Visible-Light Spectroscopy and Rock Magnetic Analyses of Iron Oxides in Mixed-Mineral Assemblages
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characteristic Wavelength Band Position of Hematite
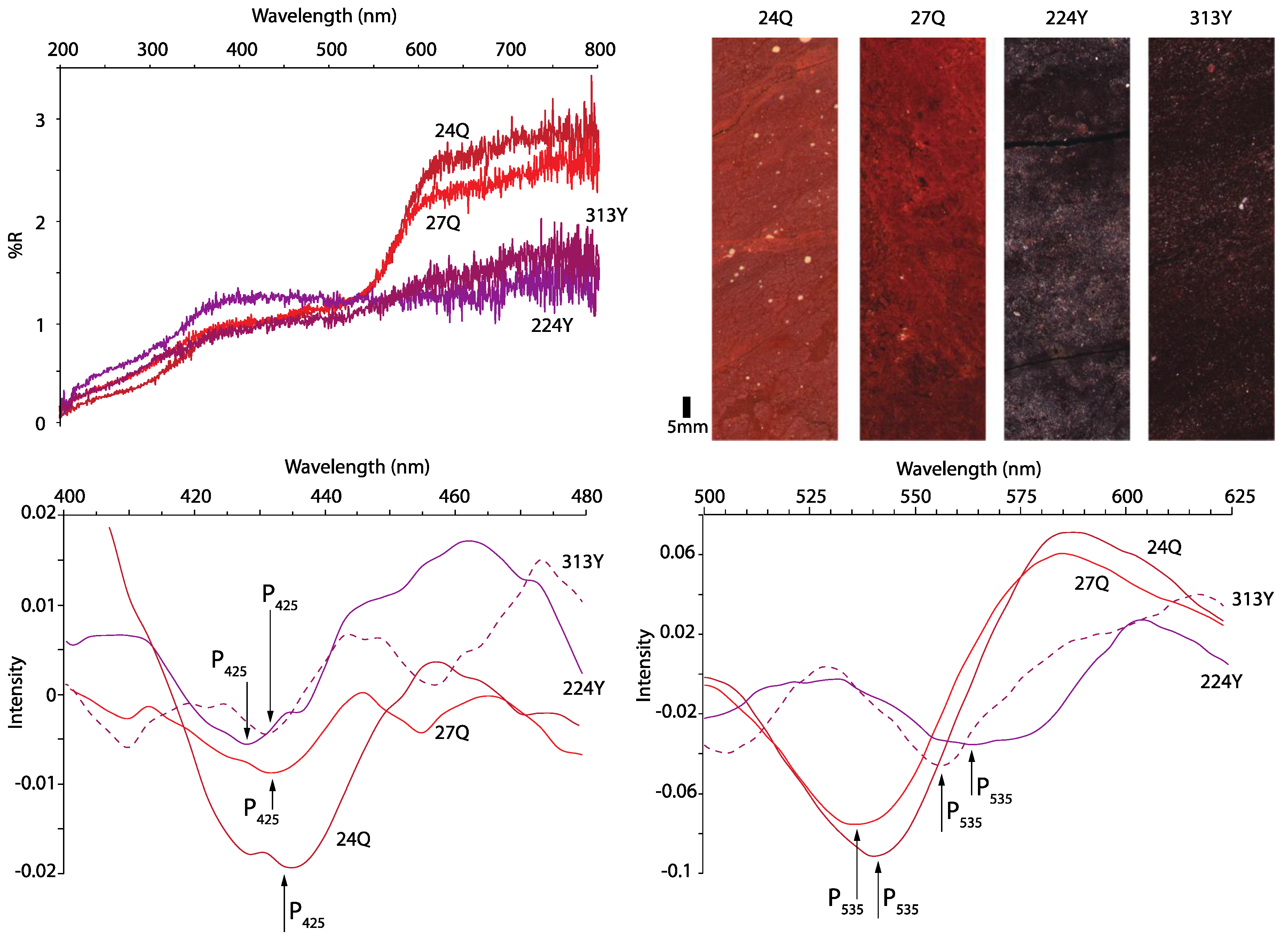
3.2. Goethite and Maghemite Discrimination
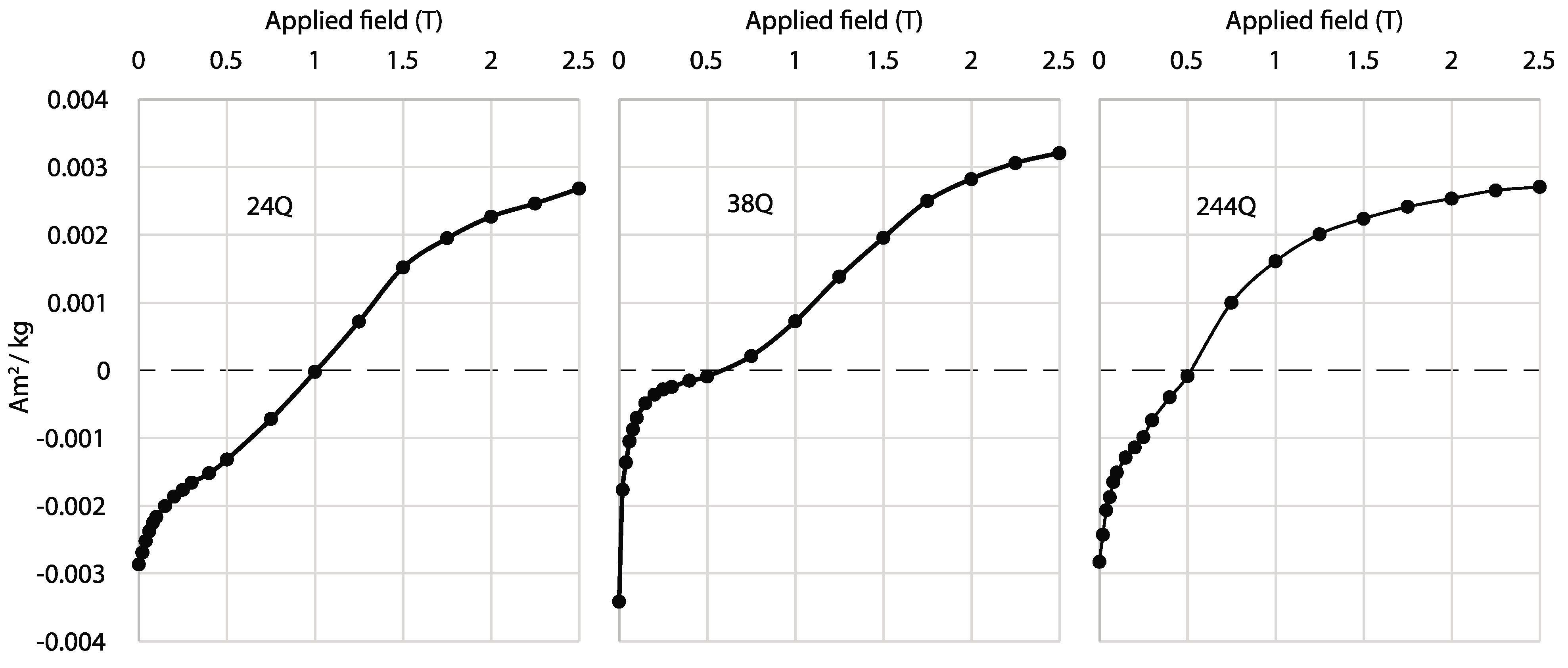
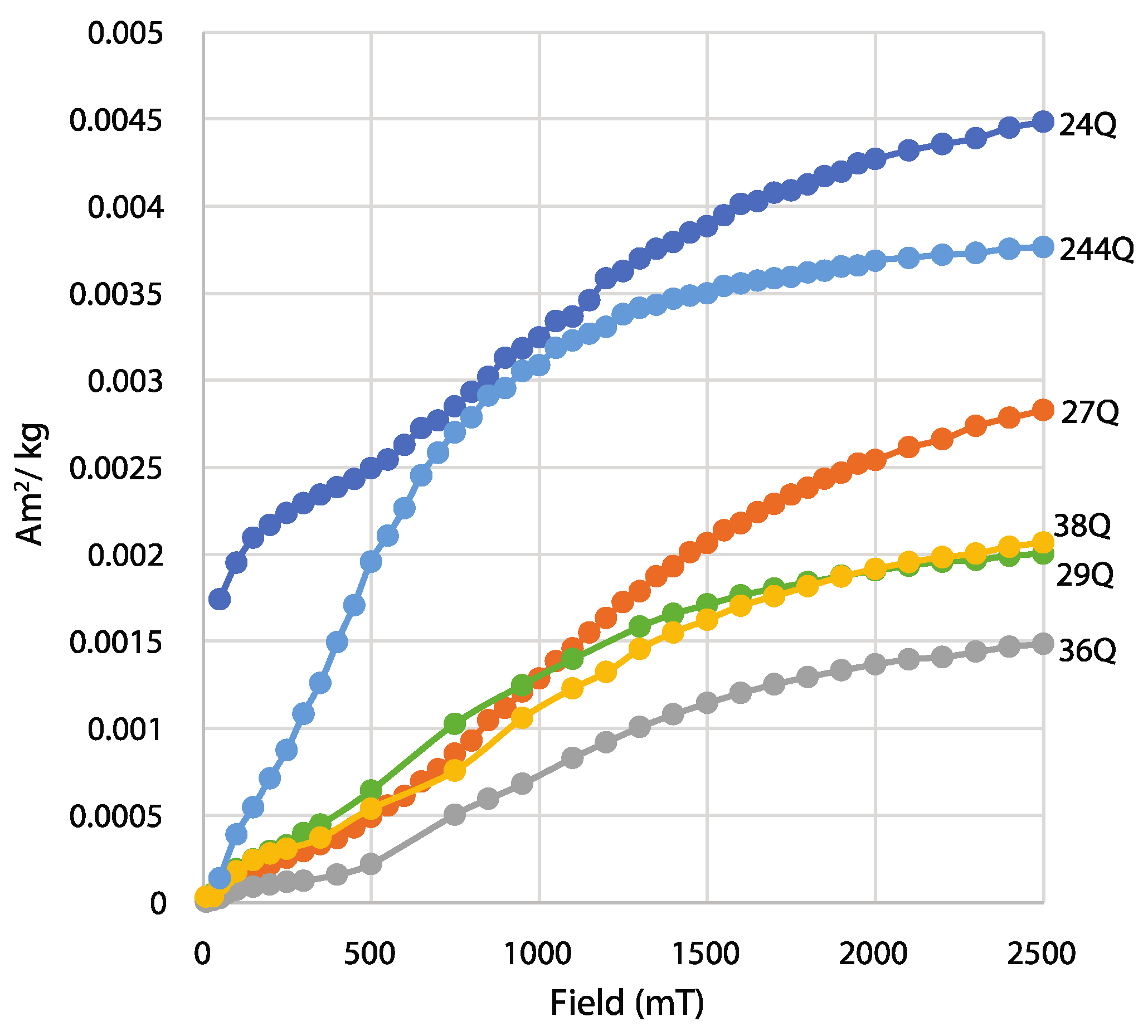
3.3. Combining Magnetic and DRS Methods to Analyze Paleoclimate
4. Conclusions
- o
- First-derivative analysis introduces shifts in the characteristic wavelength band position that are related to the math of the algorithm. This first-derivative measurement bias may be exacerbated by the layering of mineral aggregates that are produced by high concentrations of hematite. The Kubelka–Munk remission function, which is a data pretreatment step for second-derivative analysis, parameterizes the layering effects before the visible-light data are transformed by second-derivative analysis. This provides second-derivative analysis of visible-light spectra with more resolution for understanding hematite concentration and crystal size by increasing the detection sensitivity to natural shifts (<30 nm) in the characteristic wavelength band positions that are associated with hematite colors.
- o
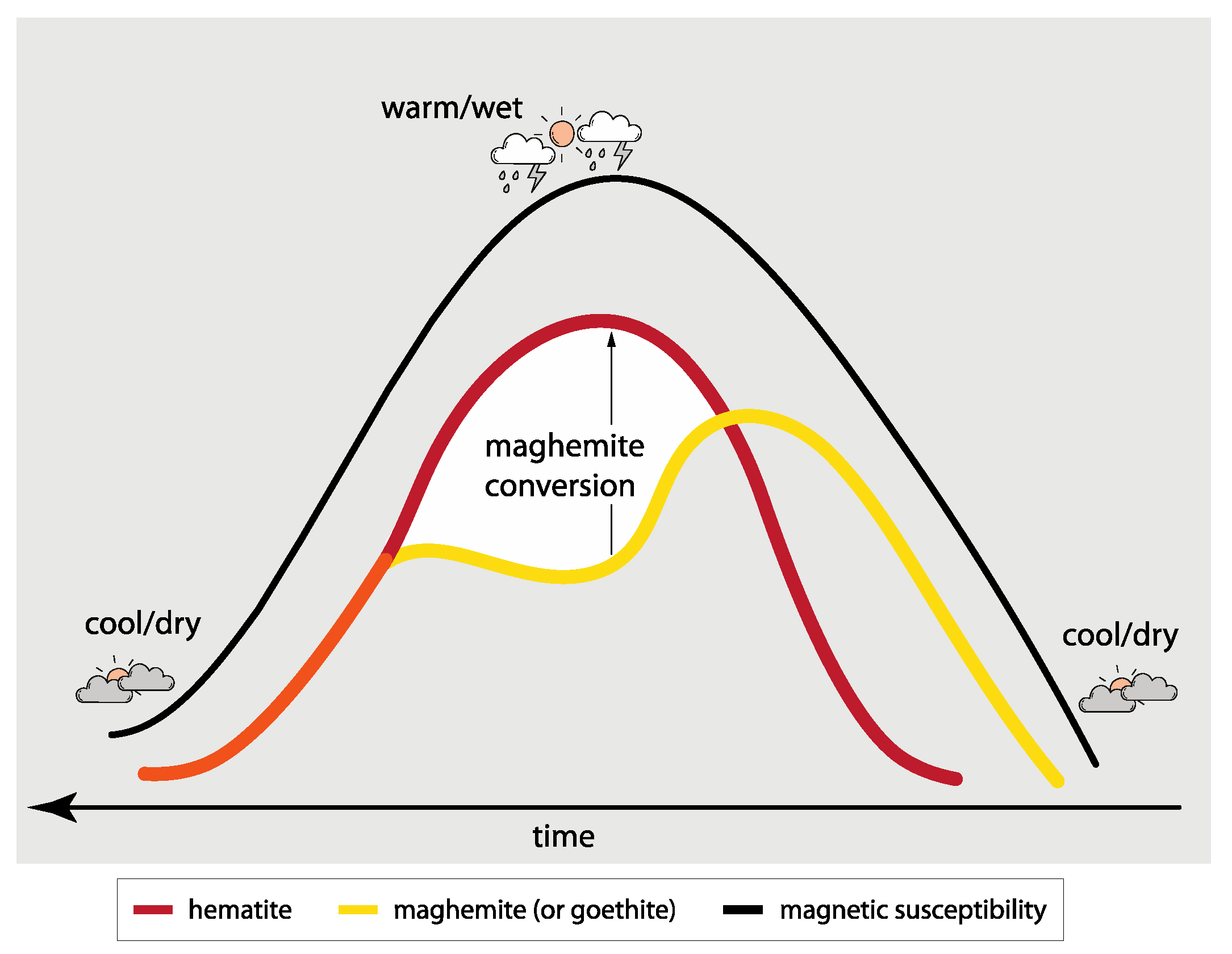
- Previous studies using visible-light data have provided examples of hematite concentration increasing under warm/wet climates. The increase in the colorful hematite is probably derived from the transformation of maghemite, and as more colorful hematite is generated, the amount of maghemite is depleted. This may be recorded by visible-light spectra as decreasing intensity at the I425 characteristic wavelength position that maghemite shares with goethite. Perceived changes in colorful goethite may be due to the loss of maghemite as it converts to hematite under warm/wet climates.
- o
- A challenge for studying maghemite and goethite from visible-light data is that the characteristic wavelength band positions of these minerals overlap at longer wavelengths near ~425 nm. To increase resolution for discriminating between maghemite and goethite, it is suggested to combine visible-light data with rock magnetic methods because of the different magnetic coercivities these minerals possess. This may improve concertation estimates for both minerals in natural assemblages and increase the understanding of the environmental conditions and the composition of mixed mineral assemblages in which goethite and maghemite form. The next steps of this research may include using the methods outlined in this paper to investigate synthetic samples doped with known concentrations of maghemite and goethite and comparing these results with natural rocks, sediment, and soil that have been validated through quantitative geochemistry.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, W.; Jiang, Z.; Gai, C.; Barrón, V.; Torrent, J.; Zhong, Y.; Liu, Q. Re-Visiting the Quantification of Hematite by Diffuse Reflectance Spectroscopy. Minerals 2022, 12, 872. [Google Scholar] [CrossRef]
- Gao, X.; Hao, Q.; Wang, L.; Song, Y.; Ge, J.; Wu, H.; Xu, B.; Han, L.; Fu, Y.; Wu, X.; et al. Changes in monsoon precipitation in East Asia under a 2 °C interglacial warming. Sci. Adv. 2024, 10, eadm7694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, Q.; Roberts, A.P.; Dekkers, M.J.; Barrón, V.; Torrent, J.; Li, S. The Magnetic and Color Reflectance Properties of Hematite: From Earth to Mars. Rev. Geophys. 2022, 60, e2020RG000698. [Google Scholar] [CrossRef]
- Boily, J.-F.; Song, X. Direct identification of reaction sites on ferrihydrite. Commun. Chem. 2020, 3, 79. [Google Scholar] [CrossRef]
- Joshi, P.; Fantle, M.S.; Larese-Casanova, P.; Gorski, C.A. Susceptibility of Goethite to Fe2+-Catalyzed Recrystallization over Time. Environ. Sci. Technol. 2017, 51, 11681–11691. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, K.; Guggenberger, G. Mineral surfaces and soil organic matter. Eur. J. Soil Sci. 2003, 54, 219–236. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 130, pp. 1–140. ISBN 978-0-12-802137-8. [Google Scholar]
- Marshall, T.A.; Morris, K.; Law, G.T.W.; Livens, F.R.; Mosselmans, J.F.W.; Bots, P.; Shaw, S. Incorporation of Uranium into Hematite during Crystallization from Ferrihydrite. Environ. Sci. Technol. 2014, 48, 3724–3731. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Long, X.; Ji, J.; Barrón, V.; Torrent, J.; Wang, Y.; Xie, S. Different Enrichment Patterns of Magnetic Particles Modulated by Primary Iron-Phosphorous Input. Geophys. Res. Lett. 2020, 47, e2020GL090439. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Tiecher, T.; Barrón, V. Iron oxides and organic matter on soil phosphorus availability. Ciênc. E Agrotecnologia 2016, 40, 369–379. [Google Scholar] [CrossRef]
- Handler, R.M.; Frierdich, A.J.; Johnson, C.M.; Rosso, K.M.; Beard, B.L.; Wang, C.; Latta, D.E.; Neumann, A.; Pasakarnis, T.; Premaratne, W.A.P.J.; et al. Fe(II)-Catalyzed Recrystallization of Goethite Revisited. Environ. Sci. Technol. 2014, 48, 11302–11311. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Plaza, C.; Saiz, H.; Manzano, R.; Flagmeier, M.; Maestre, F.T. Aridity and reduced soil micronutrient availability in global drylands. Nat. Sustain. 2019, 2, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Amelung, W.; Xing, Y.; Bol, R.; Berns, A.E. Iron cycling and isotope fractionation in terrestrial ecosystems. Earth-Sci. Rev. 2019, 190, 323–352. [Google Scholar] [CrossRef]
- Ye, C.; Huang, W.; Hall, S.J.; Hu, S. Association of Organic Carbon with Reactive Iron Oxides Driven by Soil pH at the Global Scale. Glob. Biogeochem. Cycles 2022, 36, e2021GB007128. [Google Scholar] [CrossRef]
- Zhao, M.; Mills, B.J.W.; Homoky, W.B.; Peacock, C.L. Oxygenation of the Earth aided by mineral–organic carbon preservation. Nat. Geosci. 2023, 16, 262–267. [Google Scholar] [CrossRef]
- Di Iorio, E.; Circelli, L.; Angelico, R.; Torrent, J.; Tan, W.; Colombo, C. Environmental implications of interaction between humic substances and iron oxide nanoparticles: A review. Chemosphere 2022, 303, 135172. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zeng, Q.; Sheng, Y.; Chen, C.; Yu, G.; Kappler, A. Coupled iron cycling and organic matter transformation across redox interfaces. Nat. Rev. Earth Environ. 2023, 4, 659–673. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The transformation of ferrihydrite into goethite or hematite, revisited. J. Solid State Chem. 2006, 179, 716–722. [Google Scholar] [CrossRef]
- Kadlec, R. Large Constructed Wetlands for Phosphorus Control: A Review. Water 2016, 8, 243. [Google Scholar] [CrossRef]
- Schwertmann, U. Transformation of hematite to goethite in soils. Nature 1971, 232, 624–625. [Google Scholar] [CrossRef]
- Bigham, J.M.; Fitzpatrick, R.W.; Schulze, D.G. Iron Oxides. In Soil Mineralogy with Environmental Applications; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 2002; pp. 323–366. ISBN 978-0-89118-891-9. [Google Scholar]
- Font, E.; Carlut, J.; Rémazeilles, C.; Mather, T.A.; Nédélec, A.; Mirão, J.; Casale, S. End-Cretaceous akaganéite as a mineral marker of Deccan volcanism in the sedimentary record. Sci. Rep. 2017, 7, 11453. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Cornell, R.M. (Eds.) Frontmatter. In Iron Oxides in the Laboratary; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; pp. i–xviii. ISBN 978-3-527-61322-9. [Google Scholar]
- Stucki, J.W. Properties and Behaviour of Iron in Clay Minerals. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 559–611. ISBN 978-0-08-099364-5. [Google Scholar]
- Maher, B.A. Palaeoclimatic records of the loess/palaeosol sequences of the Chinese Loess Plateau. Quat. Sci. Rev. 2016, 154, 23–84. [Google Scholar] [CrossRef]
- Evans, M.E.; Heller, F. Environmental Magnetism: Principles and Applications of Enviromagnetics; International geophysics series; Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2003; ISBN 978-0-12-243851-6. [Google Scholar]
- Huang, W.; Jackson, M.J.; Dekkers, M.J.; Solheid, P.; Zhang, B.; Guo, Z.; Ding, L. Nanogoethite as a Potential Indicator of Remagnetization in Red Beds. Geophys. Res. Lett. 2019, 46, 12841–12850. [Google Scholar] [CrossRef]
- Kumar, N.; Lezama Pacheco, J.; Noël, V.; Dublet, G.; Brown, G.E. Sulfidation mechanisms of Fe(III)-(oxyhydr)oxide nanoparticles: A spectroscopic study. Environ. Sci. Nano 2018, 5, 1012–1026. [Google Scholar] [CrossRef]
- Li, J.; Lü, B.; Chen, T.; Liu, X.; Tang, J.; Yan, H. A New Perspective on the Applicability of Diffuse Reflectance Spectroscopy for Determining the Hematite Content of Fe-Rich Soils in the Tropical Margins of China. Minerals 2024, 14, 242. [Google Scholar] [CrossRef]
- Meng, X.; Li, G.K.; Long, X.; Li, S.; Ji, J. Quantifying Soil Goethite/Hematite Ratios: A New Method Based on Diffuse Reflectance Spectra. Geophys. Res. Lett. 2023, 50, e2022GL102280. [Google Scholar] [CrossRef]
- Nagano; Nakashima Study of colors and degrees of weathering of granitic rocks by visible diffuse reflectance spectroscopy. Geochem. J. 1989, 23, 75–83. [CrossRef]
- Poggere, G.C.; Inda, A.V.; Barrón, V.; Kämpf, N.; de Brito, A.D.B.; Barbosa, J.Z.; Curi, N. Maghemite quantification and magnetic signature of Brazilian soils with contrasting parent materials. Appl. Clay Sci. 2018, 161, 385–394. [Google Scholar] [CrossRef]
- Pohl, L.; Kölbl, A.; Werner, F.; Mueller, C.W.; Höschen, C.; Häusler, W.; Kögel-Knabner, I. Imaging of Al/Fe ratios in synthetic Al-goethite revealed by nanoscale secondary ion mass spectrometry. Rapid Commun. Mass Spectrom. 2018, 32, 619–628. [Google Scholar] [CrossRef]
- Thompson, R.; Oldfield, F. Environmental Magnetism; Allen & Unwin: London, UK, 1986; ISBN 978-94-011-8038-2. [Google Scholar]
- Tishchenko, V.; Meile, C.; Scherer, M.M.; Pasakarnis, T.S.; Thompson, A. Fe2+ catalyzed iron atom exchange and re-crystallization in a tropical soil. Geochim. Cosmochim. Acta 2015, 148, 191–202. [Google Scholar] [CrossRef]
- Bógalo, M.F.; Heller, F.; Osete, M.L. Isothermal remanence experiments at room and at liquid nitrogen temperature: Application to soil studies. Geophys. Res. Lett. 2001, 28, 419–422. [Google Scholar] [CrossRef]
- Roberts, A.P.; Zhao, X.; Heslop, D.; Abrajevitch, A.; Chen, Y.-H.; Hu, P.; Jiang, Z.; Liu, Q.; Pillans, B.J. Hematite (α-Fe2O3) quantification in sedimentary magnetism: Limitations of existing proxies and ways forward. Geosci. Lett. 2020, 7, 8. [Google Scholar] [CrossRef]
- Collinson, D.W. The Role of Pigment and Specularite in the Remanent Magnetism of Red Sandstones. Geophys. J. Int. 1974, 38, 253–264. [Google Scholar] [CrossRef]
- Swanson-Hysell, N.L.; Fairchild, L.M.; Slotznick, S.P. Primary and Secondary Red Bed Magnetization Constrained by Fluvial Intraclasts. J. Geophys. Res. Solid Earth 2019, 124, 4276–4289. [Google Scholar] [CrossRef]
- Tauxe, L.; Kent, D.V.; Opdyke, N.D. Magnetic components contributing to the NRM of Middle Siwalik red beds. Earth Planet. Sci. Lett. 1980, 47, 279–284. [Google Scholar] [CrossRef]
- Dearing, J.A.; Dann, R.J.L.; Hay, K.; Lees, J.A.; Loveland, P.J.; Maher, B.A.; O’Grady, K. Frequency-dependent susceptibility measurements of environmental materials. Geophys. J. Int. 1996, 124, 228–240. [Google Scholar] [CrossRef]
- Egli, R. Characterization of Individual Rock Magnetic Components by Analysis of Remanence Curves, 1. Unmixing Natural Sediments. Stud. Geophys. Geod. 2004, 48, 391–446. [Google Scholar] [CrossRef]
- Gao, X.; Hao, Q.; Wang, L.; Oldfield, F.; Bloemendal, J.; Deng, C.; Song, Y.; Ge, J.; Wu, H.; Xu, B.; et al. The different climatic response of pedogenic hematite and ferrimagnetic minerals: Evidence from particle-sized modern soils over the Chinese Loess Plateau. Quat. Sci. Rev. 2018, 179, 69–86. [Google Scholar] [CrossRef]
- Geiss, C.E.; Egli, R.; Zanner, C.W. Direct estimates of pedogenic magnetite as a tool to reconstruct past climates from buried soils. J. Geophys. Res. 2008, 113, B11102. [Google Scholar] [CrossRef]
- Orgeira, M.J.; Egli, R.; Compagnucci, R.H. A Quantitative Model of Magnetic Enhancement in Loessic Soils. In The Earth’s Magnetic Interior; Petrovský, E., Ivers, D., Harinarayana, T., Herrero-Bervera, E., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 361–397. ISBN 978-94-007-0322-3. [Google Scholar]
- Slotznick, S.P.; Winston, D.; Webb, S.M.; Kirschvink, J.L.; Fischer, W.W. Iron mineralogy and redox conditions during deposition of the mid-Proterozoic Appekunny Formation, Belt Supergroup, Glacier National Park. In Geological Society of America Special Papers; Geological Society of America: Boulder, CO, USA, 2016; Volume 522, pp. 221–242. ISBN 978-0-8137-2522-2. [Google Scholar]
- Cai, Y.; Long, X.; Meng, X.; Ji, J.; Wang, Y.; Xie, S. Coordinated and Competitive Formation of Soil Magnetic Particles Driven by Contrary Climate Development. Geophys. Res. Lett. 2021, 48, e2021GL094506. [Google Scholar] [CrossRef]
- Jordanova, N.; Jordanova, D. Rock-magnetic and geochemical characteristics of relict Vertisols—Signs of past climate and recent pedogenic development. Geophys. J. Int. 2016, 205, 1437–1454. [Google Scholar] [CrossRef]
- Lepre, C.J. Constraints on Fe-Oxide Formation in Monsoonal Vertisols of Pliocene Kenya Using Rock Magnetism and Spectroscopy. Geochem. Geophys. Geosystems 2019, 20, 4998–5013. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.; Yang, J.; Gao, F.; Tian, W.; Ma, X.; Xia, D. Magnetic properties of surface soils in the upper and middle reaches of the Yarlung Zangbo River Basin, southern Tibetan Plateau, and their environmental significance. Geophys. J. Int. 2023, 233, 1178–1190. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Roberts, A.P.; Barrón, V.; Torrent, J.; Zhang, Q. A new model for transformation of ferrihydrite to hematite in soils and sediments. Geology 2018, 46, 987–990. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Maher, B.A. Identification and paleoclimatic significance of magnetite nanoparticles in soils. Proc. Natl. Acad. Sci. USA 2018, 115, 1736–1741. [Google Scholar] [CrossRef]
- Liu, Q.; Barrón, V.; Torrent, J.; Eeckhout, S.G.; Deng, C. Magnetism of intermediate hydromaghemite in the transformation of 2-line ferrihydrite into hematite and its paleoenvironmental implications. J. Geophys. Res. 2008, 113, B01103. [Google Scholar] [CrossRef]
- Balsam, W.; Ji, J.; Chen, J. Climatic interpretation of the Luochuan and Lingtai loess sections, China, based on changing iron oxide mineralogy and magnetic susceptibility. Earth Planet. Sci. Lett. 2004, 223, 335–348. [Google Scholar] [CrossRef]
- Berner, R.A. Goethite stability and the origin of red beds. Geochim. Cosmochim. Acta 1969, 33, 267–273. [Google Scholar] [CrossRef]
- Grygar, T.; Dědeček, J.; Kruiver, P.P.; Dekkers, M.J.; Bezdička, P.; Schneeweiss, O. Iron oxide mineralogy in late Miocene red beds from La Gloria, Spain: Rock-magnetic, voltammetric and Vis spectroscopy analyses. CATENA 2003, 53, 115–132. [Google Scholar] [CrossRef]
- Guo, X.; Retallack, G.J.; Lü, B.; He, L.; Wang, R.; Song, H. Paleosols in Devonian red-beds from northwest China and their paleoclimatic characteristics. Sediment. Geol. 2019, 379, 16–24. [Google Scholar] [CrossRef]
- Sierra-Rojas, M.I.; Molina-Garza, R.S. Detrital and early chemical remanent magnetization in redbeds and their rock magnetic signature: Zicapa Formation, southern Mexico. Geophys. J. Int. 2018, 213, 1701–1719. [Google Scholar] [CrossRef]
- Torrent, J.; Schwertmann, U. Influence of Hematite on the Color of Red Beds. J. Sediment. Petrol. 1987, 57, 682–686. [Google Scholar]
- Van Houten, F.B. Origin of Red Beds A Review-1961-1972. Annu. Rev. Earth Planet. Sci. 1973, 1, 39–61. [Google Scholar] [CrossRef]
- Walker, T.R.; Larson, E.E.; Hoblitt, R.P. Nature and origin of hematite in the Moenkopi Formation (Triassic), Colorado Plateau: A contribution to the origin of magnetism in red beds. J. Geophys. Res. 1981, 86, 317. [Google Scholar] [CrossRef]
- He, W.; Yang, Z.; Du, H.; Hu, J.; Zhang, K.; Hou, W.; Li, H. Micro-Mechanisms and Implications of Continental Red Beds. Minerals 2022, 12, 934. [Google Scholar] [CrossRef]
- Lepre, C.J.; Olsen, P.E. Hematite reconstruction of Late Triassic hydroclimate over the Colorado Plateau. Proc. Natl. Acad. Sci. USA 2021, 118, 6. [Google Scholar] [CrossRef] [PubMed]
- Bahr, A.; Kolber, G.; Kaboth-Bahr, S.; Reinhardt, L.; Friedrich, O.; Pross, J. Mega-monsoon variability during the late Triassic: Re-assessing the role of orbital forcing in the deposition of playa sediments in the Germanic Basin. Sedimentology 2020, 67, 951–970. [Google Scholar] [CrossRef]
- Olsen, P.; Laskar, J.; Kent, D.V.; Kinney, S.T.; Reynolds, D.J.; Sha, J.; Whiteside, J.H. Mapping Solar System chaos with the Geological Orrery. Proc. Natl. Acad. Sci. USA 2019, 116, 10664–10673. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Hendry, M.J.; Essilfie-Dughan, J. Transformation of Two-Line Ferrihydrite to Goethite and Hematite as a Function of pH and Temperature. Environ. Sci. Technol. 2011, 45, 268–275. [Google Scholar] [CrossRef]
- Hanesch, M.; Stanjek, H.; Petersen, N. Thermomagnetic measurements of soil iron minerals: The role of organic carbon. Geophys. J. Int. 2006, 165, 53–61. [Google Scholar] [CrossRef]
- Löhr, S.C.; Grigorescu, M.; Cox, M.E. Iron nodules in ferric soils of the Fraser Coast, Australia: Relicts of laterisation or features of contemporary weathering and pedogenesis? Soil Res. 2013, 51, 77. [Google Scholar] [CrossRef]
- Spinola, D.N.; de Castro Portes, R.; Srivastava, P.; Torrent, J.; Barrón, V.; Kühn, P. Diagenetic reddening of Early Eocene paleosols on King George Island, Antarctica. Geoderma 2018, 315, 149–159. [Google Scholar] [CrossRef]
- Srivastava, P.; Sangode, S.J.; Torrent, J. Mineral magnetic and diffuse reflectance spectroscopy characteristics of the Deccan volcanic bole beds: Implications to genesis and transformations of iron oxides. Geoderma 2015, 239–240, 317–330. [Google Scholar] [CrossRef]
- Till, J.L.; Nowaczyk, N. Authigenic magnetite formation from goethite and hematite and chemical remanent magnetization acquisition. Geophys. J. Int. 2018, 213, 1818–1831. [Google Scholar] [CrossRef]
- Barron, V.; Torrent, J. Evidence for a simple pathway to maghemite in Earth and Mars soils. Geochim. Cosmochim. Acta 2002, 66, 2801–2806. [Google Scholar] [CrossRef]
- Schwertmann, U. Relations between Iron Oxides, Soil Color, and Soil Formation. In Soil Color; SSSA Special Publications: Madison, WI, USA, 1993; pp. 51–69. [Google Scholar]
- Olsen, P.E.; Geissman, J.W.; Kent, D.V.; Gehrels, G.E.; Mundil, R.; Irmis, R.B.; Lepre, C.; Rasmussen, C.; Giesler, D.; Parker, W.G.; et al. Colorado Plateau Coring Project, Phase I (CPCP-I): A continuously cored, globally exportable chronology of Triassic continental environmental change from western North America. Sci. Drill. 2018, 24, 15–40. [Google Scholar] [CrossRef]
- Chan, M.A.; Beitler, B.; Parry, W.T.; Ormö, J.; Komatsu, G. A possible terrestrial analogue for haematite concretions on Mars. Nature 2004, 429, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.V.; Olsen, P.E.; Lepre, C.; Rasmussen, C.; Mundil, R.; Gehrels, G.E.; Giesler, D.; Irmis, R.B.; Geissman, J.W.; Parker, W.G. Magnetochronology of the Entire Chinle Formation (Norian Age) in a Scientific Drill Core From Petrified Forest National Park (Arizona, USA) and Implications for Regional and Global Correlations in the Late Triassic. Geochem. Geophys. Geosystems 2019, 20, 4654–4664. [Google Scholar] [CrossRef]
- Ji, J.; Balsam, W.; Chen, J. Mineralogic and Climatic Interpretations of the Luochuan Loess Section (China) Based on Diffuse Reflectance Spectrophotometry. Quat. Res. 2001, 56, 23–30. [Google Scholar] [CrossRef]
- Heller, F.; Tung-sheng, L. Magnetostratigraphical dating of loess deposits in China. Nature 1982, 300, 431–433. [Google Scholar] [CrossRef]
- Lepre, C. Identifying Temperature and Moisture Controls on Fe Oxide Origins. Geophys. Res. Lett. 2023, 50, e2023GL102761. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. Diffuse Reflectance Spectroscopy. Methods Soil Anal. 2008, 5, 19. [Google Scholar]
- Scheinost, A.C.; Chavernas, A.; Barron, V.; Torrent, J. Use and Limitations of Second-Derivative Diffuse Reflectance Spectroscopy in the Visible to Near-Infrared Range to Identify and Quantify Fe Oxide Minerals in Soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Kruiver, P.P.; Dekkers, M.J.; Heslop, D. Quantification of magnetic coercivity components by the analysis of acquisition curves of isothermal remanent magnetisation. Earth Planet. Sci. Lett. 2001, 189, 269–276. [Google Scholar] [CrossRef]
- Maxbauer, D.P.; Feinberg, J.M.; Fox, D.L. MAX UnMix: A web application for unmixing magnetic coercivity distributions. Comput. Geosci. 2016, 95, 140–145. [Google Scholar] [CrossRef]
- Lowrie, W. Identification of ferromagnetic minerals in a rock by coercivity and unblocking temperature properties. Geophys. Res. Lett. 1990, 17, 159–162. [Google Scholar] [CrossRef]
- Liu, Q.S.; Torrent, J.; Barrón, V.; Duan, Z.Q.; Bloemendal, J. Quantification of hematite from the visible diffuse reflectance spectrum: Effects of aluminium substitution and grain morphology. Clay Miner. 2011, 46, 137–147. [Google Scholar] [CrossRef]
- Dos Santos, I.F.S.; Edwards, H.G.M.; De Faria, D.L.A. Hematite colour revisited: Particle size and electronic transitions. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2024, 310, 123810. [Google Scholar] [CrossRef]
- Morris, R.V.; Lauer, H.V.; Lawson, C.A.; Gibson, E.K.; Nace, G.A.; Stewart, C. Spectral and other physicochemical properties of submicron powders of hematite (α-Fe2O3), maghemite (γ-Fe O3), magnetite (Fe3O4), goethite (α-FeOOH), and lepidocrocite (γ-FeOOH). J. Geophys. Res. 1985, 90, 3126. [Google Scholar] [CrossRef]
- Rossman, G.R. Why hematite is red: Correlation of optical absorption intensities and magnetic moments of Fe3+ minerals. Miner. Spectrosc. Tribute Roger G Bums 1996, 5, 23–27. [Google Scholar]
- Sherman, D.M.; Waite, T.D. Waite Electronic spectra of Fe3+ oxides and oxide hydroxides in the near IR to near UV. Am. Mineral. 1985, 70, 1262–1269. [Google Scholar]
- Bañuelos, J.L.; Borguet, E.; Brown, G.E.; Cygan, R.T.; DeYoreo, J.J.; Dove, P.M.; Gaigeot, M.-P.; Geiger, F.M.; Gibbs, J.M.; Grassian, V.H.; et al. Oxide– and Silicate–Water Interfaces and Their Roles in Technology and the Environment. Chem. Rev. 2023, 123, 6413–6544. [Google Scholar] [CrossRef] [PubMed]
- Coduri, M.; Masala, P.; Del Bianco, L.; Spizzo, F.; Ceresoli, D.; Castellano, C.; Cappelli, S.; Oliva, C.; Checchia, S.; Allieta, M.; et al. Local Structure and Magnetism of Fe2O3 Maghemite Nanocrystals: The Role of Crystal Dimension. Nanomaterials 2020, 10, 867. [Google Scholar] [CrossRef] [PubMed]
- OSF. Available online: https://osf.io/5vd8u/ (accessed on 30 April 2024).
- Scheinost, A.C.; Schwertmann, U. Color Identification of Iron Oxides and Hydroxysulfates: Use and Limitations. Soil Sci. Soc. Am. J. 1999, 63, 1463–1471. [Google Scholar] [CrossRef]
- Kerker, M.; Scheiner, P.; Cooke, D.D.; Kratohvil, J.P. Absorption index and color of colloidal hematite. J. Colloid Interface Sci. 1979, 71, 176–187. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. The visible diffuse reflectance spectrum in relation to the color and crystal properties of hematite. Clays Clay Miner. 2003, 51, 309–317. [Google Scholar] [CrossRef]
- Deaton, B.C.; Balsam, W.L. Visible Spectroscopy—A Rapid Method for Determining Hematite and Goethite Concentration in Geological Materials. J. Sediment. Petrol. 1991, 61, 628–632. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- Hu, P.; Jiang, Z.; Liu, Q.; Heslop, D.; Roberts, A.P.; Torrent, J.; Barrón, V. Estimating the concentration of aluminum-substituted hematite and goethite using diffuse reflectance spectrometry and rock magnetism: Feasibility and limitations: Al-Hematite/Al-Goethite Quantification. J. Geophys. Res. Solid Earth 2016, 121, 4180–4194. [Google Scholar] [CrossRef]
- Balsam, W.; Junfeng, J.; Renock, D.; Deaton, B.C.; Williams, E. Determining hematite content from NUV/Vis/NIR spectra: Limits of detection. Am. Mineral. 2014, 99, 2280–2291. [Google Scholar] [CrossRef]
- Long, X.; Ji, J.; Balsam, W.L. Rainfall-dependent transformations of iron oxides in a tropical saprolite transect of Hainan Island, South China: Spectral and magnetic measurements. J. Geophys. Res. 2011, 116, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Ji, J.; Balsam, W.L.; Liu, L.; Chen, J. High resolution hematite and goethite records from ODP 1143, South China Sea: Co-evolution of monsoonal precipitation and El Niño over the past 600,000 years. Earth Planet. Sci. Lett. 2007, 264, 136–150. [Google Scholar] [CrossRef]
- Nagano, T.; Isobe, H.; Nakashima, S.; Ashizaki, M. Characterization of Iron Hydroxides in a Weathered Rock Surface by Visible Microspectroscopy. Appl. Spectrosc. 2002, 56, 651–657. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Colombo, C.; Barrón, V.; Torrent, J.; Hu, P. Quantification of Al-goethite from diffuse reflectance spectroscopy and magnetic methods. Geophys. J. Int. 2014, 196, 131–144. [Google Scholar] [CrossRef]
- Torrent, J.; Liu, Q.; Bloemendal, J.; Barrón, V. Magnetic Enhancement and Iron Oxides in the Upper Luochuan Loess–Paleosol Sequence, Chinese Loess Plateau. Soil Sci. Soc. Am. J. 2007, 71, 1570. [Google Scholar] [CrossRef]
- Moskowitz, B.M.; Reynolds, R.L.; Goldstein, H.L.; Berquó, T.S.; Kokaly, R.F.; Bristow, C.S. Iron oxide minerals in dust-source sediments from the Bodélé Depression, Chad: Implications for radiative properties and Fe bioavailability of dust plumes from the Sahara. Aeolian Res. 2016, 22, 93–106. [Google Scholar] [CrossRef]
- Peters, C.; Dekkers, M.J. Selected room temperature magnetic parameters as a function of mineralogy, concentration and grain size. Phys. Chem. Earth Parts ABC 2003, 28, 659–667. [Google Scholar] [CrossRef]
- France, D.E.; Oldfield, F. Identifying goethite and hematite from rock magnetic measurements of soils and sediments. J. Geophys. Res. Solid Earth 2000, 105, 2781–2795. [Google Scholar] [CrossRef]
- Özdemir, Ö. High-temperature hysteresis and thermoremanence of single-domain maghemite. Phys. Earth Planet. Inter. 1990, 65, 125–136. [Google Scholar] [CrossRef]
- Gehring, A.U.; Fischer, H.; Louvel, M.; Kunze, K.; Weidler, P.G. High temperature stability of natural maghemite: A magnetic and spectroscopic study. Geophys. J. Int. 2009, 179, 1361–1371. [Google Scholar] [CrossRef]
- van Velzen, A.J.; Dekkers, M.J. Low-Temperature Oxidation of Magnetite in Loess-Paleosol Sequences: A Correction of Rock Magnetic Parameters. Studia Geoph. Geod. 1999, 43, 357–375. [Google Scholar] [CrossRef]
- Fitzpatrick, R.W.; Schwertmann, U. Al-Substituted Goethite—An Indicator of Pedogenic and other Weathering Environments in South Africa. Geoderma 1982, 27, 335–347. [Google Scholar] [CrossRef]
- Liu, Q.; Roberts, A.P.; Torrent, J.; Horng, C.-S.; Larrasoaña, J.C. What do the HIRM and S-ratio really measure in environmental magnetism? Geochem. Geophys. Geosystems 2007, 8, 1–10. [Google Scholar] [CrossRef]
- Tibbits, D.; Chang, C.; Kinney, S.T.; Bebo, C.R.; Pinnella, M.; Witkowski, R.; Prabhakar, L. Leveraging Scanning X-ray Fluorescence Spectroscopy and High-Resolution X-ray Computed Tomography to Map Density Changes Along Cores from the Colorado Plateau Coring Project Phase 1: Implications for Early Mesozoic Climate and Geogenic Groundwater Contamination. In Proceedings of the AGU Annual Meeting, San Francisco, CA, USA, 11–15 December 2023. [Google Scholar]
- Irving, E.; Opdyke, N.D. The Palaeomagnetism of the Bloomsburg Red Beds and its Possible Application to the Tectonic History of the Appalachians. Geophys. J. Int. 1965, 9, 153–167. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, Q.; Dekkers, M.J.; Tauxe, L.; Qin, H.; Barrón, V.; Torrent, J. Acquisition of chemical remanent magnetization during experimental ferrihydrite–hematite conversion in Earth-like magnetic field—Implications for paleomagnetic studies of red beds. Earth Planet. Sci. Lett. 2015, 428, 1–10. [Google Scholar] [CrossRef]
- Dunlop, D.J.; Özdemir, Ö. Magnetizations in rocks and minerals. In Geomagnetism; Treatise on Geophysics; Elsevier: Amsterdam, The Netherlands, 2009; Volume 5, pp. 277–336. [Google Scholar]
- Forster, T.; Evans, M.E.; Heller, F. The frequency dependence of low field susceptibility in loess sediments. Geophys. J. Int. 1994, 118, 636–642. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J. Hysteresis and coercivity of hematite: Hysteresis and Coercivity of Hematite. J. Geophys. Res. Solid Earth 2014, 119, 2582–2594. [Google Scholar] [CrossRef]
- Lagroix, F.; Guyodo, Y. A New Tool for Separating the Magnetic Mineralogy of Complex Mineral Assemblages from Low Temperature Magnetic Behavior. Front. Earth Sci. 2017, 5, 61. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Dunlop, D.J.; Berquó, T.S. Morin transition in hematite: Size dependence and thermal hysteresis. Geochem. Geophys. Geosystems 2008, 9, 2008GC002110. [Google Scholar] [CrossRef]
- Nie, J.; Garzione, C.; Su, Q.; Liu, Q.; Zhang, R.; Heslop, D.; Necula, C.; Zhang, S.; Song, Y.; Luo, Z. Dominant 100,000-year precipitation cyclicity in a late Miocene lake from northeast Tibet. Sci. Adv. 2017, 3, e1600762. [Google Scholar] [CrossRef]
- Retallack, G.J.; Sheldon, N.D.; Cogoini, M.; Elmore, R.D. Magnetic susceptibility of early Paleozoic and Precambrian paleosols. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003, 198, 373–380. [Google Scholar] [CrossRef]
- Yin, K.; Hong, H.; Algeo, T.J.; Churchman, G.J.; Li, Z.; Zhu, Z.; Fang, Q.; Zhao, L.; Wang, C.; Ji, K.; et al. Fe-oxide mineralogy of the Jiujiang red earth sediments and implications for Quaternary climate change, southern China. Sci. Rep. 2018, 8, 3610. [Google Scholar] [CrossRef] [PubMed]
- Ao, H.; Dupont-Nivet, G.; Rohling, E.J.; Zhang, P.; Ladant, J.-B.; Roberts, A.P.; Licht, A.; Liu, Q.; Liu, Z.; Dekkers, M.J.; et al. Orbital climate variability on the northeastern Tibetan Plateau across the Eocene–Oligocene transition. Nat. Commun. 2020, 11, 5249. [Google Scholar] [CrossRef] [PubMed]
- Zhisheng, A.; Guoxiong, W.; Jianping, L.; Youbin, S.; Yimin, L.; Weijian, Z.; Yanjun, C.; Anmin, D.; Li, L.; Jiangyu, M.; et al. Global Monsoon Dynamics and Climate Change. Annu. Rev. Earth Planet. Sci. 2015, 43, 29–77. [Google Scholar] [CrossRef]
- Liu, Q.; Bloemendal, J.; Torrent, J.; Deng, C. Contrasting behavior of hematite and goethite within paleosol S5 of the Luochuan profile, Chinese Loess Plateau. Geophys. Res. Lett. 2006, 33, L20301. [Google Scholar] [CrossRef]
- Ao, H.; Rohling, E.J.; Stringer, C.; Roberts, A.P.; Dekkers, M.J.; Dupont-Nivet, G.; Yu, J.; Liu, Q.; Zhang, P.; Liu, Z.; et al. Two-stage mid-Brunhes climate transition and mid-Pleistocene human diversification. Earth-Sci. Rev. 2020, 210, 103354. [Google Scholar] [CrossRef]
- Kukla, G.J.; Bender, M.L.; de Beaulieu, J.-L.; Bond, G.; Broecker, W.S.; Cleveringa, P.; Gavin, J.E.; Herbert, T.D.; Imbrie, J.; Jouzel, J.; et al. Last Interglacial Climates. Quat. Res. 2002, 58, 2–13. [Google Scholar] [CrossRef]
- Chen, J.; An, Z.; Head, J. Variation of Rb/Sr Ratios in the Loess-Paleosol Sequences of Central China during the Last 130,000 Years and Their Implications for Monsoon Paleoclimatology. Quat. Res. 1999, 51, 215–219. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, H.; Liu, J.; Fang, Q.; Yao, Y.; Tan, W.; Yin, K.; Wang, C.; Chen, M.; Algeo, T.J. Assessing the utility of visible-to-shortwave infrared reflectance spectroscopy for analysis of soil weathering intensity and paleoclimate reconstruction. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 512, 80–94. [Google Scholar] [CrossRef]
- Evans, M.E.; Heller, F. Magnetic Enhancement and Palaeoclimate: Study of A Loess/Palaeosol Couplet Across the Loess Plateau of China. Geophys. J. Int. 1994, 117, 257–264. [Google Scholar] [CrossRef]
- Kukla, G.; Heller, F.; Ming, L.X.; Chun, X.T.; Sheng, L.T.; Sheng, A.Z. Pleistocene climates in China dated by magnetic susceptibility. Geology 1988, 16, 811–814. [Google Scholar] [CrossRef]
- Muxworthy, A.R.; Turney, J.N.; Qi, L.; Baker, E.B.; Perkins, J.R.; Abdulkarim, M.A. Interpreting high-temperature magnetic susceptibility data of natural systems. Front. Earth Sci. 2023, 11, 1171200. [Google Scholar] [CrossRef]
- Hao, Q.; Oldfield, F.; Bloemendal, J.; Torrent, J.; Guo, Z. The record of changing hematite and goethite accumulation over the past 22 Myr on the Chinese Loess Plateau from magnetic measurements and diffuse reflectance spectroscopy. J. Geophys. Res. 2009, 114, B12101. [Google Scholar] [CrossRef]
- Zhang, R.; Nie, J. Goethite Concentration Variations in the Red Clay Sequence on the Chinese Loess Plateau: Variations of Goethite in the Red Clay. Geochem. Geophys. Geosystems 2017, 18, 4179–4185. [Google Scholar] [CrossRef]
- Long, X.; Ji, J.; Barrón, V.; Torrent, J. Climatic thresholds for pedogenic iron oxides under aerobic conditions: Processes and their significance in paleoclimate reconstruction. Quat. Sci. Rev. 2016, 150, 264–277. [Google Scholar] [CrossRef]
- OSF. Available online: https://osf.io/b25h3/ (accessed on 30 April 2024).





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lepre, C.J.; Yazzie, O.M.; Klaus, B.R. Visible-Light Spectroscopy and Rock Magnetic Analyses of Iron Oxides in Mixed-Mineral Assemblages. Crystals 2024, 14, 644. https://doi.org/10.3390/cryst14070644
Lepre CJ, Yazzie OM, Klaus BR. Visible-Light Spectroscopy and Rock Magnetic Analyses of Iron Oxides in Mixed-Mineral Assemblages. Crystals. 2024; 14(7):644. https://doi.org/10.3390/cryst14070644
Chicago/Turabian StyleLepre, Christopher J., Owen M. Yazzie, and Benjamin R. Klaus. 2024. "Visible-Light Spectroscopy and Rock Magnetic Analyses of Iron Oxides in Mixed-Mineral Assemblages" Crystals 14, no. 7: 644. https://doi.org/10.3390/cryst14070644
APA StyleLepre, C. J., Yazzie, O. M., & Klaus, B. R. (2024). Visible-Light Spectroscopy and Rock Magnetic Analyses of Iron Oxides in Mixed-Mineral Assemblages. Crystals, 14(7), 644. https://doi.org/10.3390/cryst14070644




