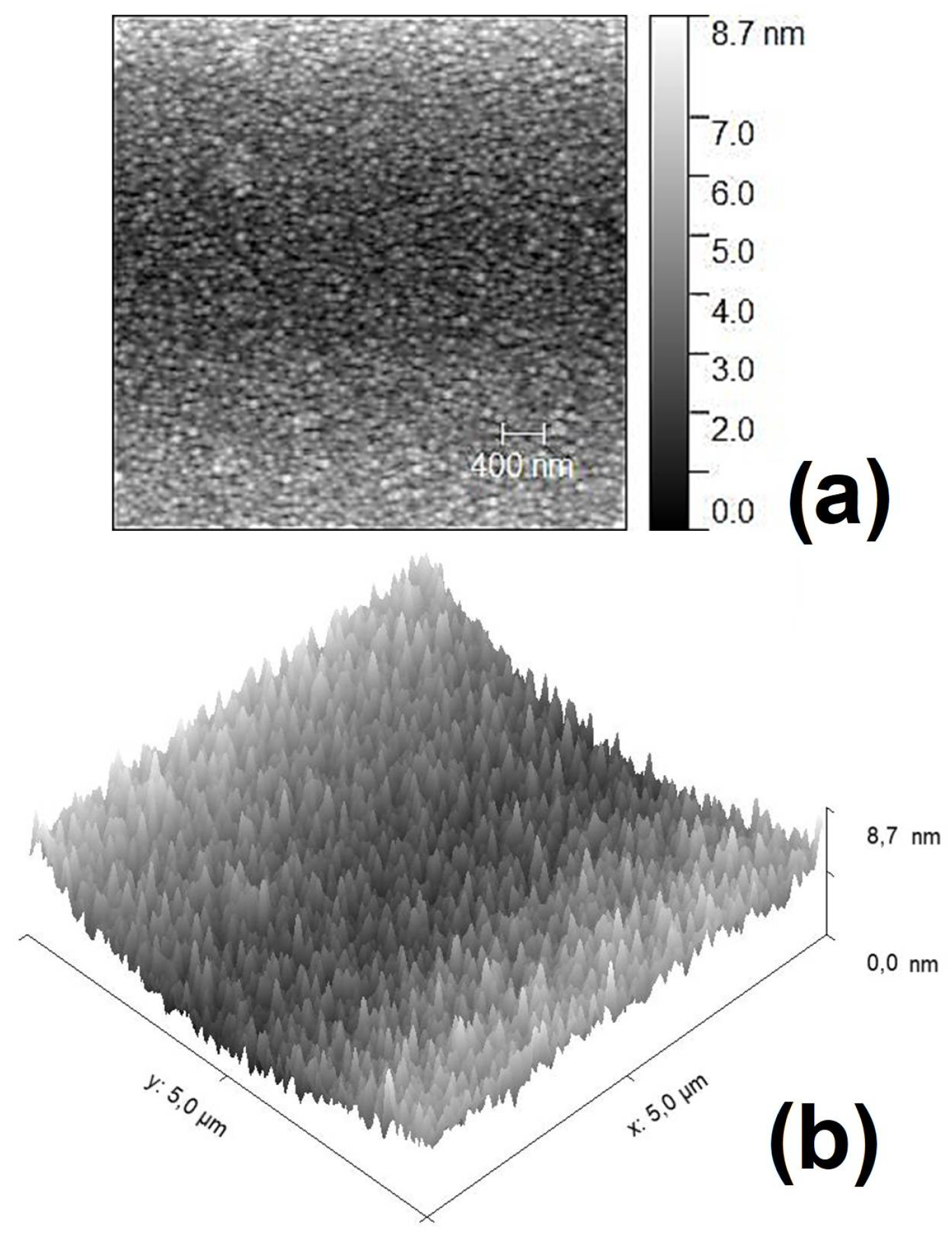
3.2. Electron Microscopy
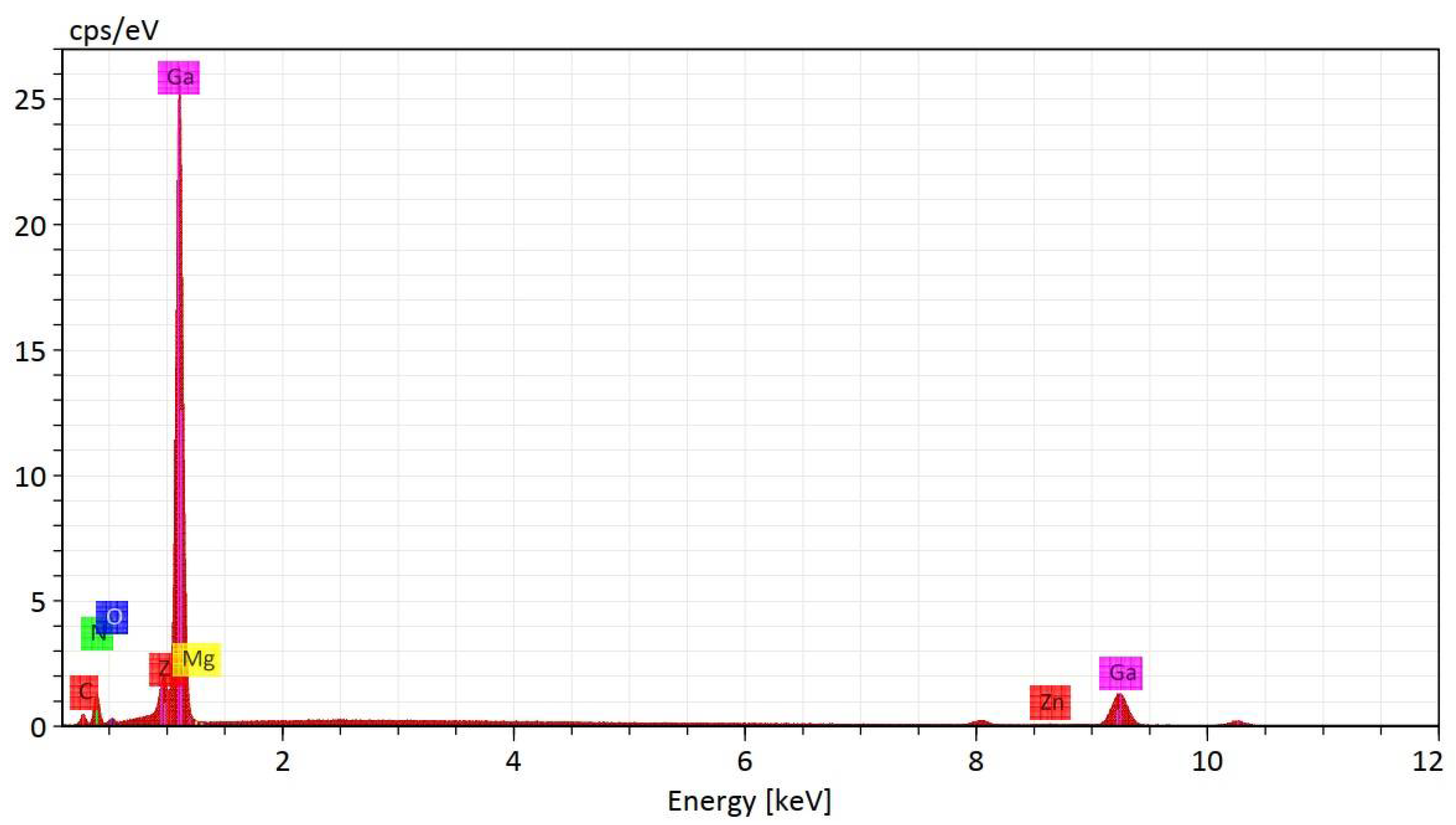
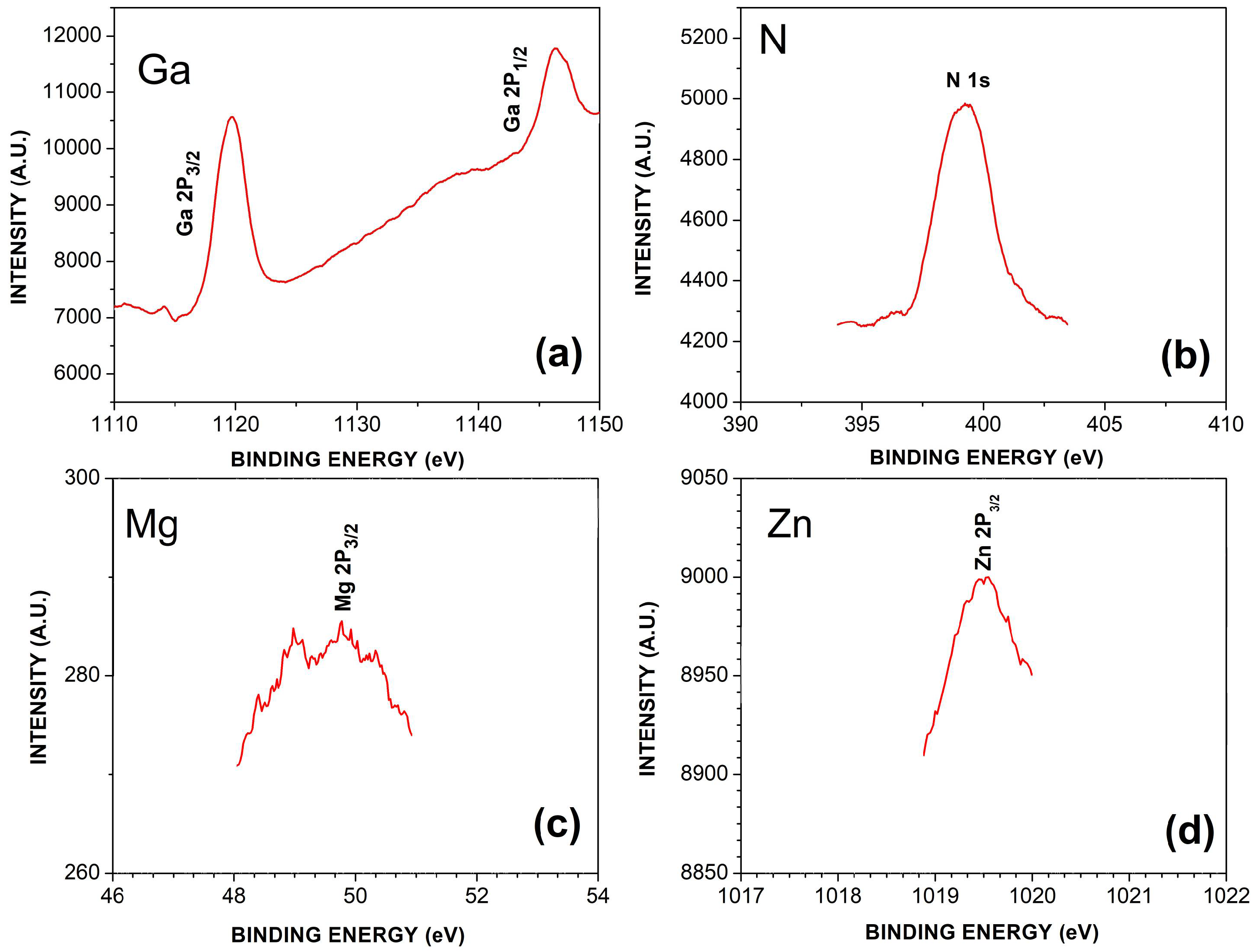
3.3. X-ray Photoelectron Spectroscopy
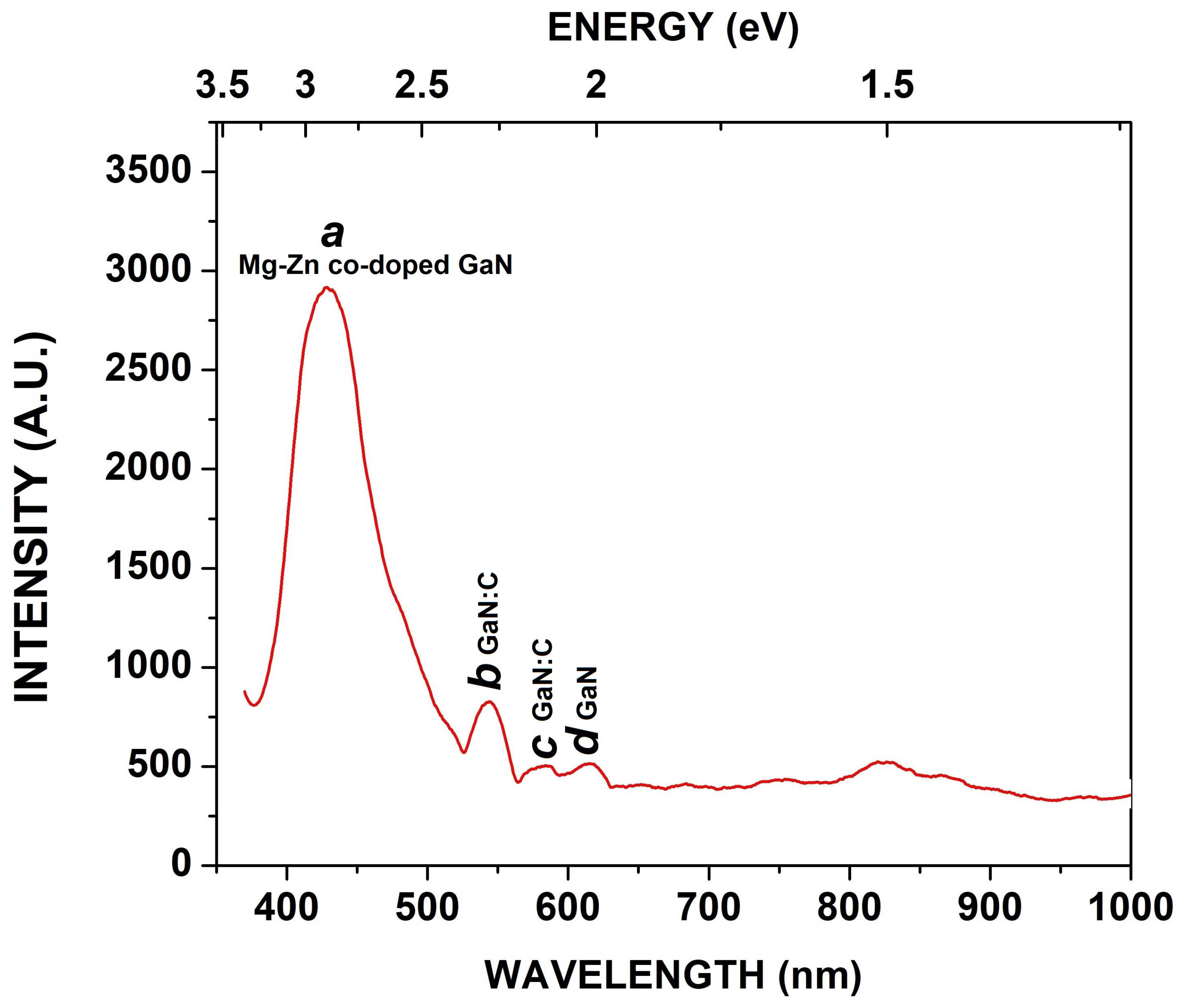
3.4. Photoluminescence
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wassen, M.; Adel, E.; Laoucine, K. The Global Semiconductor Chip Shortage: Causes, Implications, and Potential Remedies. IFAC-PapersOnLine 2022, 55, 476–483. [Google Scholar] [CrossRef]
- Kwon, W.; Kawasaki, S.; Watanabe, H.; Tanaka, A.; Honda, Y.; Ikeda, H.; Iso, K.; Amano, H. Reverse Leakage Mechanism of Dislocation-Free GaN Vertical p-n Diodes. IEEE Electron Device Lett. 2023, 44, 1172–1175. [Google Scholar] [CrossRef]
- Fanciulli, M.; Lei, T.; Moustakas, T.D. Conduction-electron spin resonance in zinc-blende GaN thin films. Phys. Rev. B 1993, 20, 15-144–15-147. [Google Scholar] [CrossRef] [PubMed]
- Sami, B.; Burak, T.; Cagla, O.-A.; Necmi, B.; Kemal, O.A. Electronic and optical device applications of hollow cathode plasma assisted atomic layer deposition based GaN thin films. J. Vac. Sci. Technol. A Vac. Surf. Film. 2015, 33, 01A143. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, J.H.; Lee, Y.H.; Lee, D.D. GaN thin films as gas sensors. Sens. Actuators B 2003, 89, 305–310. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, Y.; Leung, B.; Yuan, G.; Wang, G.; Jiang, H.; Fan, Y.; Sun, Q.; Wang, J.; Xu, K.; et al. Mechanical Properties of Nanoporous GaN and Its Application for Separation and Transfer of GaN Thin Films. ACS Appl. Mater. Interfaces 2013, 5, 11074–11079. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Hong, Y.J.; Li, Y.; Cho, J.; Kim, Y.J.; Yi, G.C. Photocatalysis Using GaN Nanowires. ACS Nano 2008, 2, 637–642. [Google Scholar] [CrossRef]
- Liu, W.-S.; Chang, Y.-L.; Tan, C.-Y.; Tsai, C.-T.; Kuo, H.-C. Properties of N-Type GaN Thin Film with Si-Ti Codoping on a Glass Substrate. Crystals 2020, 10, 582. [Google Scholar] [CrossRef]
- Sun, L.; Liu, C.; Li, J.; Wang, J.; Yan, F.; Zeng, Y.; Li, J. Structural and magnetic properties of GaN:Sm:Eu films fabricated by co-implantation method. Mater. Lett. 2010, 64, 1031–1033. [Google Scholar] [CrossRef]
- Jeong, M.C.; Ham, M.H.; Myoung, J.M.; Noh, S.K. Room-temperature ferromagnetism of Mg and Mn co-doped GaN films grown by PEMBE. Appl. Surf. Sci. 2004, 222, 322–326. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, G.M.; Lee, H.J. The study on the growth and properties of Mg doped and Mg–Si codoped p-type GaN. Solid-State Electron. 1999, 43, 1807–1812. [Google Scholar] [CrossRef]
- Kim, K.S.; Han, M.S.; Yang, G.M.; Youn, C.J.; Lee, H.J.; Cho, H.K.; Lee, J. Codoping characteristics of Zn with Mg in GaN. Appl. Phys. Lett. 2000, 77, 1123–1125. [Google Scholar] [CrossRef]
- Naito, A.; Ueno, K.; Kobayashi, A.; Fujioka, H. Hole Conduction Mechanism in In–Mg-Codoped GaN Prepared via Pulsed Sputtering Deposition. Phys. Status Solidi A 2024, 221, 2300806. [Google Scholar] [CrossRef]
- Gastellóu, E.; García, R.; Herrera, A.M.; Ramos, A.; García, G.; Hirata, G.A.; Luna, J.A.; Carrillo, R.C.; Rodríguez, J.A.; Robles, M.; et al. Obtaining of Mg-Zn Co-Doped GaN Powders via Nitridation of the Ga-Mg-Zn Metallic Solution and Their Structural and Optical Properties. Materials 2023, 16, 3272. [Google Scholar] [CrossRef]
- Gastellóu, E.; García, G.; Herrera, A.M.; Morales, C.; García, R.; Hirata, G.A.; Rosendo, E.; Luna, J.A.; Robles, M.; Rodríguez, J.A.; et al. Effects in the Optical and Structural Properties Caused by Mg or Zn Doping of GaN Films Grown via Radio-Frequency Magnetron Sputtering Using Laboratory-Prepared Targets. Appl. Sci. 2021, 11, 6990. [Google Scholar] [CrossRef]
- Jewett, S.A.; Makowski, M.S.; Andrews, B.; Manfra, M.J.; Ivanisevic, A. Gallium nitride is biocompatible and non-toxic before and after functionalization with peptides. Acta Biomater. 2012, 8, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Hirata, G.A.; Thomas, A.C.; Ponce, F.A. Structure and luminescence of nanocrystalline gallium nitride synthesized by a novel polymer pyrolysis route. Opt. Mater. 2006, 29, 19–23. [Google Scholar] [CrossRef]
- Fang, J.; Yang, W.; Zhang, X.; Tian, A.; Lu, S.; Liu, J.; Yang, H. The Effect of Periodic Duty Cyclings in Metal-Modulated Epitaxy on GaN:Mg Film. Materials 2023, 16, 1730. [Google Scholar] [CrossRef]
- Kumar, A.; Berg, M.; Wang, Q.; Salter, M.; Ramvall, P. Growth of p-type GaN—The role of oxygen in activation of Mg-doping. Power Electron. Devices Compon. 2023, 5, 100036. [Google Scholar] [CrossRef]
- Reshchikov, M.A.; Morkoç, H. Luminescence properties of defects in GaN. J. Appl. Phys. 2005, 97, 061301-1–061301-95. [Google Scholar] [CrossRef]
- Furqan, C.M.; Ho Jacob, Y.L.; Kwok, H.S. GaN thin film: Growth and Characterizations by Magnetron Sputtering. Surf. Interfaces 2021, 26, 101364. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gastellóu, E.; García, R.; Herrera, A.M.; Ramos, A.; García, G.; Hirata, G.A.; Luna, J.A.; Rodríguez, J.A.; Robles, M.; Ramírez, Y.D.; et al. Deposition and Structural Characterization of Mg-Zn Co-Doped GaN Films by Radio-Frequency Magnetron Sputtering in a N2-Ar2 Environment. Crystals 2024, 14, 618. https://doi.org/10.3390/cryst14070618
Gastellóu E, García R, Herrera AM, Ramos A, García G, Hirata GA, Luna JA, Rodríguez JA, Robles M, Ramírez YD, et al. Deposition and Structural Characterization of Mg-Zn Co-Doped GaN Films by Radio-Frequency Magnetron Sputtering in a N2-Ar2 Environment. Crystals. 2024; 14(7):618. https://doi.org/10.3390/cryst14070618
Chicago/Turabian StyleGastellóu, Erick, Rafael García, Ana M. Herrera, Antonio Ramos, Godofredo García, Gustavo A. Hirata, José A. Luna, Jorge A. Rodríguez, Mario Robles, Yani D. Ramírez, and et al. 2024. "Deposition and Structural Characterization of Mg-Zn Co-Doped GaN Films by Radio-Frequency Magnetron Sputtering in a N2-Ar2 Environment" Crystals 14, no. 7: 618. https://doi.org/10.3390/cryst14070618
APA StyleGastellóu, E., García, R., Herrera, A. M., Ramos, A., García, G., Hirata, G. A., Luna, J. A., Rodríguez, J. A., Robles, M., Ramírez, Y. D., & García, I. E. (2024). Deposition and Structural Characterization of Mg-Zn Co-Doped GaN Films by Radio-Frequency Magnetron Sputtering in a N2-Ar2 Environment. Crystals, 14(7), 618. https://doi.org/10.3390/cryst14070618







