Synthesis, Characterisation, and Applications of TiO and Other Black Titania Nanostructures Species (Review)
Abstract
1. Introduction
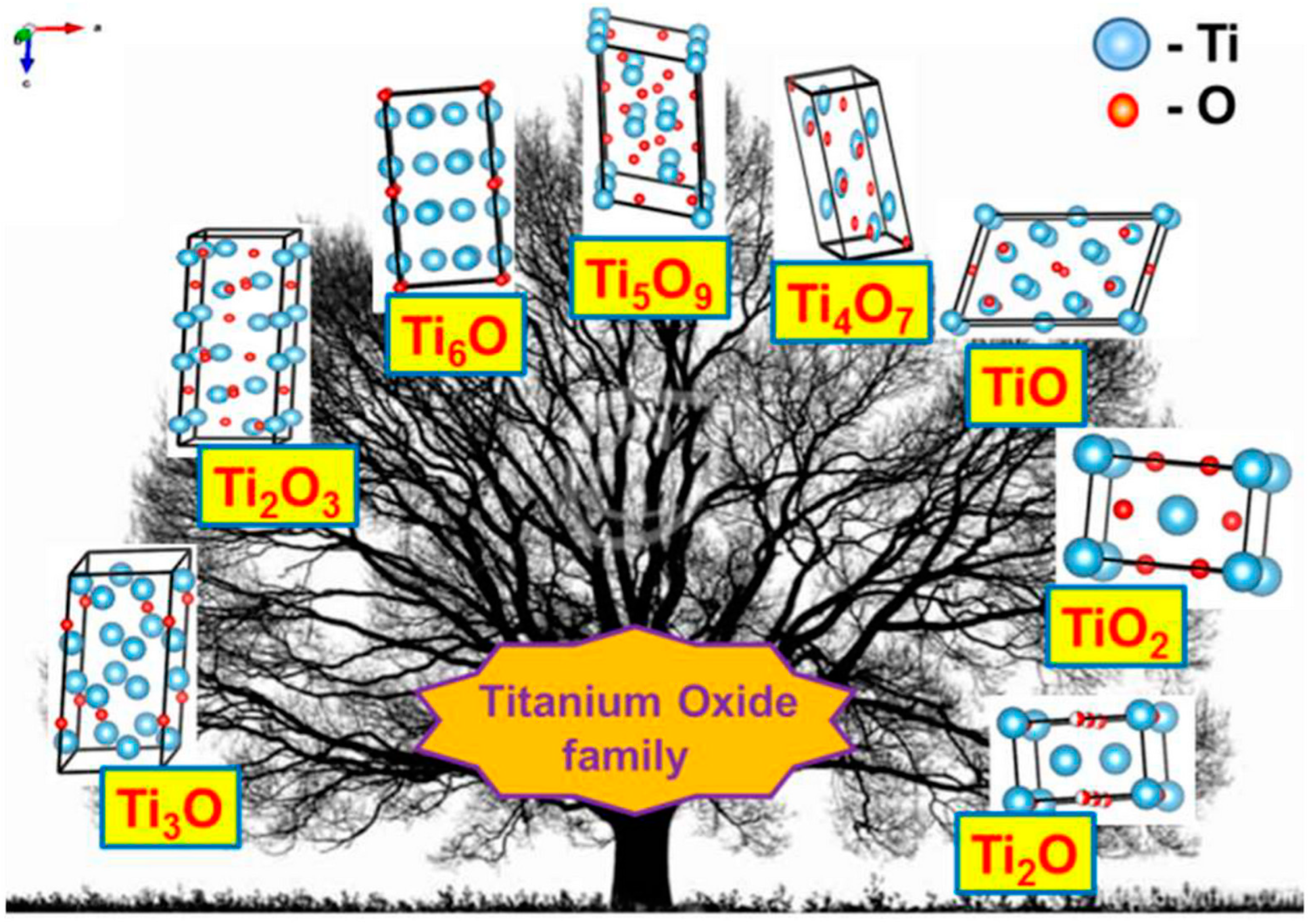
2. The Family of Black Titania Materials
3. Synthesis Routes for the Formation of Black Titania
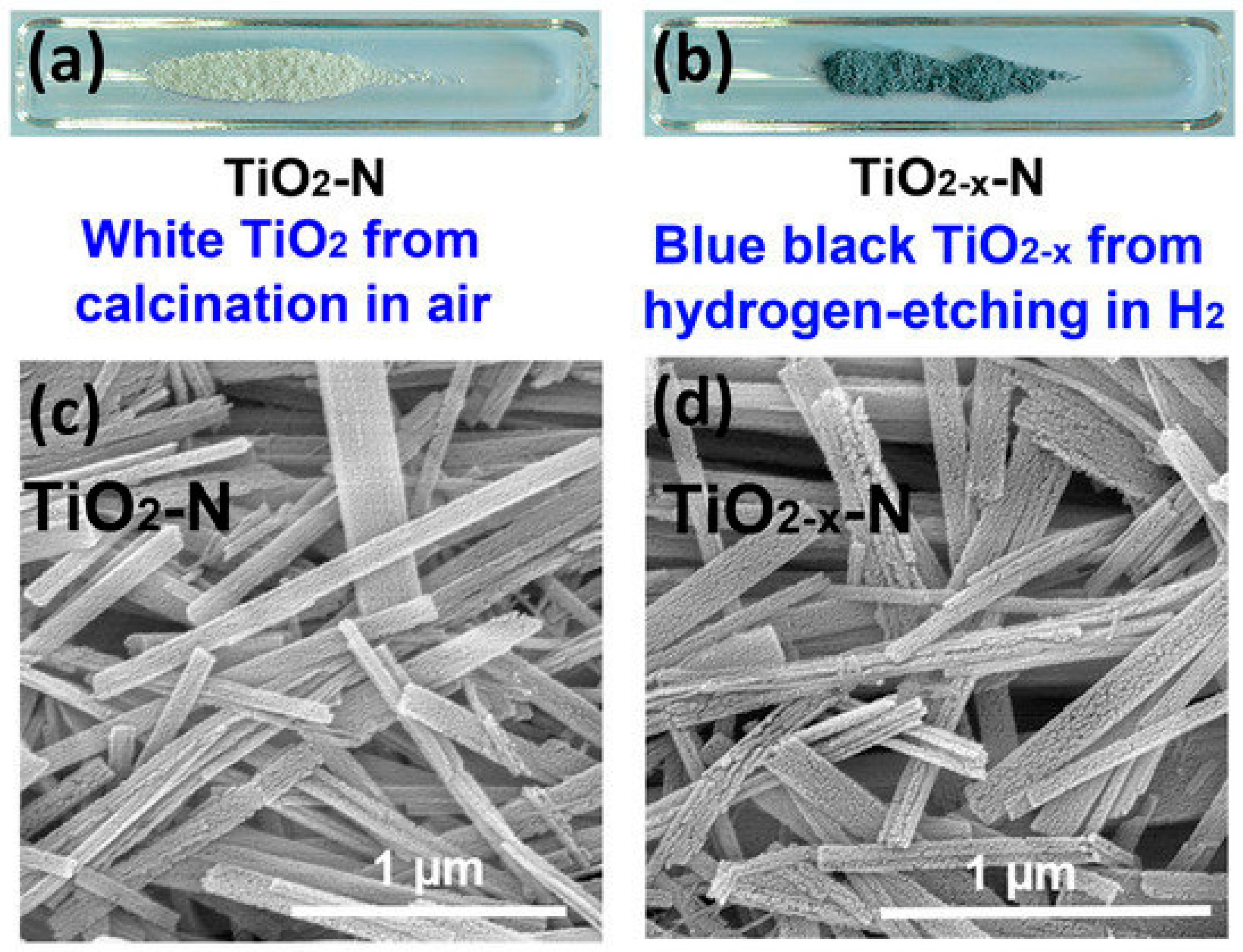
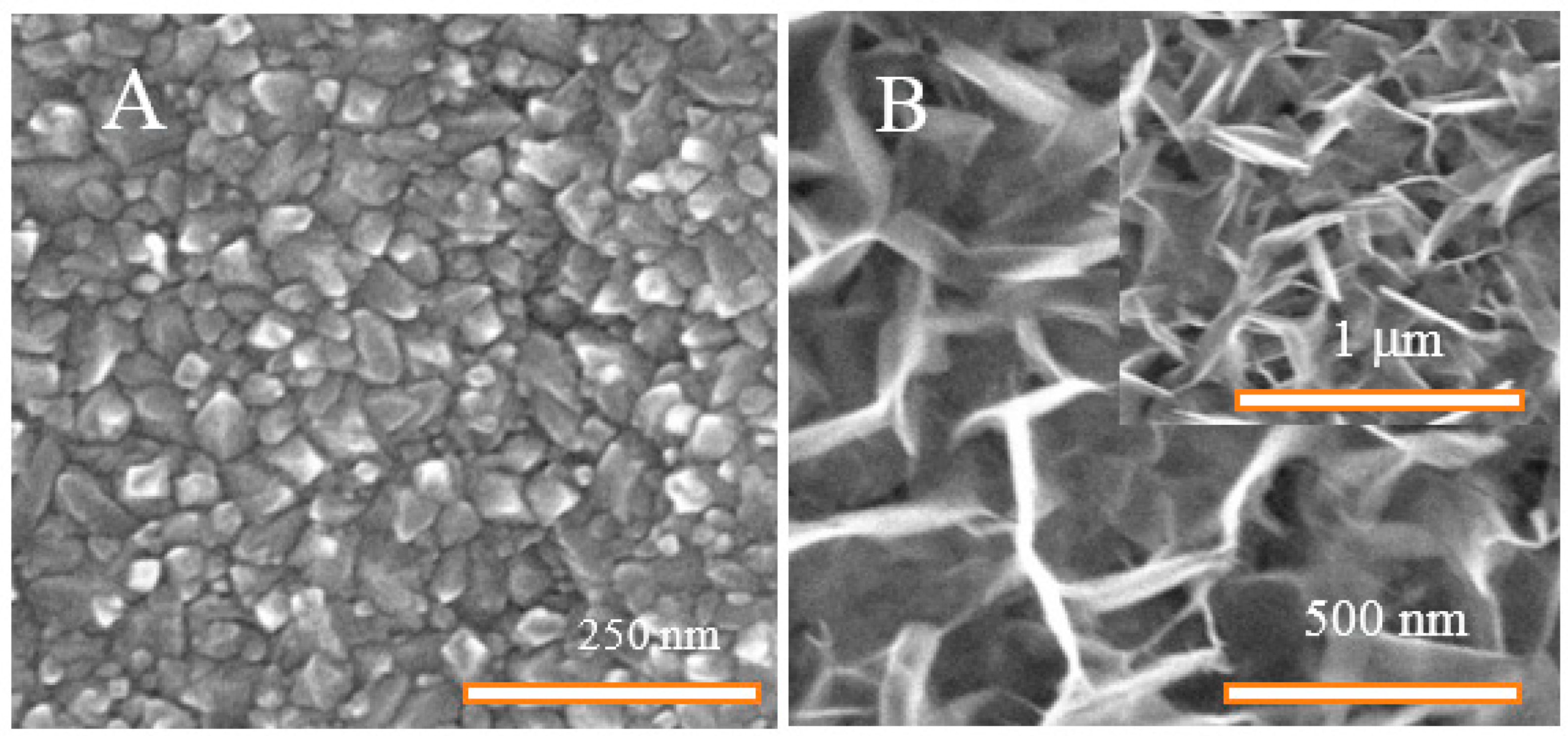
3.1. Annealing under Various Atmospheres
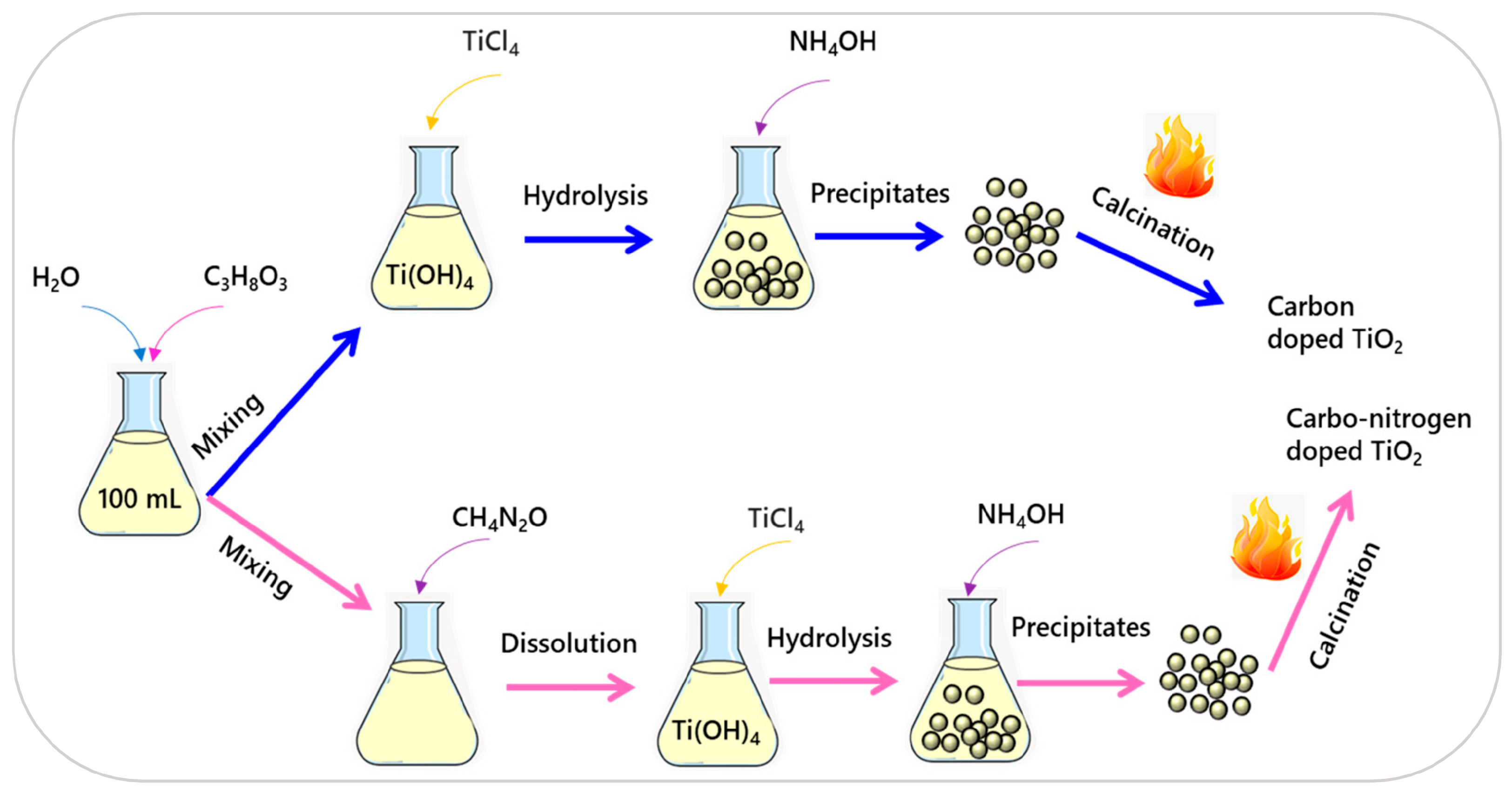
3.2. Wet Chemical Methods
3.3. Laser Ablation
3.4. Molten Salt Method
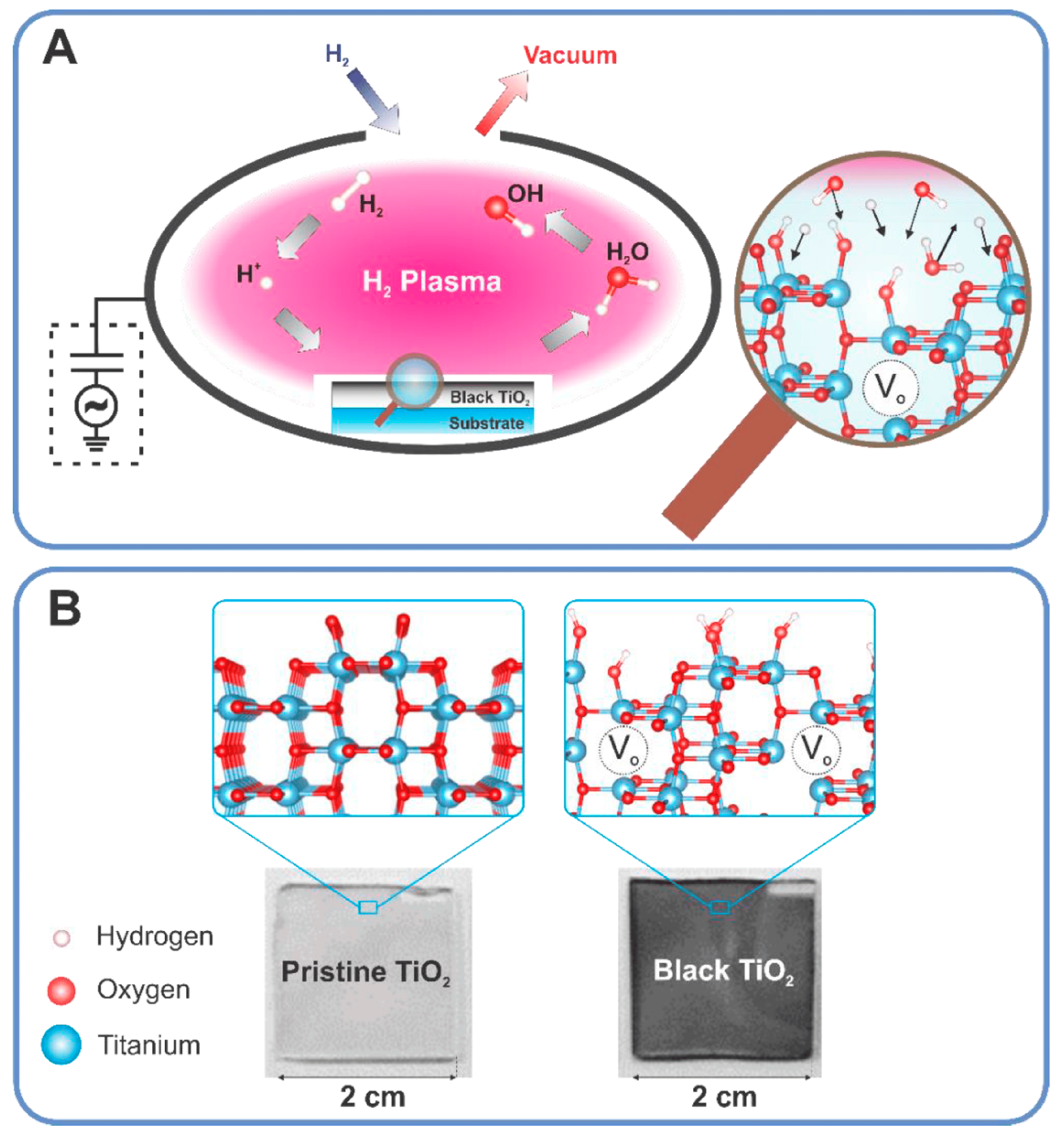
3.5. Plasma Treatment
4. Identification of Magneli Phase Black Titania
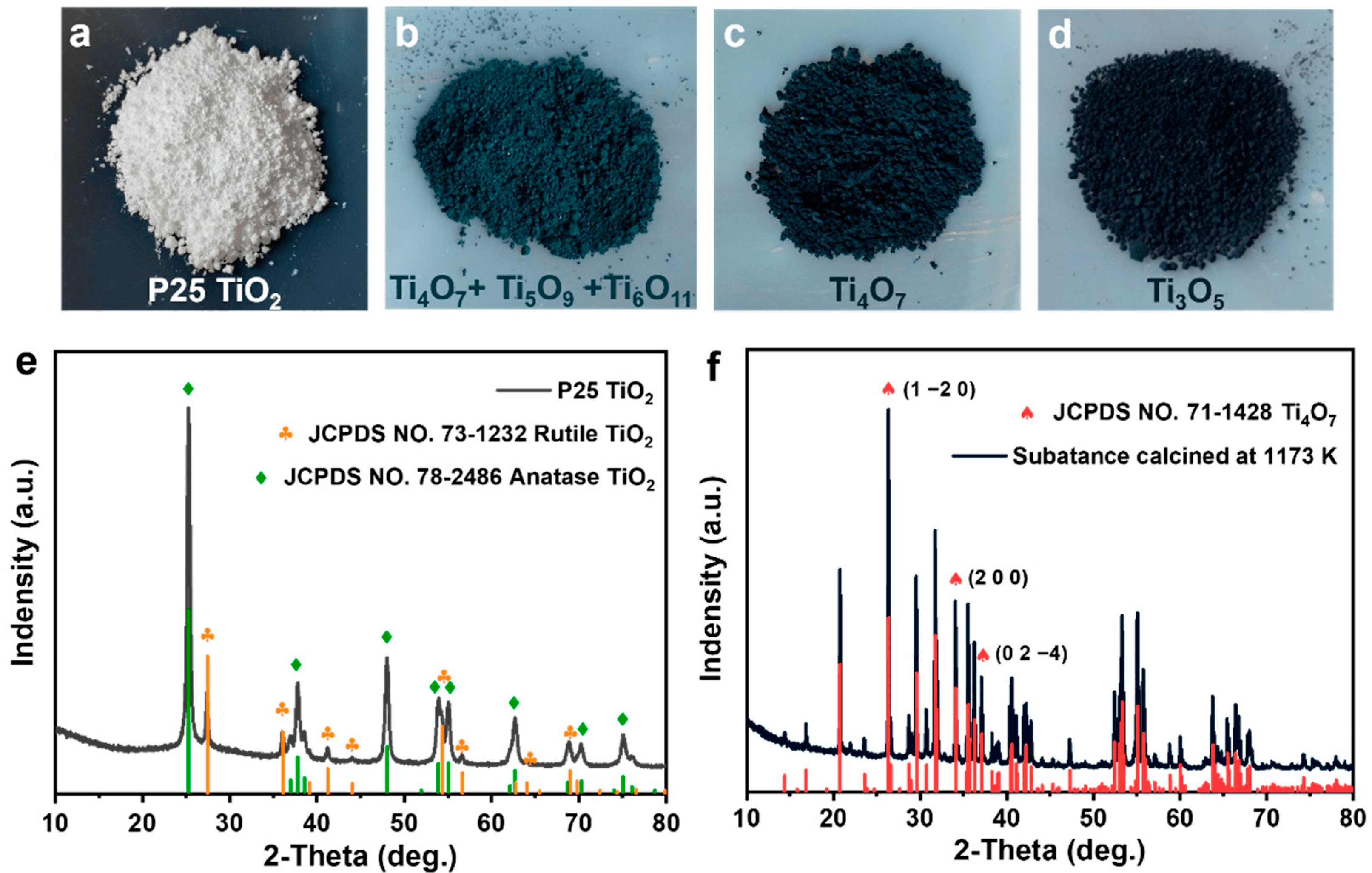
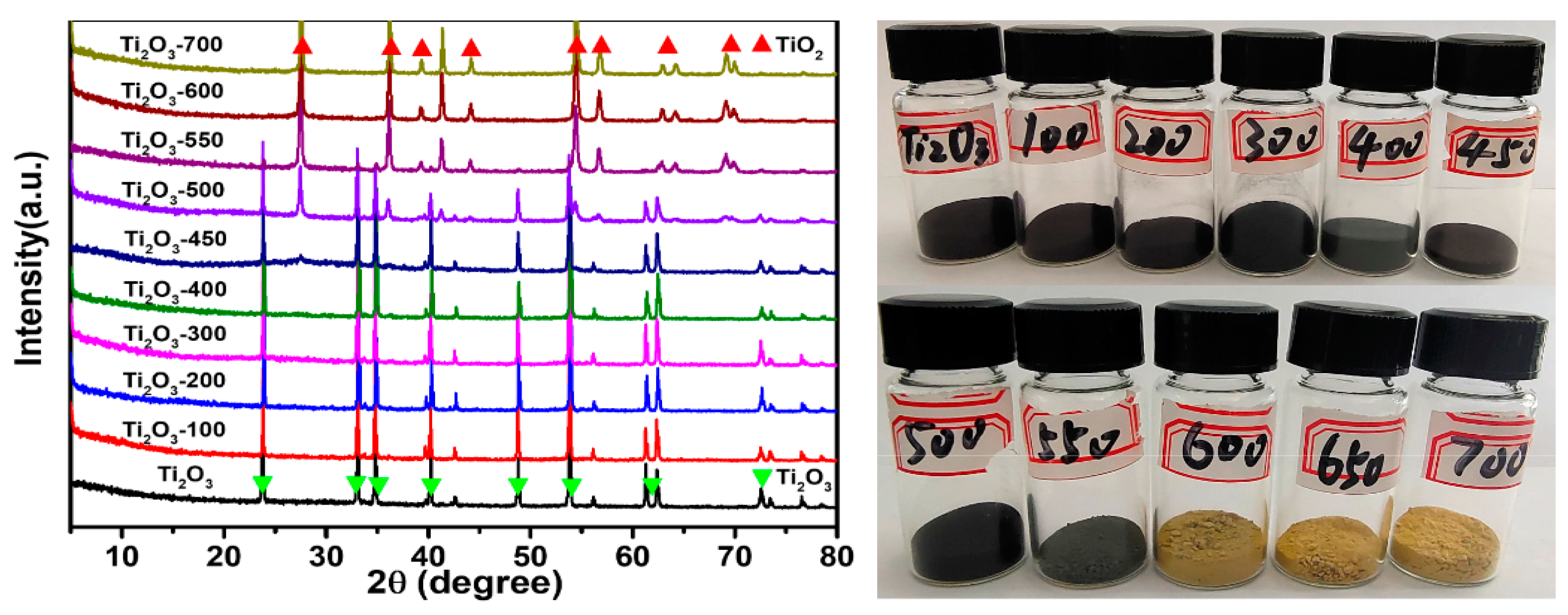
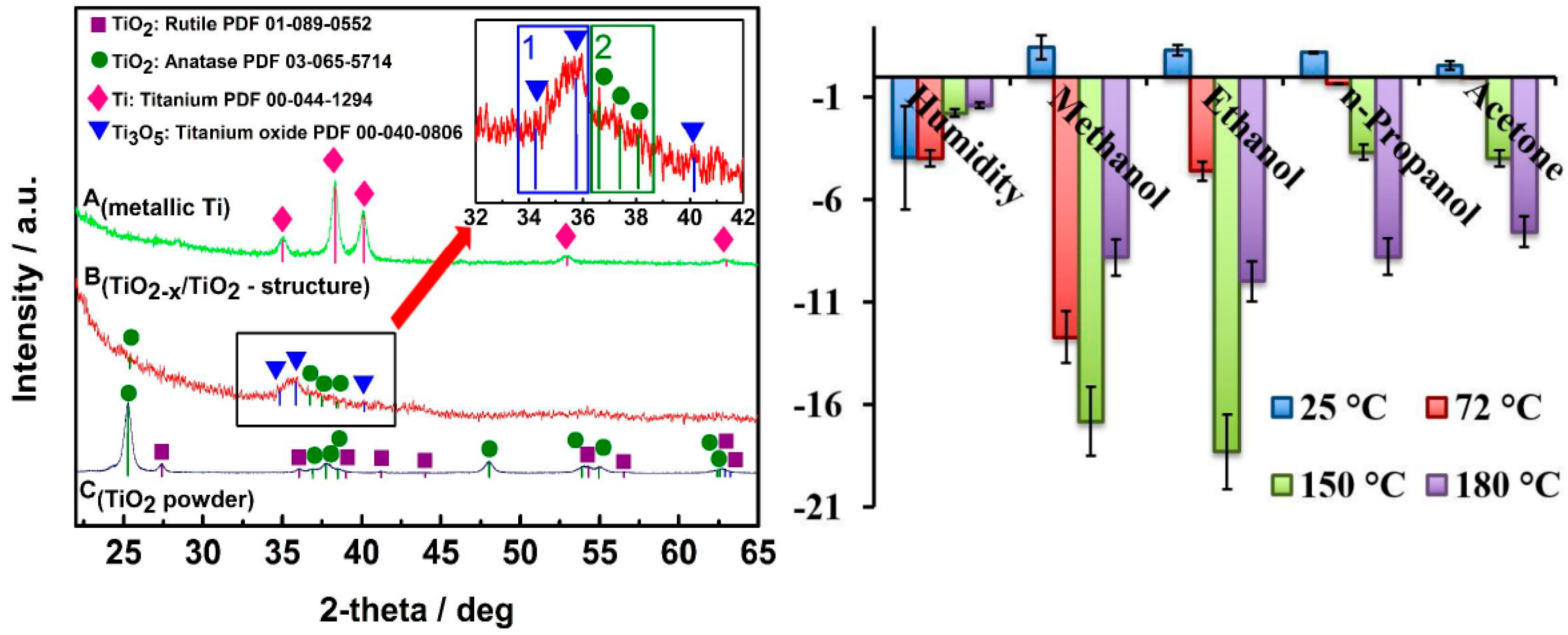
4.1. Identification through X-ray Diffraction (XRD)
4.2. Identification through Raman Spectroscopy
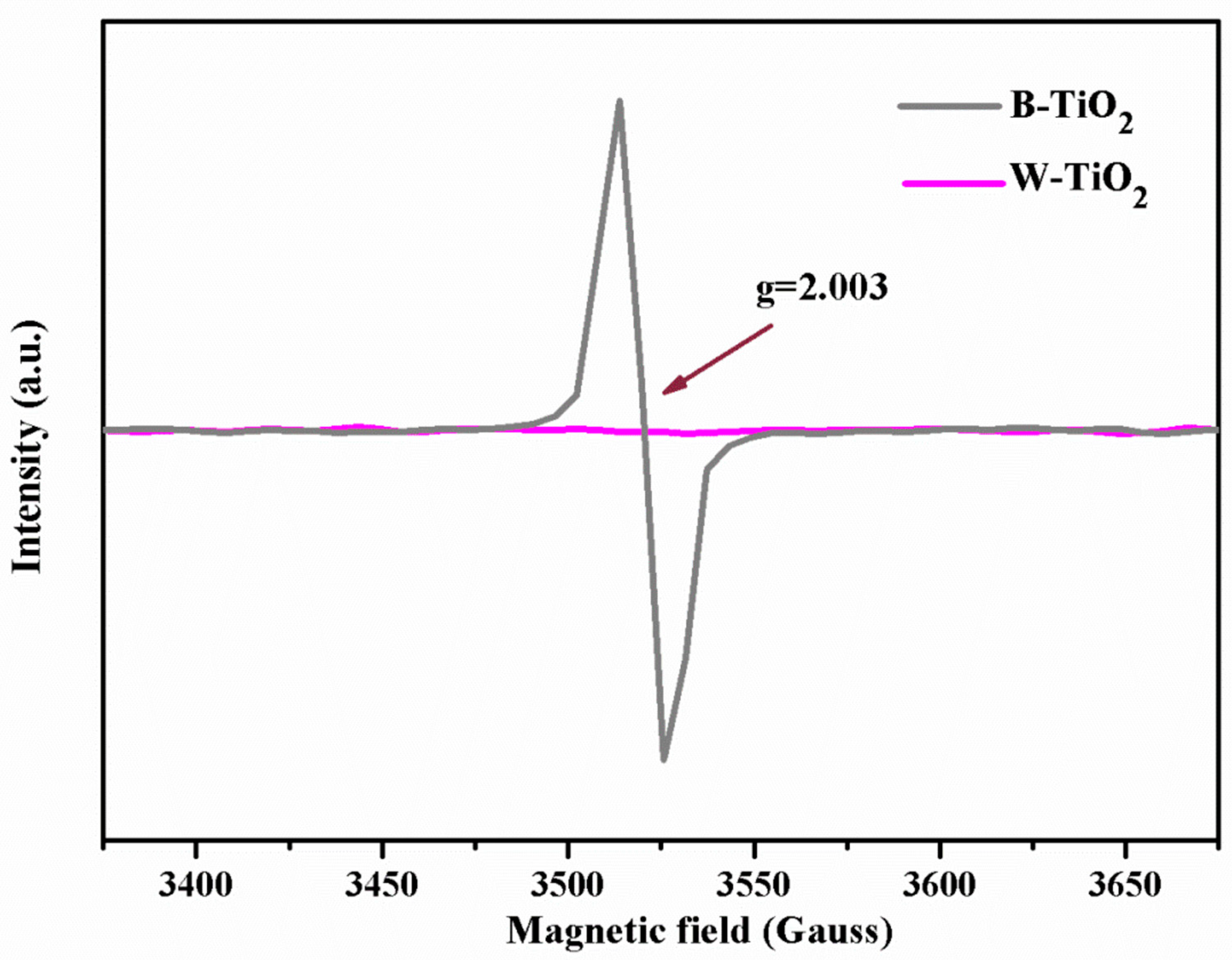
4.3. Identification through Electron Paramagnetic Resonance (EPR)
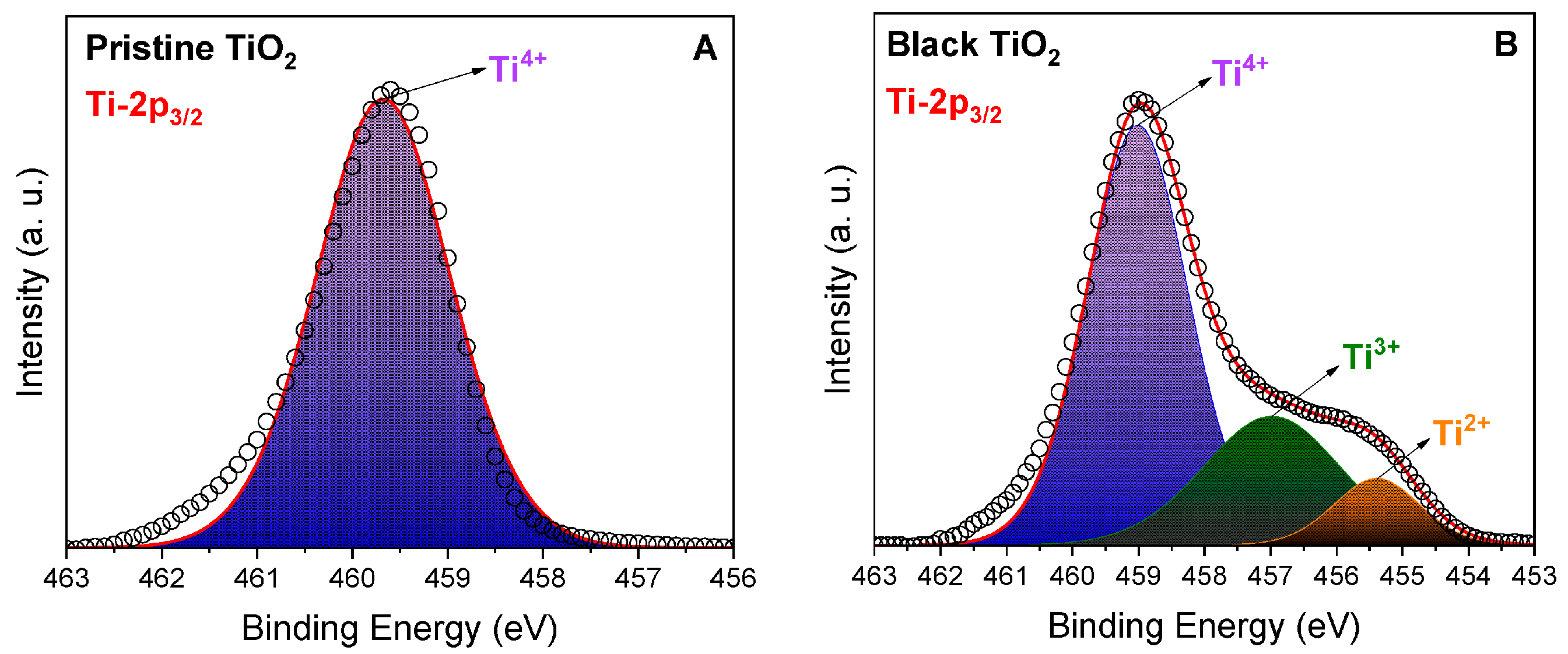
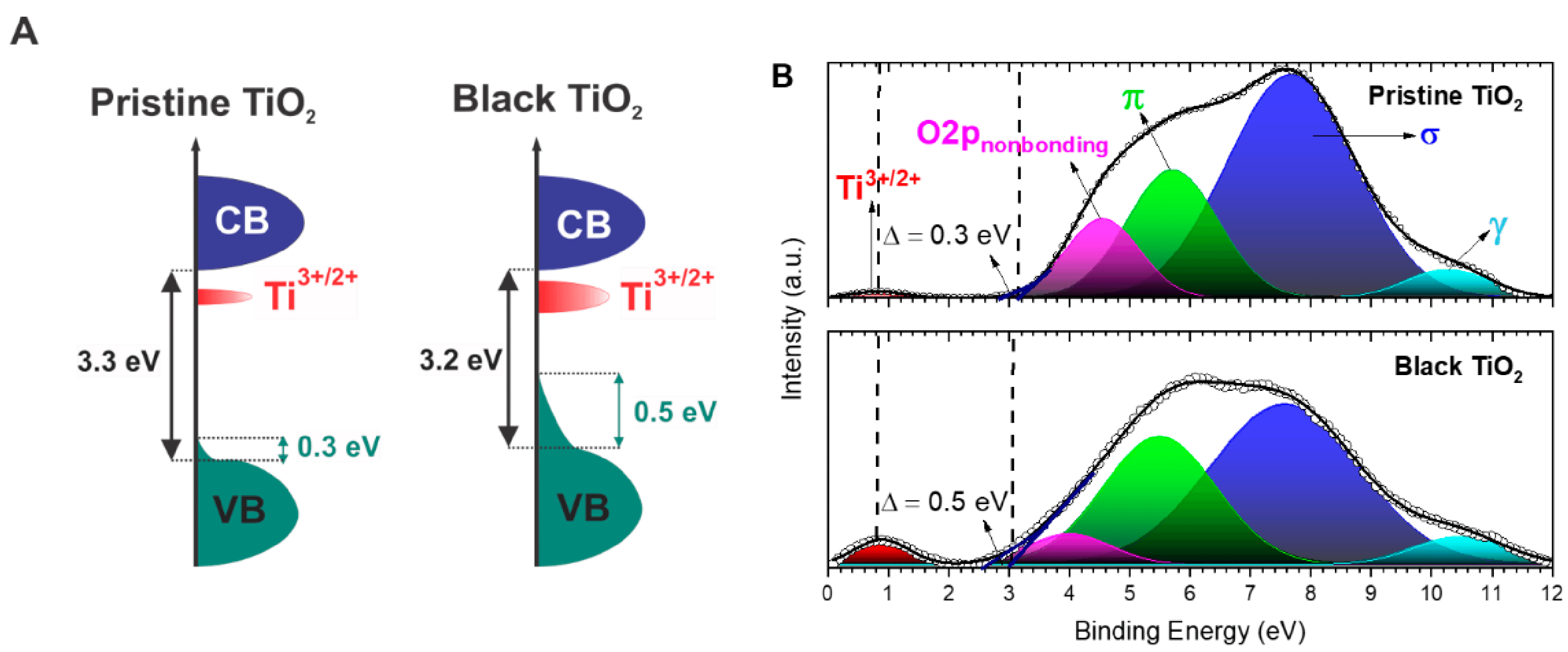
4.4. Identification through X-ray Photoelectron Spectroscopy (XPS)
5. Applications
5.1. Supercapacitors
5.2. Self-Heating Gas Sensors
5.3. Fuel Cells
5.4. Surface-Enhanced Raman Spectroscopy
5.5. Visible Light Photocatalytic Applications
5.6. Medicine
5.7. Microwave Absorption
5.8. Commercial Applications
6. Conclusions and Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, A. Phase Behavior of Empirical Potentials of Titanium Dioxide. J. Chem. Phys. 2019, 151, 064505. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Andersson, S.; Collén, B.; Kuylenstierna, U.; Magnéli, A. Phase Analysis Studies on the Titanium-Oxygen System. Acta Chem. Scand. 1957, 11, 1641–1652. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, L.; Tan, X.; Li, X.; Chen, X. Synthesis, Properties, and Applications of Black Titanium Dioxide Nanomaterials. Sci. Bull. (Beijing) 2017, 62, 431–441. [Google Scholar] [CrossRef]
- Tian, M.; Liu, C.; Ge, J.; Geohegan, D.; Duscher, G.; Eres, G. Recent Progress in Characterization of the Core–Shell Structure of Black Titania. J. Mater. Res. 2019, 34, 1138–1153. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A Review of Its Properties and Conflicting Trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Mao, C.-C.; Weng, H.-S. Effect of Heat Treatment on Photocatalytic Activity of Titania Incorporated with Carbon Black for Degradation of Methyl Orange. Environ. Prog. Sustain. Energy 2012, 31, 306–317. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Chernyakova, K.; Ovodok, E.; Poznyak, S.; Kotsikau, D.; Micusik, M. Synthesis and Structural Features of Black TiO2 Nanotubes after Annealing in Hydrogen. Mater. Chem. Phys. 2023, 297, 127416. [Google Scholar] [CrossRef]
- Khanam, R.; Taparia, D.; Mondal, B.; Mohanta, D. Black Titania: Effect of Hydrogenation on Structural and Thermal Stability of Nanotitania. Appl. Phys. A 2016, 122, 92. [Google Scholar] [CrossRef]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with Reduced TiO2: From Black TiO2 to Cocatalyst-Free Hydrogen Production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Xu, J.; Zhao, W.; Huang, F. Constructing Black Titania with Unique Nanocage Structure for Solar Desalination. ACS Appl. Mater. Interfaces 2016, 8, 31716–31721. [Google Scholar] [CrossRef] [PubMed]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Black TiO2 Nanomaterials: A Review of Recent Advances. Chem. Eng. J. 2018, 343, 708–736. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Lin, T.; Yin, H.; Lü, X.; Wan, D.; Xu, T.; Zheng, C.; Lin, J.; Huang, F.; et al. Core-Shell Nanostructured “Black” Rutile Titania as Excellent Catalyst for Hydrogen Production Enhanced by Sulfur Doping. J. Am. Chem. Soc. 2013, 135, 17831–17838. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-D.; Li, J.; Lizundia, E.; Niederberger, M.; Hamad, W.Y.; MacLachlan, M.J. Black Titania with Nanoscale Helicity. Adv. Funct. Mater. 2019, 29, 1904639. [Google Scholar] [CrossRef]
- Jagminas, A.; Ramanavičius, S.; Jasulaitiene, V.; Šimėnas, M. Hydrothermal Synthesis and Characterization of Nanostructured Titanium Monoxide Films. RSC Adv. 2019, 9, 40727–40735. [Google Scholar] [CrossRef]
- Sabirovas, T.; Ramanavicius, S.; Naujokaitis, A.; Niaura, G.; Jagminas, A. Design and Characterization of Nanostructured Titanium Monoxide Films Decorated with Polyaniline Species. Coatings 2022, 12, 1615. [Google Scholar] [CrossRef]
- Shu, G.; Wang, H.; Zhao, H.-X.; Zhang, X. Microwave-Assisted Synthesis of Black Titanium Monoxide for Synergistic Tumor Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 3323–3333. [Google Scholar] [CrossRef] [PubMed]
- Sener, M.E.; Quesada-Cabrera, R.; Parkin, I.P.; Caruana, D.J. Facile Formation of Black Titania Films Using an Atmospheric-Pressure Plasma Jet. Green. Chem. 2022, 24, 2499–2505. [Google Scholar] [CrossRef]
- Teng, F.; Li, M.; Gao, C.; Zhang, G.; Zhang, P.; Wang, Y.; Chen, L.; Xie, E. Preparation of Black TiO2 by Hydrogen Plasma Assisted Chemical Vapor Deposition and Its Photocatalytic Activity. Appl. Catal. B 2014, 148–149, 339–343. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Watthanaphanit, A.; Ishizaki, T.; Saito, N. Water-Plasma-Assisted Synthesis of Black Titania Spheres with Efficient Visible-Light Photocatalytic Activity. Phys. Chem. Chem. Phys. 2015, 17, 13794–13799. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 565489. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Z.; Yin, G.; Lin, T.; Huang, F. Controllable Reduced Black Titania with Enhanced Photoelectrochemical Water Splitting Performance. Dalton Trans. 2017, 46, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhu, G.; Lin, T.; Hong, Z.; Wang, J.; Huang, F. Molten Salt Assisted Synthesis of Black Titania Hexagonal Nanosheets with Tuneable Phase Composition and Morphology. RSC Adv. 2015, 5, 85928–85932. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Mansingh, S.; Babu, P.; Parida, K. Black Titania an Emerging Photocatalyst: Review Highlighting the Synthesis Techniques and Photocatalytic Activity for Hydrogen Generation. Nanoscale Adv. 2021, 3, 5487–5524. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Ghasemi, J.B.; Badiei, A. Black Titania; Novel Researches in Synthesis and Applications. Inorg. Chem. Commun. 2022, 135, 109092. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2-x/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2020, 20, 74. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Jagminas, A.; Naujokaitis, A.; Gaigalas, P.; Ramanavičius, S.; Kurtinaitienė, M.; Trusovas, R. Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals 2020, 10, 1251. [Google Scholar] [CrossRef]
- Varnagiris, S.; Medvids, A.; Lelis, M.; Milcius, D.; Antuzevics, A. Black Carbon-Doped TiO2 Films: Synthesis, Characterization and Photocatalysis. J. Photochem. Photobiol. A Chem. 2019, 382, 111941. [Google Scholar] [CrossRef]
- Veremchuk, I.; Antonyshyn, I.; Candolfi, C.; Feng, X.; Burkhardt, U.; Baitinger, M.; Zhao, J.-T.; Grin, Y. Diffusion-Controlled Formation of Ti2O3 during Spark-Plasma Synthesis. Inorg. Chem. 2013, 52, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Ohwada, M.; Kimoto, K.; Suenaga, K.; Sato, Y.; Ebina, Y.; Sasaki, T. Synthesis and Atomic Characterization of a Ti2O3 Nanosheet. J. Phys. Chem. Lett. 2011, 2, 1820–1823. [Google Scholar] [CrossRef]
- Zhao, P.F.; Li, G.S.; Li, W.; Cheng, P.; Pang, Z.; Xiong, L.; Zou, X.L.; Xu, Q.; Lu, X.G. Progress in Ti3O5: Synthesis, Properties and Applications. Trans. Nonferrous Met. Soc. China 2021, 31, 3310–3327. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ma, S.; Ye, J.; Zhang, X.; Wang, G.; Qiu, Y. The Synthesis and Gas Sensitivity of the β-Ti3O5 Powder: Experimental and DFT Study. J. Alloys Compd. 2015, 649, 939–948. [Google Scholar] [CrossRef]
- Jayashree, S.; Ashokkumar, M. Switchable Intrinsic Defect Chemistry of Titania for Catalytic Applications. Catalysts 2018, 8, 601. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Yao, H.; Bu, K.; Pan, J.; He, J.; Xu, F.; Hong, Z.; Chen, X.; Huang, F. Nano Titanium Monoxide Crystals and Unusual Superconductivity at 11 K. Adv. Mater. 2018, 30, 1706240. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, M.G.; Lukoyanov, A.V.; Zhukov, V.P.; Rempel, A.A. Vacancies in Ordered and Disordered Titanium Monoxide: Mechanism of B1 Structure Stabilization. J. Solid State Chem. 2013, 204, 146–152. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Wang, X.; Yuan, X.; Lin, T.; Huang, F. Progress in Black Titania: A New Material for Advanced Photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Gulino, A.; Cantarella, M.; Privitera, V.; Grimaldi, M.G. Hydrogenated Black-TiOx: A Facile and Scalable Synthesis for Environmental Water Purification. Catal. Today 2019, 321–322, 146–157. [Google Scholar] [CrossRef]
- Peng, X.; Chen, A. Large-Scale Synthesis and Characterization of TiO2-Based Nanostructures on Ti Substrates. Adv. Funct. Mater. 2006, 16, 1355–1362. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lee, T.; Soon, A. Phase Stability Diagrams of Group 6 Magnéli Oxides and Their Implications for Photon-Assisted Applications. Chem. Mater. 2019, 31, 4282–4290. [Google Scholar] [CrossRef]
- Serratos, M.; Bronson, A. The Effect of Oxygen Partial Pressure on the Stability of Magneli Phases in High Temperature Corrosive Wear. Wear 1996, 198, 267–270. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The Continuing Development of Magnéli Phase Titanium Sub-Oxides and Ebonex® Electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Andersson, S.; Collén, B.; Kruuse, G.; Kuylenstierna, U.; Magnéli, A.; Pestmalis, H.; Åsbrink, S. Identification of Titanium Oxides by X-Ray Powder Patterns. Acta Chem. Scand. (Den.) Divid. Into Acta Chem. Scand. Ser. A Ser. B 1957, 1, 1653–1657. [Google Scholar] [CrossRef]
- Åsbrink, S.; Magnéli, A. Crystal Structure Studies on Trititanium Pentoxide, Ti3O5. Acta Crystallogr. 1959, 12, 575–581. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Yan, W.; Zhou, C. Study of the Relationship between Metal–Support Interactions and the Electrocatalytic Performance of Pt/Ti4O7 with Different Loadings. Catalysts 2022, 12, 480. [Google Scholar] [CrossRef]
- Hu, T.; Feng, P.; Guo, L.; Chu, H.; Liu, F. Construction of Built-In Electric Field in TiO2@Ti2O3 Core-Shell Heterojunctions toward Optimized Photocatalytic Performance. Nanomaterials 2023, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Lu, Z.; Zhang, Y.; Su, Q.; Li, L. Hydrogen-Etched TiO2−x as Efficient Support of Gold Catalysts for Water–Gas Shift Reaction. Catalysts 2018, 8, 26. [Google Scholar] [CrossRef]
- Agarwal, R.; Himanshu; Patel, S.L.; Verma, M.; Chander, S.; Ameta, C.; Dhaka, M.S. Tailoring the Physical Properties of Titania Thin Films with Post Deposition Air and Vacuum Annealing. Opt. Mater. (Amst.) 2021, 116, 111033. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Davood Abadi Farahani, M.H.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a New Type of Black TiO2 under a Vacuum Atmosphere for Sunlight Photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, M.; Cui, N.; Chen, G.; Zou, G.; Zhou, L. Defective Black TiO2: Effects of Annealing Atmospheres and Urea Addition on the Properties and Photocatalytic Activities. Nanomaterials 2021, 11, 2648. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-Light Photocatalytic, Solar Thermal and Photoelectrochemical Properties of Aluminium-Reduced Black Titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Ye, M.; Jia, J.; Wu, Z.; Qian, C.; Chen, R.; O’Brien, P.G.; Sun, W.; Dong, Y.; Ozin, G.A. Synthesis of Black TiOx Nanoparticles by Mg Reduction of TiO2 Nanocrystals and Their Application for Solar Water Evaporation. Adv. Energy Mater. 2017, 7, 1601811. [Google Scholar] [CrossRef]
- Gao, J.; Shen, Q.; Guan, R.; Xue, J.; Liu, X.; Jia, H.; Li, Q.; Wu, Y. Oxygen Vacancy Self-Doped Black TiO2 Nanotube Arrays by Aluminothermic Reduction for Photocatalytic CO2 Reduction under Visible Light Illumination. J. CO2 Util. 2020, 35, 205–215. [Google Scholar] [CrossRef]
- Janczarek, M.; Endo-Kimura, M.; Wang, K.; Wei, Z.; Akanda, M.M.A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Is Black Titania a Promising Photocatalyst? Catalysts 2022, 12, 1320. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 Nanotube Arrays for Photoelectrochemical Water Splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Ding, S.; Lin, T.; Wang, Y.; Lü, X.; Huang, F. New Facile Synthesis of TiO2 Hollow Sphere with an Opening Hole and Its Enhanced Rate Performance in Lithium-Ion Batteries. New J. Chem. 2013, 37, 784–789. [Google Scholar] [CrossRef]
- Bottein, T.; Wood, T.; David, T.; Claude, J.B.; Favre, L.; Berbézier, I.; Ronda, A.; Abbarchi, M.; Grosso, D. “Black” Titania Coatings Composed of Sol–Gel Imprinted Mie Resonators Arrays. Adv. Funct. Mater. 2017, 27, 1604924. [Google Scholar] [CrossRef]
- Wu, M.C.; Chang, I.C.; Hsiao, K.C.; Huang, W.K. Highly Visible-Light Absorbing Black TiO2 Nanocrystals Synthesized by Sol–Gel Method and Subsequent Heat Treatment in Low Partial Pressure H2. J. Taiwan Inst. Chem. Eng. 2016, 63, 430–435. [Google Scholar] [CrossRef]
- Nawaz, R.; Sahrin, N.T.; Haider, S.; Ullah, H.; Junaid, M.; Akhtar, M.S.; Khan, S. Photocatalytic Performance of Black Titanium Dioxide for Phenolic Compounds Removal from Oil Refinery Wastewater: Nanoparticles vs Nanowires. Appl. Nanosci. 2022, 12, 3499–3515. [Google Scholar] [CrossRef]
- Rahman, S.; Nawaz, R.; Khan, J.A.; Ullah, H.; Irfan, M.; Glowacz, A.; Lyp-Wronska, K.; Wzorek, L.; Asif Khan, M.K.; Jalalah, M.; et al. Synthesis and Characterization of Carbon and Carbon-Nitrogen Doped Black TiO2 Nanomaterials and Their Application in Sonophotocatalytic Remediation of Treated Agro-Industrial Wastewater. Materials 2021, 14, 6175. [Google Scholar] [CrossRef] [PubMed]
- Mills, A.; Elliott, N.; Hill, G.; Fallis, D.; Durrant, J.R.; Willis, R.L. Preparation and Characterisation of Novel Thick Sol-Gel Titania Film Photocatalysts. Photochem. Photobiol. Sci. 2003, 2, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Ullattil, S.G.; Periyat, P. A ‘One Pot’ Gel Combustion Strategy towards Ti3+ Self-Doped ‘Black’ Anatase TiO2−x Solar Photocatalyst. J. Mater. Chem. A 2016, 4, 5854–5858. [Google Scholar] [CrossRef]
- Siuzdak, K.; Haryński, Ł.; Wawrzyniak, J.; Grochowska, K. Review on Robust Laser Light Interaction with Titania—Patterning, Crystallisation and Ablation Processes. Prog. Solid. State Chem. 2021, 62, 100297. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Z.; Zheng, R.; Li, J.; Wang, L.; Yang, X.; Lü, W.; Xu, H. Black TiO2-Based Dual Photoanodes Boost the Efficiency of Quantum Dot-Sensitized Solar Cells to 11.7%. Nanomaterials 2022, 12, 4294. [Google Scholar] [CrossRef] [PubMed]
- Raveendran Nair, P.; Rosa Santiago Ramirez, C.; Angel Gracia Pinilla, M.; Krishnan, B.; Avellaneda Avellaneda, D.; Fabian Cienfuegos Pelaes, R.; Shaji, S. Black Titanium Dioxide Nanocolloids by Laser Irradiation in Liquids for Visible Light Photo-Catalytic/Electrochemical Applications. Appl. Surf. Sci. 2023, 623, 157096. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Boutinguiza, M.; Privitera, V.; Grimaldi, M.G. Laser Irradiation in Water for the Novel, Scalable Synthesis of Black TiOx Photocatalyst for Environmental Remediation. Beilstein J. Nanotechnol. 2017, 8, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, D.; Liu, K.; Wang, C.; Liu, L.; Li, B.; Zhang, Z.; Shen, D. Laser-Modified Black Titanium Oxide Nanospheres and Their Photocatalytic Activities under Visible Light. ACS Appl. Mater. Interfaces 2015, 7, 16070–16077. [Google Scholar] [CrossRef] [PubMed]
- Lepcha, A.; Maccato, C.; Mettenbörger, A.; Andreu, T.; Mayrhofer, L.; Walter, M.; Olthof, S.; Ruoko, T.-P.; Klein, A.; Moseler, M.; et al. Electrospun Black Titania Nanofibers: Influence of Hydrogen Plasma-Induced Disorder on the Electronic Structure and Photoelectrochemical Performance. J. Phys. Chem. C 2015, 119, 18835–18842. [Google Scholar] [CrossRef]
- Tian, Z.; Cui, H.; Zhu, G.; Zhao, W.; Xu, J.J.; Shao, F.; He, J.; Huang, F. Hydrogen Plasma Reduced Black TiO2B Nanowires for Enhanced Photoelectrochemical Water-Splitting. J. Power Sources 2016, 325, 697–705. [Google Scholar] [CrossRef]
- Godoy Junior, A.; Pereira, A.; Gomes, M.; Fraga, M.; Pessoa, R.; Leite, D.; Petraconi, G.; Nogueira, A.; Wender, H.; Miyakawa, W.; et al. Black TiO2 Thin Films Production Using Hollow Cathode Hydrogen Plasma Treatment: Synthesis, Material Characteristics and Photocatalytic Activity. Catalysts 2020, 10, 282. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, H.; Yang, C.; Cui, H.; Wang, Z.; Xu, J.; Lin, T.; Huang, F. Black Titania for Superior Photocatalytic Hydrogen Production and Photoelectrochemical Water Splitting. ChemCatChem 2015, 7, 2614–2619. [Google Scholar] [CrossRef]
- El-Gendy, D.M.; Abdel Ghany, N.A.; Allam, N.K. Black Titania Nanotubes/Spongy Graphene Nanocomposites for High-Performance Supercapacitors. RSC Adv. 2019, 9, 12555–12566. [Google Scholar] [CrossRef]
- Tang, C.; Zhou, D.; Zhang, Q. Synthesis and Characterization of Magneli Phases: Reduction of TiO2 in a Decomposed NH3 Atmosphere. Mater. Lett. 2012, 79, 42–44. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly Conductive Nano-Sized Magnéli Phases Titanium Oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, Y.; Wang, Y.; Hu, Z.; Zhao, H.; Xie, W. A Facile Approach to Prepare Black TiO2 with Oxygen Vacancy for Enhancing Photocatalytic Activity. Nanomaterials 2018, 8, 245. [Google Scholar] [CrossRef]
- Surmacki, J.; Wroński, P.; Szadkowska-Nicze, M.; Abramczyk, H. Raman Spectroscopy of Visible-Light Photocatalyst—Nitrogen-Doped Titanium Dioxide Generated by Irradiation with Electron Beam. Chem. Phys. Lett. 2013, 566, 54–59. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and Applications of EPR Spectroscopy in the Chemical Sciences. Chem. Soc. Rev. 2018, 47, 2534–2553. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, X.; Tian, Y.; Lin, Y.; Hu, Y.H. Excellent Photocatalytic Degradation of Tetracycline over Black Anatase-TiO2 under Visible Light. Chem. Eng. J. 2021, 406, 126747. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Wang, C.; Ao, Y. Oxygen Vacancies and Phosphorus Codoped Black Titania Coated Carbon Nanotube Composite Photocatalyst with Efficient Photocatalytic Performance for the Degradation of Acetaminophen under Visible Light Irradiation. Chem. Eng. J. 2018, 352, 947–956. [Google Scholar] [CrossRef]
- Plodinec, M.; Grčić, I.; Willinger, M.G.; Hammud, A.; Huang, X.; Panžić, I.; Gajović, A. Black TiO2 Nanotube Arrays Decorated with Ag Nanoparticles for Enhanced Visible-Light Photocatalytic Oxidation of Salicylic Acid. J. Alloys Compd. 2019, 776, 883–896. [Google Scholar] [CrossRef]
- Guan, S.; Cheng, Y.; Hao, L.; Yoshida, H.; Tarashima, C.; Zhan, T.; Itoi, T.; Qiu, T.; Lu, Y. Oxygen Vacancies Induced Band Gap Narrowing for Efficient Visible-Light Response in Carbon-Doped TiO2. Sci. Rep. 2023, 13, 14105. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Shen, H.; Min, H.; Ge, J. Enhanced Acetone Sensing Performance in Black TiO2 by Ag Modification. J. Mater. Sci. 2020, 55, 10399–10411. [Google Scholar] [CrossRef]
- Naik, K.M.; Hamada, T.; Higuchi, E.; Inoue, H. Defect-Rich Black Titanium Dioxide Nanosheet-Supported Palladium Nanoparticle Electrocatalyst for Oxygen Reduction and Glycerol Oxidation Reactions in Alkaline Medium. ACS Appl. Energy Mater. 2021, 4, 12391–12402. [Google Scholar] [CrossRef]
- Touni, A.; Liu, X.; Kang, X.; Papoulia, C.; Pavlidou, E.; Lambropoulou, D.; Tsampas, M.N.; Chatzitakis, A.; Sotiropoulos, S. Methanol Oxidation at Platinum Coated Black Titania Nanotubes and Titanium Felt Electrodes. Molecules 2022, 27, 6382. [Google Scholar] [CrossRef] [PubMed]
- Plaça, L.F.; Vital, P.-L.S.; Gomes, L.E.; Roveda, A.C., Jr.; Cardoso, D.R.; Martins, C.A.; Wender, H. Black TiO2 Photoanodes for Direct Methanol Photo Fuel Cells. ACS Appl. Mater. Interfaces 2023, 15, 43259–43271. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Peng, Y.; Yang, Y.; Liu, J.; Li, Z.; Ma, Y.; Zhang, Z.; Wei, Y.; Li, S.; Huang, Z.; et al. Green and Sensitive Flexible Semiconductor SERS Substrates: Hydrogenated Black TiO2 Nanowires. ACS Appl. Nano Mater. 2018, 1, 4516–4527. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, Y.; Cao, Y.; Yin, H.; Long, N.V.; Huang, Z. Hydrogenated Black TiO2 Nanowires Decorated with Ag Nanoparticles as Sensitive and Reusable Surface-Enhanced Raman Scattering Substrates. RSC Adv. 2015, 5, 34737–34743. [Google Scholar] [CrossRef]
- Gür, T.M. Review of Electrical Energy Storage Technologies, Materials and Systems: Challenges and Prospects for Large-Scale Grid Storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for Renewable Energy Production and Storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef]
- Zhi, J.; Cui, H.; Wang, Z.; Huang, F. Surface Confined Titania Redox Couple for Ultrafast Energy Storage. Mater. Horiz. 2018, 5, 691–698. [Google Scholar] [CrossRef]
- El-Gendy, D.M.; Maafa, I.M.; Zouli, N.; Abutaleb, A.; Yousef, A. Synthesis of Black TiO2 Nanotubes Decorated Biomass-Derived Spongy Carbon as an Electrode Material for Producing Deionized Water through Capacitive Deionization Technology. Ceram. Int. 2024, 50, 7775–7788. [Google Scholar] [CrossRef]
- Jo, M.-S.; Kim, S.-H.; Park, S.-Y.; Choi, K.-W.; Kim, S.-H.; Yoo, J.-Y.; Kim, B.-J.; Yoon, J.-B. Fast-Response and Low-Power Self-Heating Gas Sensor Using Metal/Metal Oxide/Metal (MMOM) Structured Nanowires. ACS Sens. 2024, 9, 1896–1905. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, S.; Jagminas, A.; Ramanavicius, A. Gas Sensors Based on Titanium Oxides (Review). Coatings 2022, 12, 699. [Google Scholar] [CrossRef]
- Bontempi, N.; Cavaliere, E.; Cappello, V.; Pingue, P.; Gavioli, L. Ag@TiO2 Nanogranular Films by Gas Phase Synthesis as Hybrid SERS Platforms. Phys. Chem. Chem. Phys. 2019, 21, 25090–25097. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-Doped Black Titania with Very High Solar Absorption and Excellent Photocatalysis Enhanced by Localized Surface Plasmon Resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Ma, X.; Dai, Y.; Yu, L.; Huang, B. Noble-Metal-Free Plasmonic Photocatalyst: Hydrogen Doped Semiconductors. Sci. Rep. 2014, 4, 3986. [Google Scholar] [CrossRef] [PubMed]
- Djurišić, A.B.; He, Y.; Ng, A.M.C. Visible-Light Photocatalysts: Prospects and Challenges. APL Mater. 2020, 8, 030903. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts: A Review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Bi, Q.; Huang, X.; Dong, Y.; Huang, F. Conductive Black Titania Nanomaterials for Efficient Photocatalytic Degradation of Organic Pollutants. Catal. Lett. 2020, 150, 1346–1354. [Google Scholar] [CrossRef]
- Kononenko, V.; Drobne, D. In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells. Int. J. Mol. Sci. 2019, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Jemec Kokalj, A.; Novak, S.; Talaber, I.; Kononenko, V.; Bizjak Mali, L.; Vodovnik, M.; Žegura, B.; Eleršek, T.; Kalčikova, G.; Žgajnar Gotvajn, A.; et al. The First Comprehensive Safety Study of Magnéli Phase Titanium Suboxides Reveals No Acute Environmental Hazard. Environ. Sci. Nano 2019, 6, 1131–1139. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X. Activating Titanium Oxide Coatings for Orthopedic Implants. Surf. Coat. Technol. 2013, 233, 57–64. [Google Scholar] [CrossRef]
- Hasan, J.; Jain, S.; Chatterjee, K. Nanoscale Topography on Black Titanium Imparts Multi-Biofunctional Properties for Orthopedic Applications. Sci. Rep. 2017, 7, 41118. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Ren, W.; Wu, A. Cancer Theranostics of Black TiO2 Nanoparticles. In TiO2 Nanoparticles; Wiley (United States) 2020; pp. 185–215. ISBN 9783527825431. [CrossRef]
- Zhang, M.; Wu, N.; Yang, J.; Zhang, Z. Photoelectrochemical Antibacterial Platform Based on Rationally Designed Black TiO2−x Nanowires for Efficient Inactivation against Bacteria. ACS Appl. Bio Mater. 2022, 5, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, Z.; Li, Y.; Xiao, C.; Zhang, H.; Li, W.; Zhan, L.; Liang, G.; Chang, Y.; Ning, C.; et al. In Situ Construction of Black Titanium Oxide with a Multilevel Structure on a Titanium Alloy for Photothermal Antibacterial Therapy. ACS Biomater. Sci. Eng. 2022, 8, 2419–2427. [Google Scholar] [CrossRef]
- Abir, M.M.M.; Otsuka, Y.; Ohnuma, K.; Miyashita, Y. Effects of Composition of Hydroxyapatite/Gray Titania Coating Fabricated by Suspension Plasma Spraying on Mechanical and Antibacterial Properties. J. Mech. Behav. Biomed. Mater. 2022, 125, 104888. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Karges, J.; Xiong, K.; Chen, Y.; Ji, L.; Chao, H. Cancer Cell Membrane Camouflaged Iridium Complexes Functionalized Black-Titanium Nanoparticles for Hierarchical-Targeted Synergistic NIR-II Photothermal and Sonodynamic Therapy. Biomaterials 2021, 275, 120979. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Chen, W.; Wang, J.; Cheng, L.; Wang, J.; Zhang, H.; Song, L.; Hu, Y.; Ma, X. Oxygen-Deficient Titanium Dioxide-Loaded Black Phosphorus Nanosheets for Synergistic Photothermal and Sonodynamic Cancer Therapy. Biomater. Adv. 2022, 136, 212794. [Google Scholar] [CrossRef]
- Green, M.; Van Tran, A.T.; Smedley, R.; Roach, A.; Murowchick, J.; Chen, X. Microwave Absorption of Magnesium/Hydrogen-Treated Titanium Dioxide Nanoparticles. Nano Mater. Sci. 2019, 1, 48–59. [Google Scholar] [CrossRef]
- Li, Y.; Qing, Y.; Zhao, B.; Bai, P.; Zhang, R.; Yao, H.; Luo, F. Tunable Magnetic Coupling and Dipole Polarization of Core-Shell Magnéli Ti4O7 Ceramic/Magnetic Metal Possessing Broadband Microwave Absorption Properties. Ceram. Int. 2021, 47, 33373–33381. [Google Scholar] [CrossRef]
- Li, Y.; Qing, Y.; Yao, H.; Xu, H.; Wu, H. A Novel Plasma-Sprayed Ti4O7/Carbon Nanotubes/Al2O3 Coating with Bifunctional Microwave Application. J. Colloid. Interface Sci. 2023, 645, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Yang, B.; Chen, W.; Li, Z.; Yan, H.; Zhao, X.; Zuo, L. Electromagnetic Wave Absorption Performance of Ti2O3 and Vacancy Enhancement Effective Bandwidth. J. Mater. Sci. Technol. 2021, 76, 166–173. [Google Scholar] [CrossRef]
- Fu, X.; Liu, H. High Purity λ-Ti3O5 Prepared by Sc Doping for Enhanced Microwave Absorption. J. Mater. Chem. C. [CrossRef]
- Fu, X.; Chen, W.; Hao, X.; Zhang, Z.; Tang, R.; Yang, B.; Zhao, X.; Zuo, L. Preparing High Purity λ-Ti3O5 and Li/λ-Ti3O5 as High-Performance Electromagnetic Wave Absorbers. J. Mater. Chem. C 2021, 9, 7976–7981. [Google Scholar] [CrossRef]
- Wei, Y.; Shi, Y.; Zhang, X.; Li, D.; Zhang, L.; Gong, C.; Zhang, J. Preparation of Black Titanium Monoxide Nanoparticles and Their Potential in Electromagnetic Wave Absorption. Adv. Powder Technol. 2020, 31, 3458–3464. [Google Scholar] [CrossRef]
- Bejan, D.; Malcolm, J.D.; Morrison, L.; Bunce, N.J. Mechanistic Investigation of the Conductive Ceramic Ebonex® as an Anode Material. Electrochim. Acta 2009, 54, 5548–5556. [Google Scholar] [CrossRef]
- Smith, J.R.; Walsh, F.C.; Clarke, R.L. Electrodes Based on Magnéli Phase Titanium Oxides: The Properties and Applications of Ebonex® Materials. J. Appl. Electrochem. 1998, 28, 1021–1033. [Google Scholar] [CrossRef]
- Lačnjevac, U.Č.; Jović, B.M.; Jović, V.D.; Radmilović, V.R.; Krstajić, N.V. Kinetics of the Hydrogen Evolution Reaction on Ni-(Ebonex-Supported Ru) Composite Coatings in Alkaline Solution. Int. J. Hydrog. Energy 2013, 38, 10178–10190. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramanavicius, S.; Jagminas, A. Synthesis, Characterisation, and Applications of TiO and Other Black Titania Nanostructures Species (Review). Crystals 2024, 14, 647. https://doi.org/10.3390/cryst14070647
Ramanavicius S, Jagminas A. Synthesis, Characterisation, and Applications of TiO and Other Black Titania Nanostructures Species (Review). Crystals. 2024; 14(7):647. https://doi.org/10.3390/cryst14070647
Chicago/Turabian StyleRamanavicius, Simonas, and Arunas Jagminas. 2024. "Synthesis, Characterisation, and Applications of TiO and Other Black Titania Nanostructures Species (Review)" Crystals 14, no. 7: 647. https://doi.org/10.3390/cryst14070647
APA StyleRamanavicius, S., & Jagminas, A. (2024). Synthesis, Characterisation, and Applications of TiO and Other Black Titania Nanostructures Species (Review). Crystals, 14(7), 647. https://doi.org/10.3390/cryst14070647






