Electrochemical Recovery of N and P from Municipal Wastewater
Abstract
:1. Introduction
2. Materials and Methods
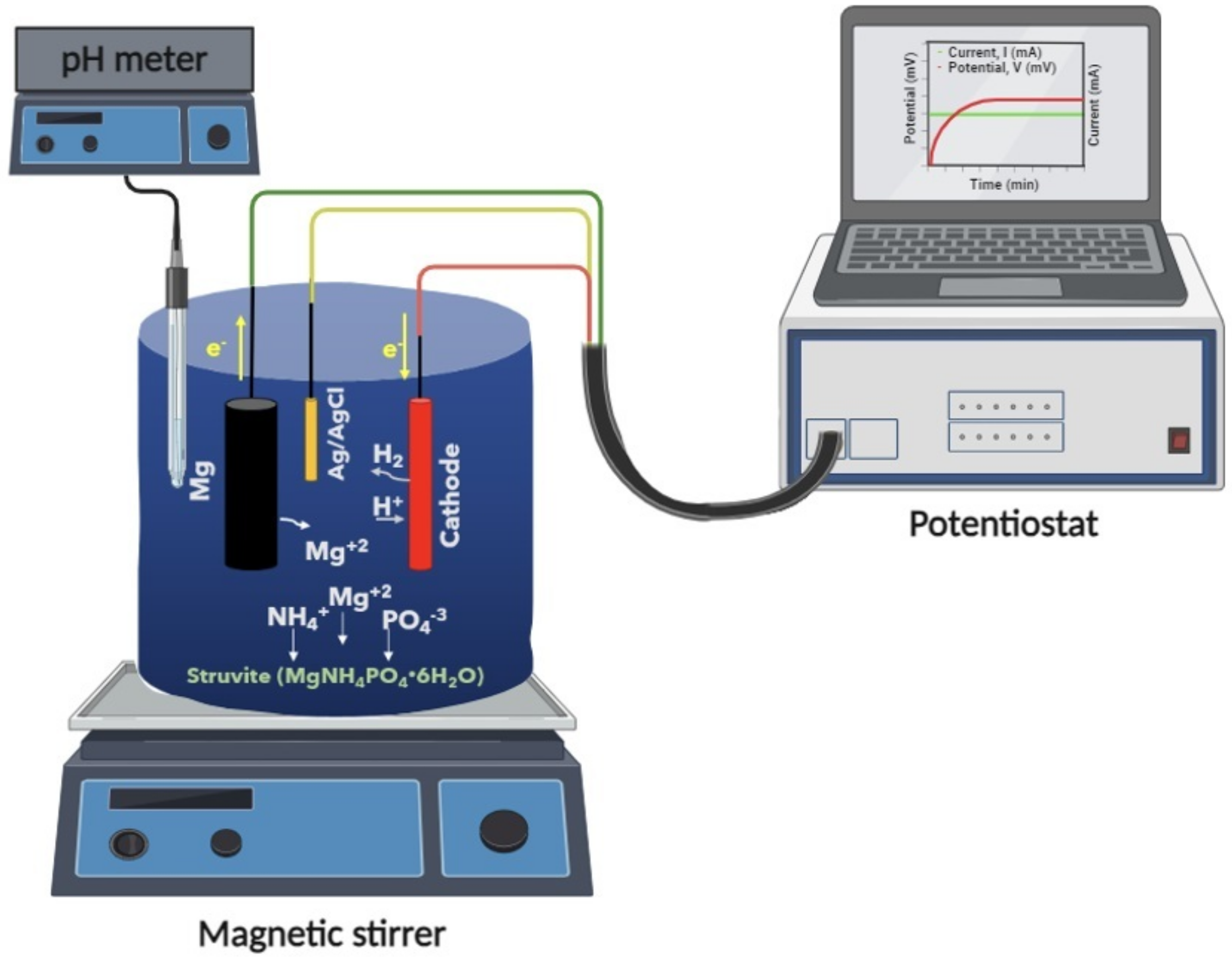
2.1. Experimental Set Up
2.2. Analysis
3. Results
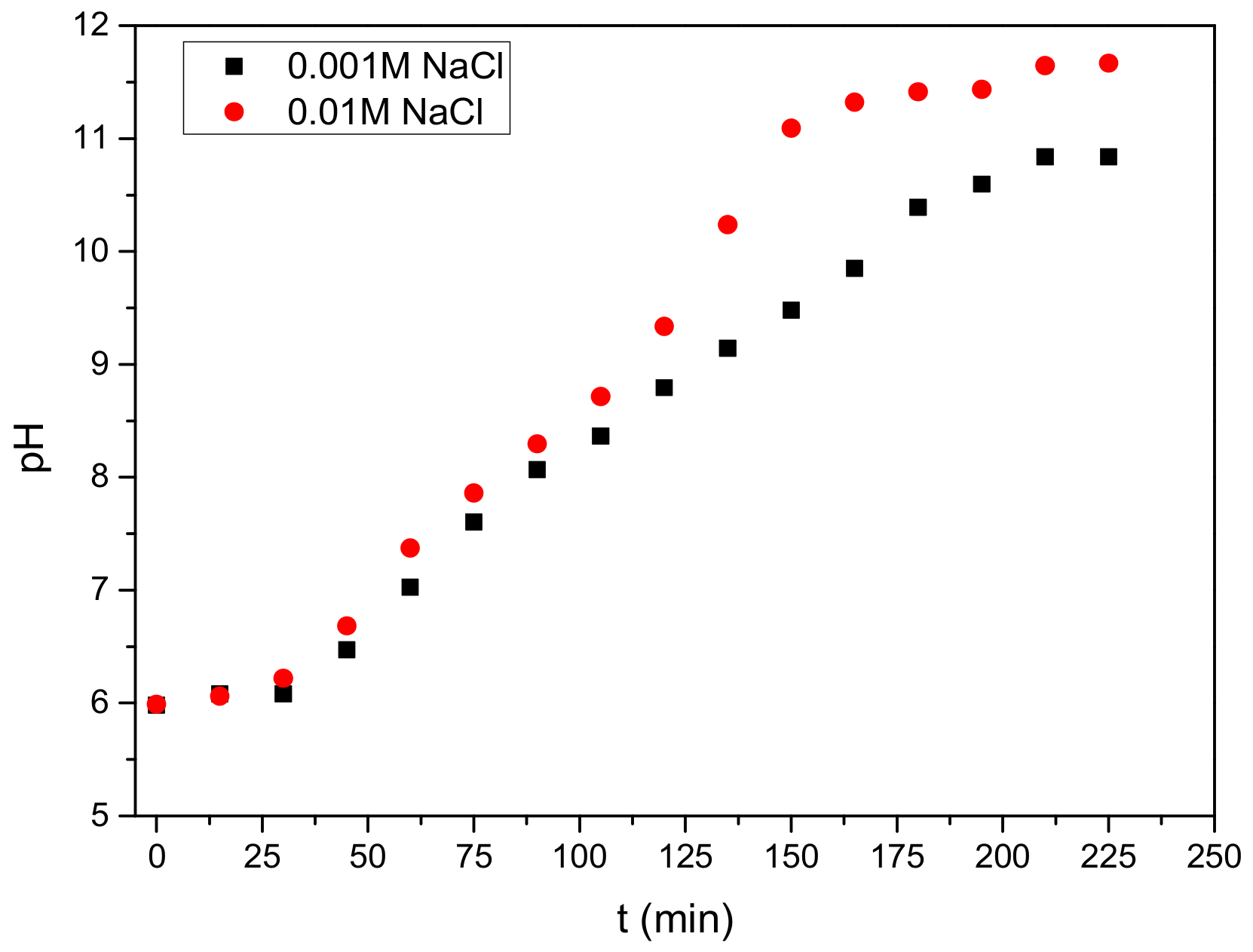
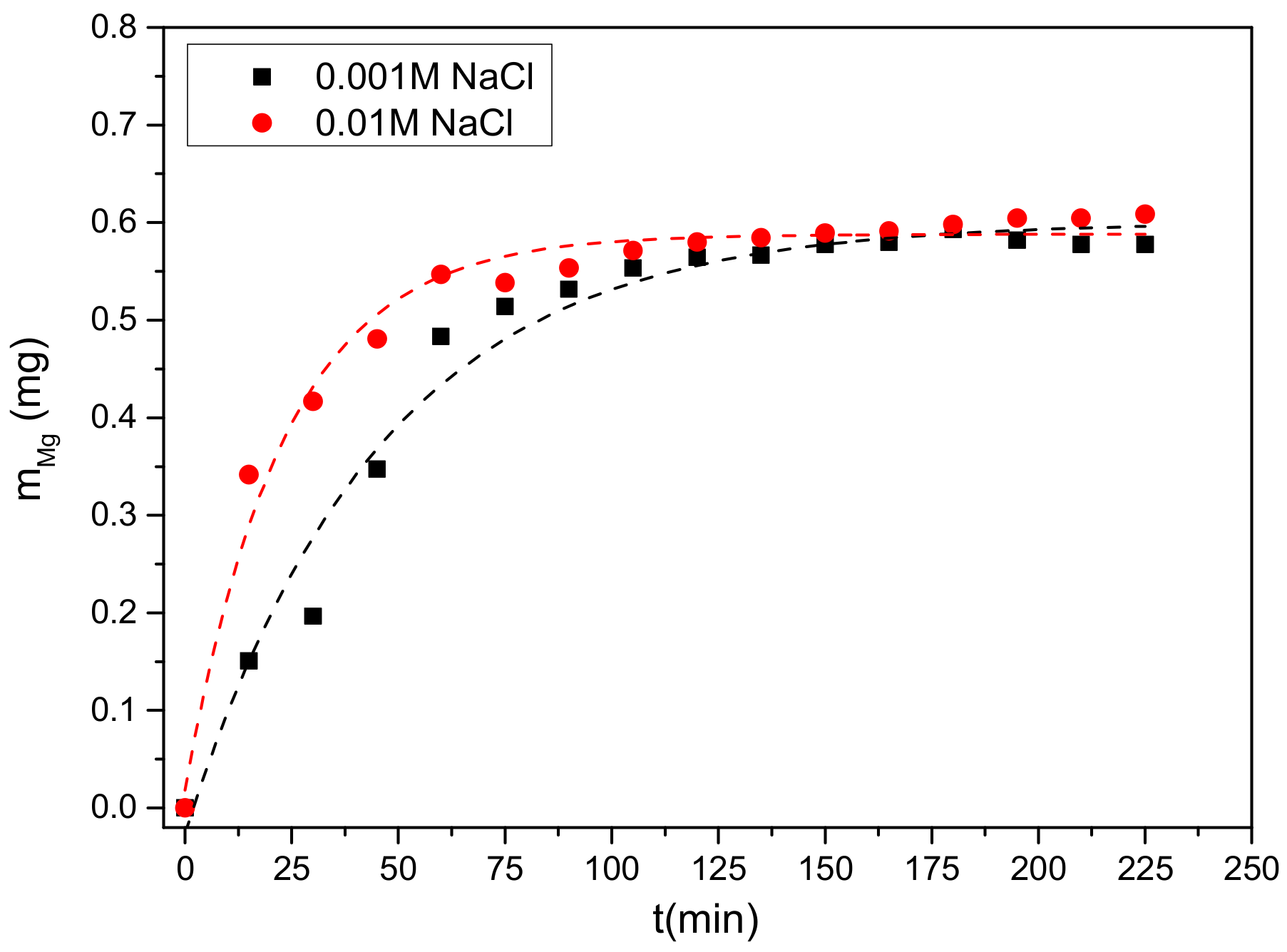
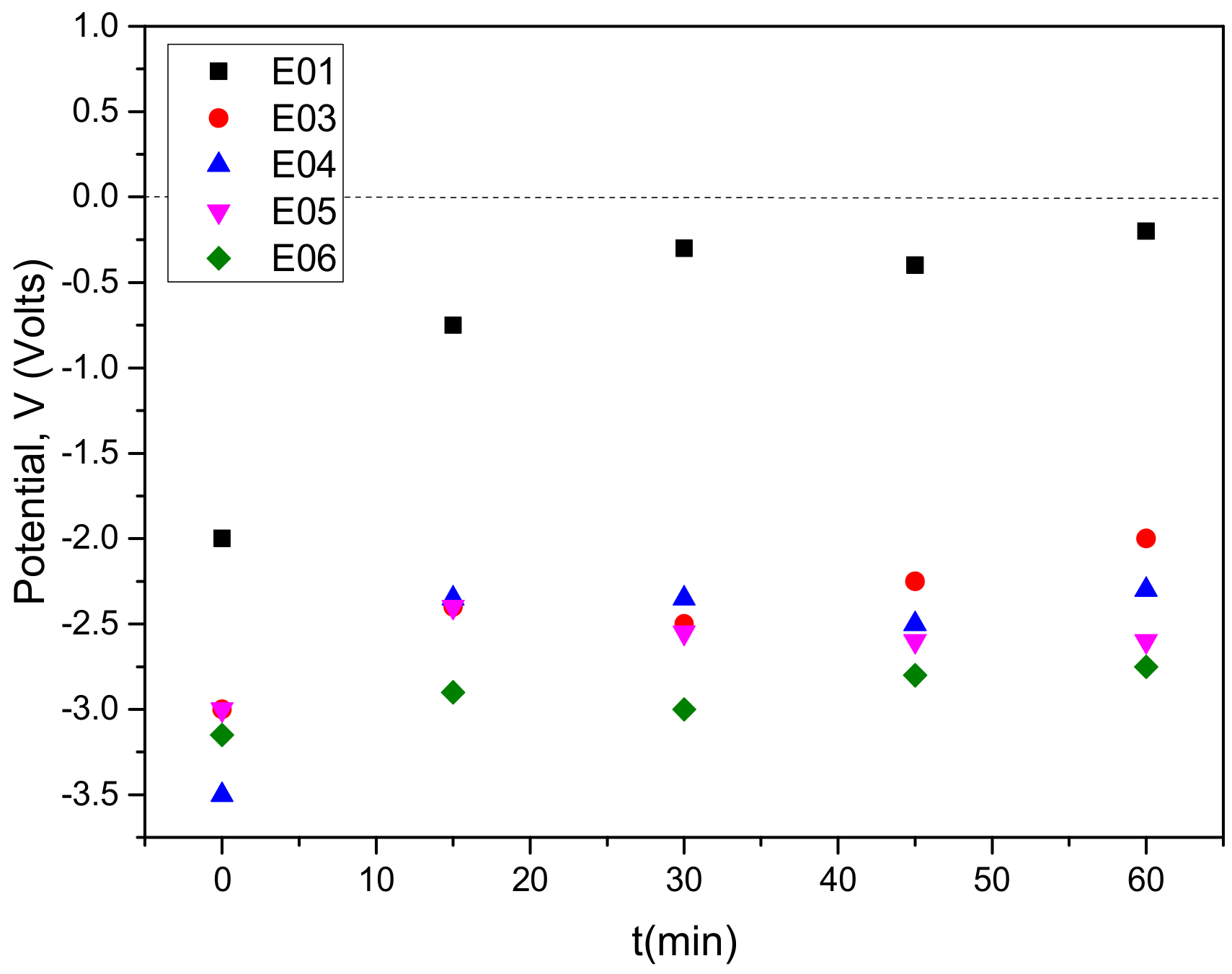
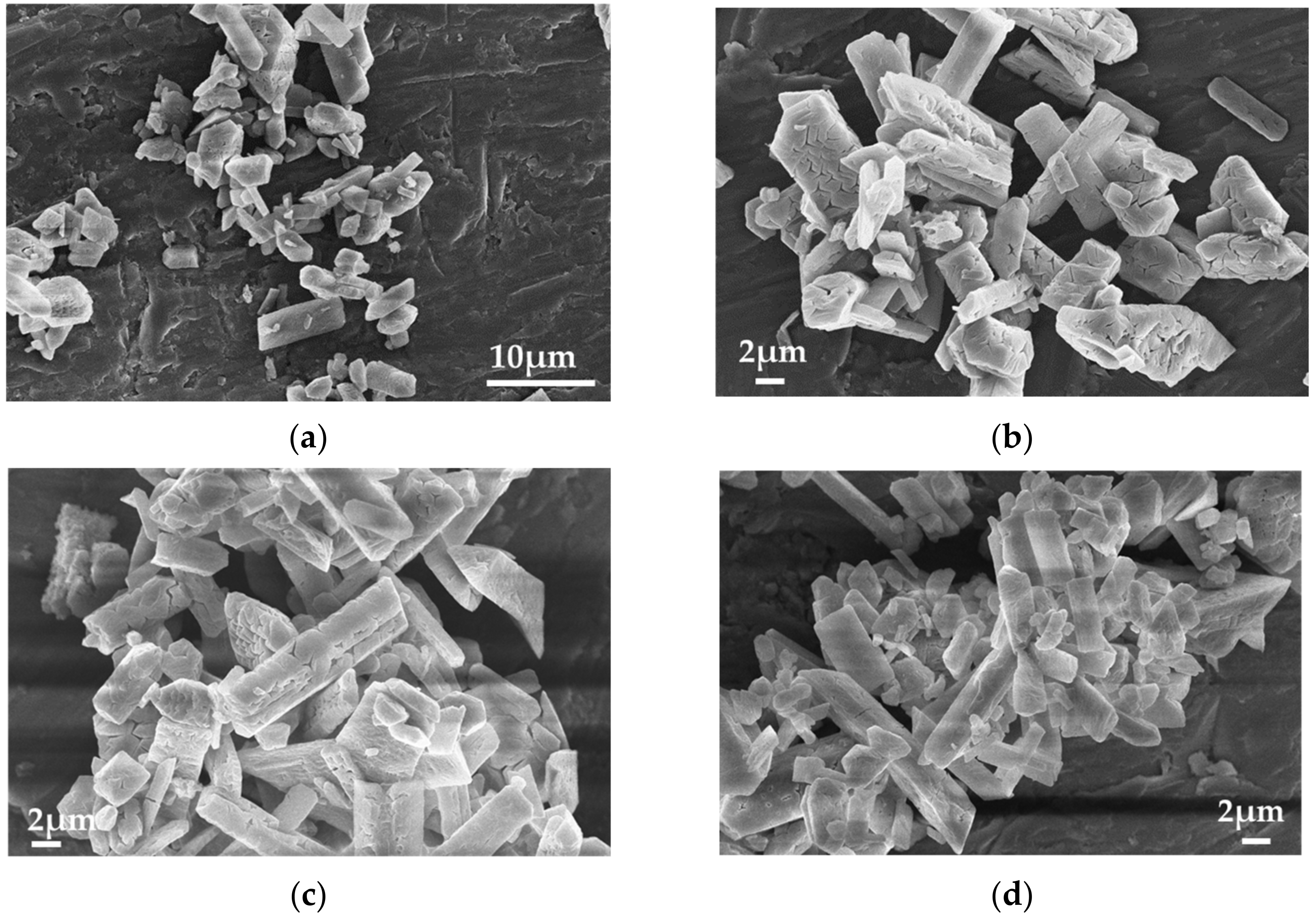
3.1. Anode Immersion Experiments at Open Circuit Potential (OCP) Conditions
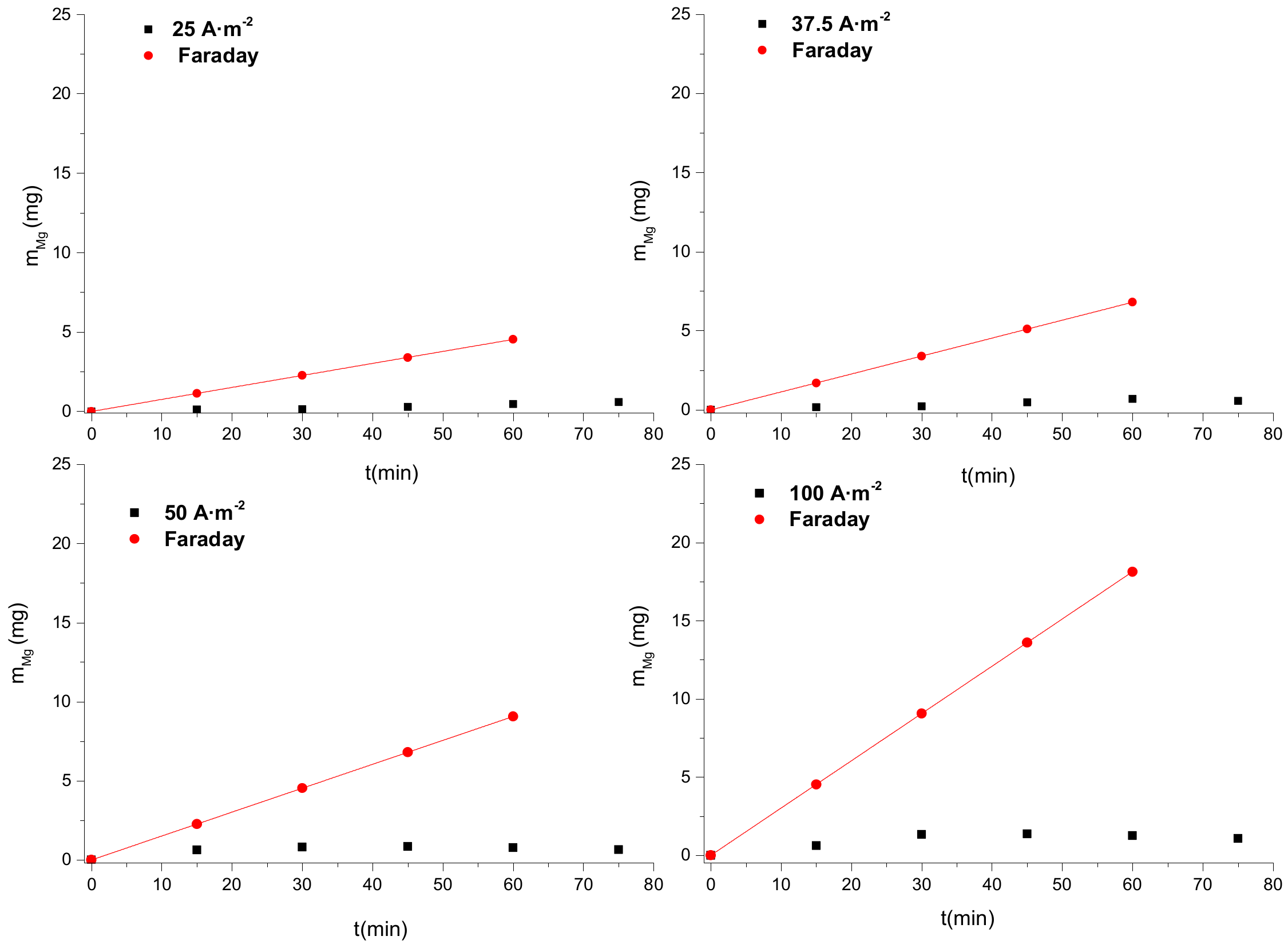
3.2. Constant Current Dissolution of Mg Anode
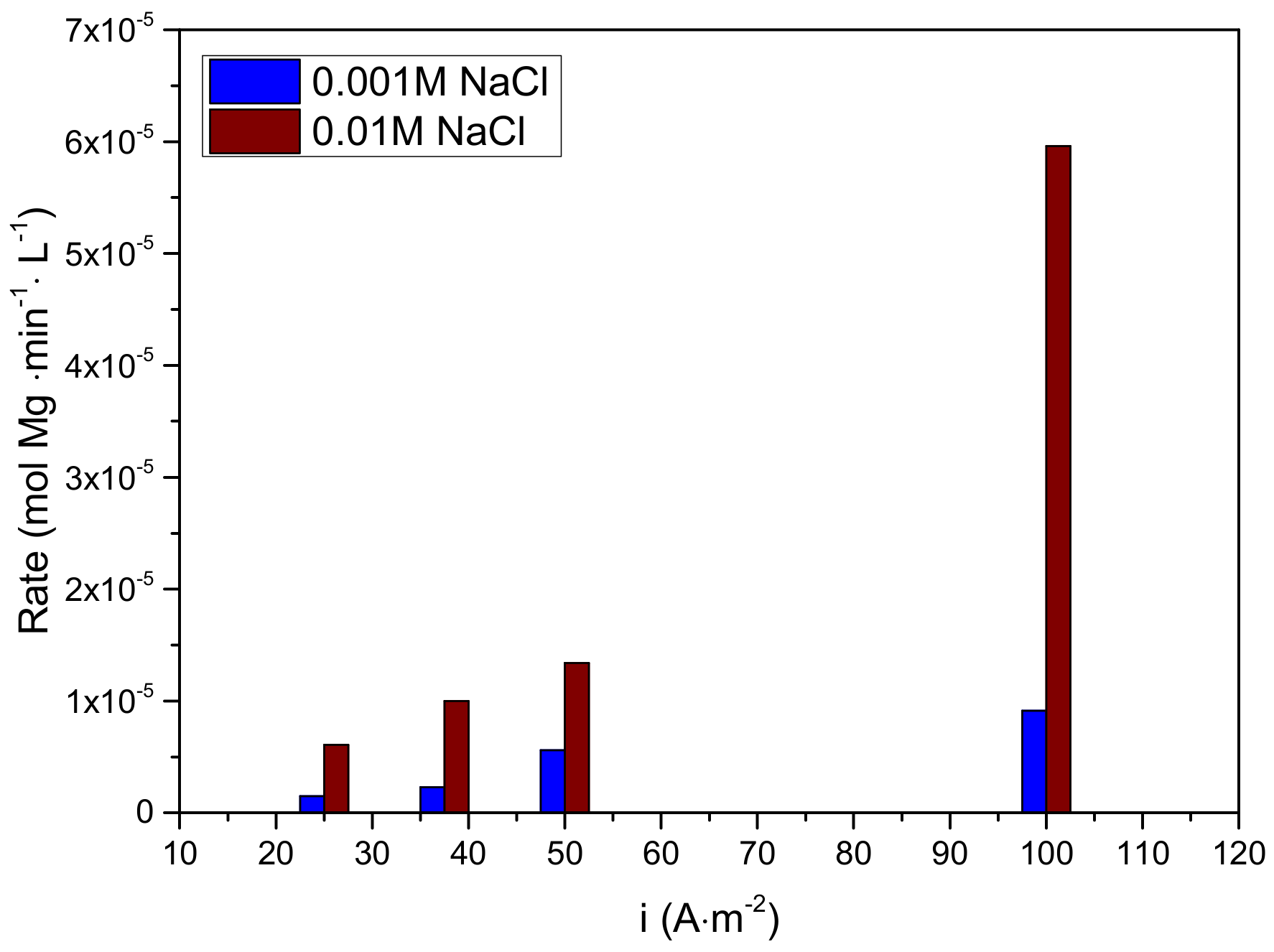
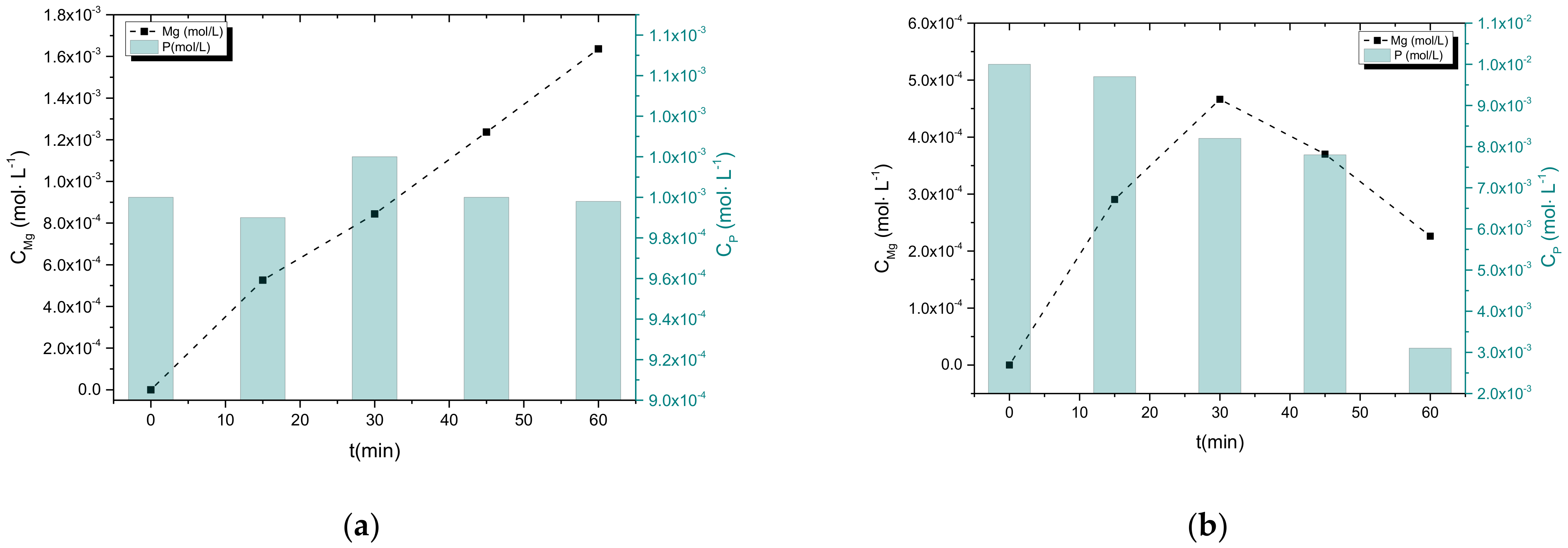
3.2.1. Electrolyte Concentration Effect
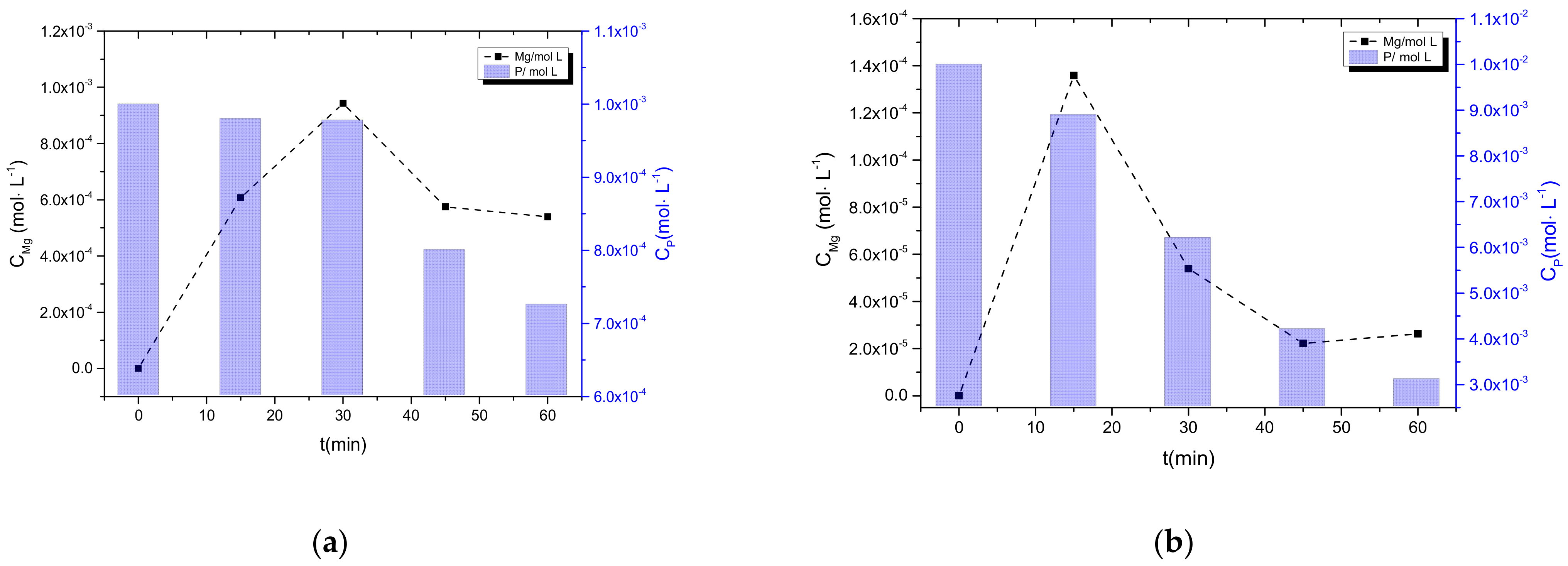
3.2.2. Precipitation in the Presence of NH4H2PO4
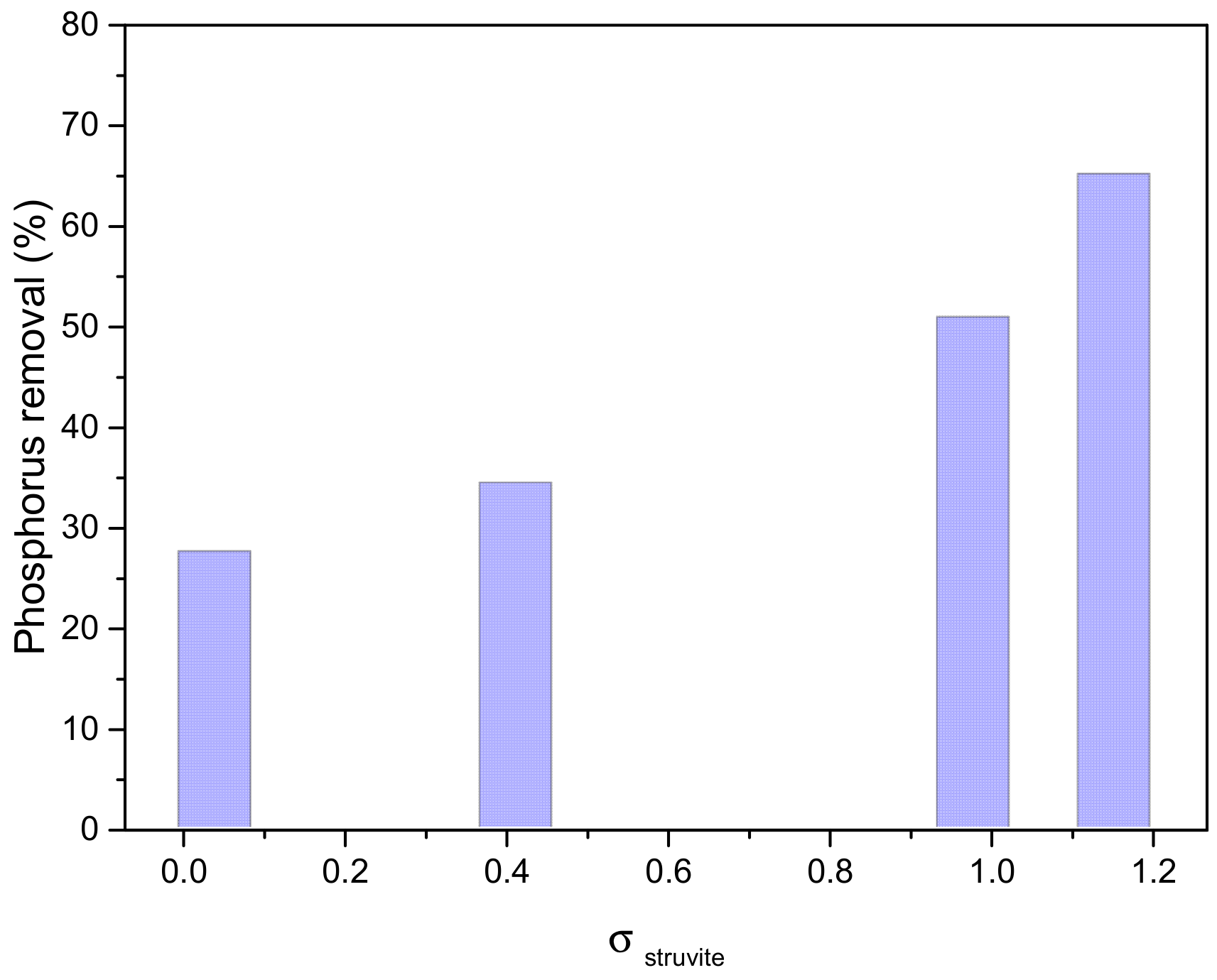
3.2.3. Struvite Precipitation in Simulated Wastewater (SWW)
3.2.4. Estimate of Process Cost
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ohtake, H.; Tsuneda, S. (Eds.) Phosphorus Recovery and Recycling; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019, 648, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Gai, S.; Liu, Z.; Sui, L.; Cheng, K.; Yang, F. Sustainable phosphorus recycling: A review of advanced recovery methods with a focus on hydrothermal humification technology and potential phosphorus resources in China for this method. Soil Use Manag. 2024, 40, e13001. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Membrane. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Liu, W.; Ke, H.; Xie, J.; Tan, H.; Luo, G.; Xu, B.; Abakari, G. Characterizing the water quality and microbial communities in different zones of a recirculating aquaculture system using biofloc biofilters. Aquaculture 2020, 529, 735624. [Google Scholar] [CrossRef]
- Suresh, K.; Tang, T.; van Vliet, M.T.H.; Bierkens, M.F.P.; Strokal, M.; Sorger-Domenigg, F.; Wad, Y. Recent advancement in water quality indicators for eutrophication in global freshwater lakes. Environ. Res. Lett. 2023, 18, 063004. [Google Scholar] [CrossRef]
- Cai, Y.; Han, Z.; Lin, X.; Duan, Y.; Du, J.; Ye, Z.; Zhu, J. Study on removal of phosphorus as struvite from synthetic wastewater using a pilot-scale electrodialysis system with magnesium anode. Sci. Total Environ. 2020, 726, 138221. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.J.; Ma, J.; Li, Y.; Jin, D.; Ali, Z.; Zhou, J.; Sun, R. Recent advances and challenges on removal and recycling of phosphate from wastewater using biomass-derived adsorbents. Chemosphere 2021, 278, 130377. [Google Scholar] [CrossRef] [PubMed]
- Al-Juboori, R.A.; Ahmed, F.E.; Khanzada, N.K.; Khatri, M.; Al-shaeli, M.; Ibrahim, Y.; Hilal, N. Burgeoning innovation and scalability activities for phosphorus recovery from wastewater treatment facilities. Sustain. Mater. Technol. 2024, 40, e00907. [Google Scholar] [CrossRef]
- Munir, M.T.; Li, B.; Mardon, I.; Young, B.R.; Baroutian, S. Integrating wet oxidation and struvite precipitation for sewage sludge treatment and phosphorus recovery. J. Clean. Prod. 2019, 232, 1043–1052. [Google Scholar] [CrossRef]
- Shakoor, B.; Ye, Z.L.; Chen, S. Engineered biochars for recovering phosphate and ammonium from wastewater: A review. Sci. Total Environ. 2021, 779, 146240. [Google Scholar] [CrossRef] [PubMed]
- Egle, L.; Rechberger, H.; Zessner, M. Overview and description of technologies for recovering phosphorus from municipal wastewater. Resour. Conserv. Recycl. 2015, 105, 325–346. [Google Scholar] [CrossRef]
- Zhang, T.; He, X.; Deng, Y.; Tsang, D.C.W.; Jiang, R.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2020, 698, 134240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jiang, R.; Deng, Y. Phosphorus Recovery by Struvite Crystallization from Livestock Wastewater and Reuse as Fertilizer: A Review. In Physico-Chemical Wastewater Treatment and Resource Recovery; Chapter 7; Farooq, R., Ahmad, Z., Eds.; IntechOpen: London, UK, 2017. [Google Scholar]
- Le Corre, K.; Valsami-Jones, E.; Hobbs, P.; Parsons, S. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Gujer, W. Struvite precipitation thermodynamics in source-separated urine. Water Res. 2007, 41, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Pastor, L.; Mangin, D.; Barat, R.; Seco, A. A pilot-scale study of struvite precipitation in a stirred tank reactor: Conditions influencing the process. Bioresour. Technol. 2008, 99, 6285–6291. [Google Scholar] [CrossRef] [PubMed]
- Moerman, W.; Carballa, M.; Vandekerckhove, A.; Derycke, D.; Verstraete, W. Phosphate removal in agro-industry: Pilot and full-scale operational considerations of struvite crystallization. Water Res. 2009, 43, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Kruk, D.J.; Elektorowicz, M.; Oleszkiewicz, J.A. Struvite precipitation and phosphorus removal using magnesium sacrificial anode. Chemosphere 2014, 101, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, P.; Zhang, Z. Simultaneous removal of ammonia nitrogen and recovery of phosphate from swine wastewater by struvite electrochemical precipitation and recycling technology. J. Clean. Prod. 2016, 127, 302–310. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Impact of calcium on struvite crystal size, shape and purity. J. Cryst. Growth 2005, 283, 514–522. [Google Scholar] [CrossRef]
- Doyle, J.D.; Parsons, S.A. Struvite formation control and recovery. Water Res. 2002, 36, 3925–3940. [Google Scholar] [CrossRef] [PubMed]
- Trotta, S.; Adani, F.; Fedele, M.; Salvatori, M. Nitrogen and phosphorus recovery from cow digestate by struvite precipitation: Process optimization to maximize phosphorus recovery. Results Eng. 2023, 20, 101478. [Google Scholar] [CrossRef]
- Nagul, E.A.; McKelvie, I.D.; Worsfold, P.; Kolev, S.D. The molybdenum blue reaction for the determination of orthophosphate revisited: Opening the black box. Anal. Chim. Acta 2015, 890, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for PHREEQC Version 3—A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. In U.S. Geological Survey Techniques and Methods, Book 6; Chapter A43; U.S. Geological Survey: Reston, VA, USA, 2013; p. 497. [Google Scholar]
- Mullin, W. Crystallization, 3rd ed.; Butterworth-Heinemann Ltd.: Oxford, UK, 2001; p. 189. [Google Scholar]
- Natsi, P.D.; Goudas, K.-A.; Koutsoukos, P.G. Phosphorus Recovery from Municipal Wastewater: Brucite from MgO Hydrothermal Treatment as Magnesium Source. Crystals 2023, 13, 208. [Google Scholar] [CrossRef]
- Sönel, O.; Garside, J. Precipitation, Basic Principles and Industrial Applications; Butterworth-Heineman Ltd.: Oxford, UK, 1992; pp. 89–92. [Google Scholar]
- Matsouki, N. Phosphorus Recovery from Municipal Wastewater. Master’s Thesis, Graduate Program in Environmental Sciences, University of Patras, Patras, Greece, 2013. Available online: https://nemertes.library.upatras.gr/search?query=%CE%9C%CE%B1%CF%84%CF%83%CE%BF%CF%8D%CE%BA%CE%B7%20%CE%9D%CE%A%CE%BA%CE%B7 (accessed on 10 April 2024).
- Bagastyo, A.Y.; Anggrainy, A.D.; Khoiruddin, K.; Ursada, R.; Warmadewanthi, I.; Wenten, I.G. Electrochemically-driven struvite recovery: Prospect and challenges for the application of magnesium sacrificial anode. Sep. Purif. Technol. 2022, 288, 120653. [Google Scholar] [CrossRef]
- Lin, X.; Han, Z.; Yu, H.; Ye, Z.; Zhu, S.; Zhu, J. Struvite precipitation from biogas digestion slurry using a two-chamber electrolysis cell with a magnesium anode. J. Clean. Prod. 2018, 174, 1598–1607. [Google Scholar] [CrossRef]
- Kofina, A.N.; Koutsoukos, P.G. Spontaneous Precipitation of Struvite from Synthetic Wastewater Solutions. Cryst. Growth Des. 2005, 5, 489–496. [Google Scholar] [CrossRef]
- Kofina, A.N. Recovery of Inorganic Phosphates from Aqueous Solutions. Ph.D. Thesis, University of Patras, Patras, Greece, 2006. Available online: https://hdl.handle.net/10889/1468 (accessed on 25 June 2024).
- Cai, Y.; Han, Z.; Lin, X.; Du, J.; Lei, Z.; Ye, Z.; Zhu, J. Mechanisms of releasing magnesium ions from a magnesium anode in an electrolysis reactor with struvite precipitation. J. Environ. Chem. Eng. 2022, 10, 106661. [Google Scholar] [CrossRef]
- Sultana, R.; Greenlee, L.F. The implications of pulsating anode potential on the electrochemical recovery of phosphate as magnesium ammonium phosphate hexahydrate (struvite). Chem. Eng. J. 2023, 459, 141522. [Google Scholar] [CrossRef]
- Sultana, R.; Kékedy-Nagy, L.; Daneshpour, R.; Greenlee, L.F. Electrochemical recovery of phosphate from synthetic wastewater with enhanced salinity. Electrochim. Acta 2022, 426, 140848. [Google Scholar] [CrossRef]
- Muys, M.; Phukan, R.; Brader, G.; Samad, A.; Moretti, M.; Haiden, B.; Pluchon, S.; Roest, K.; Vlaeminck, S.E.; Spiller, M. A systematic comparison of commercially produced struvite: Quantities, qualities and soil-maize phosphorus availability. Sci. Total Environ. 2021, 756, 143726. [Google Scholar] [CrossRef] [PubMed]








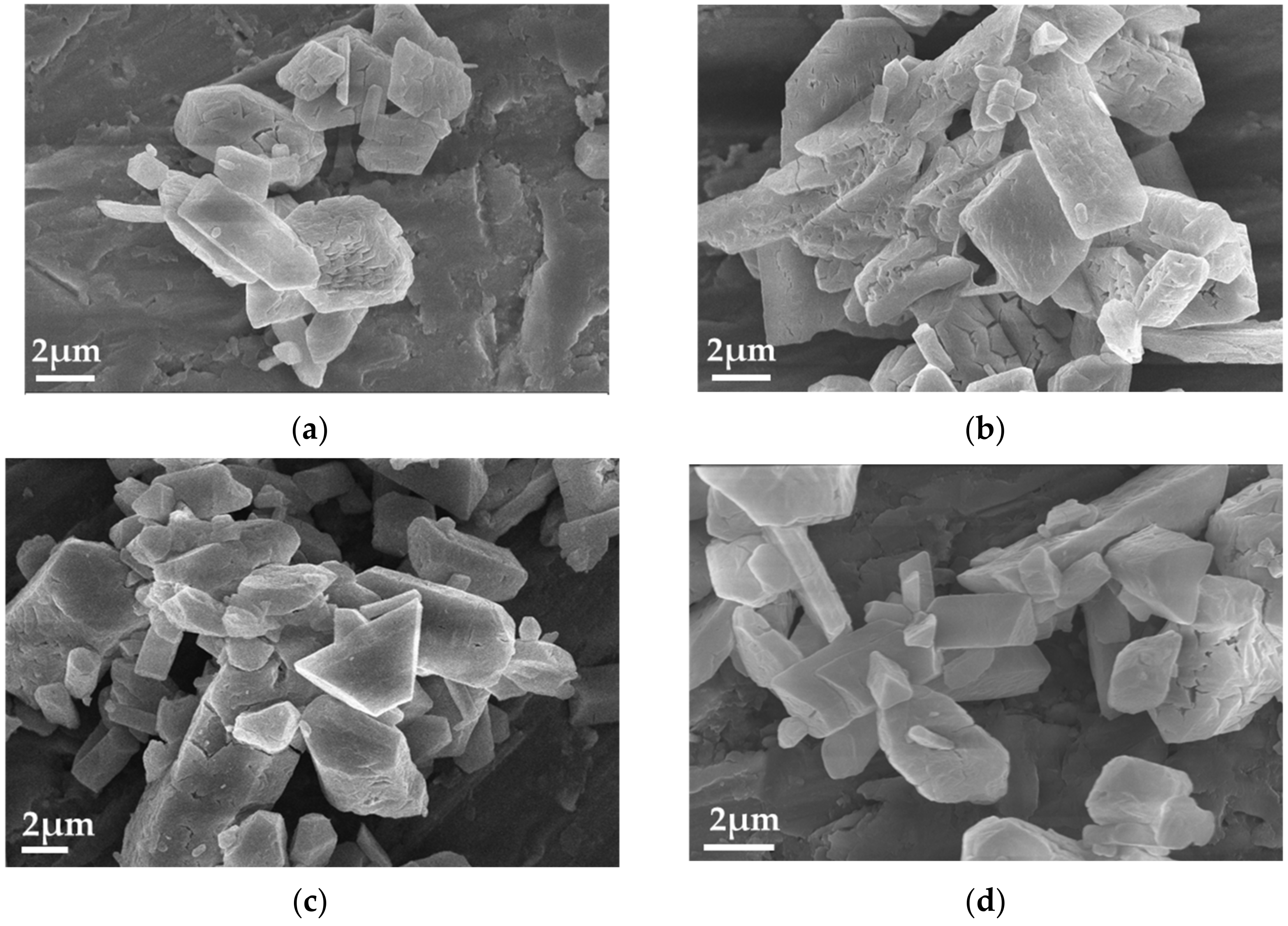

| Component | Concentration (/×10−4 mol·L−1) |
|---|---|
| Glucose | 5.17 |
| NaHCO3 | 176.8 |
| NaCl | 100 |
| NaNO3 | 5.88 |
| Na2SO4 | 120 |
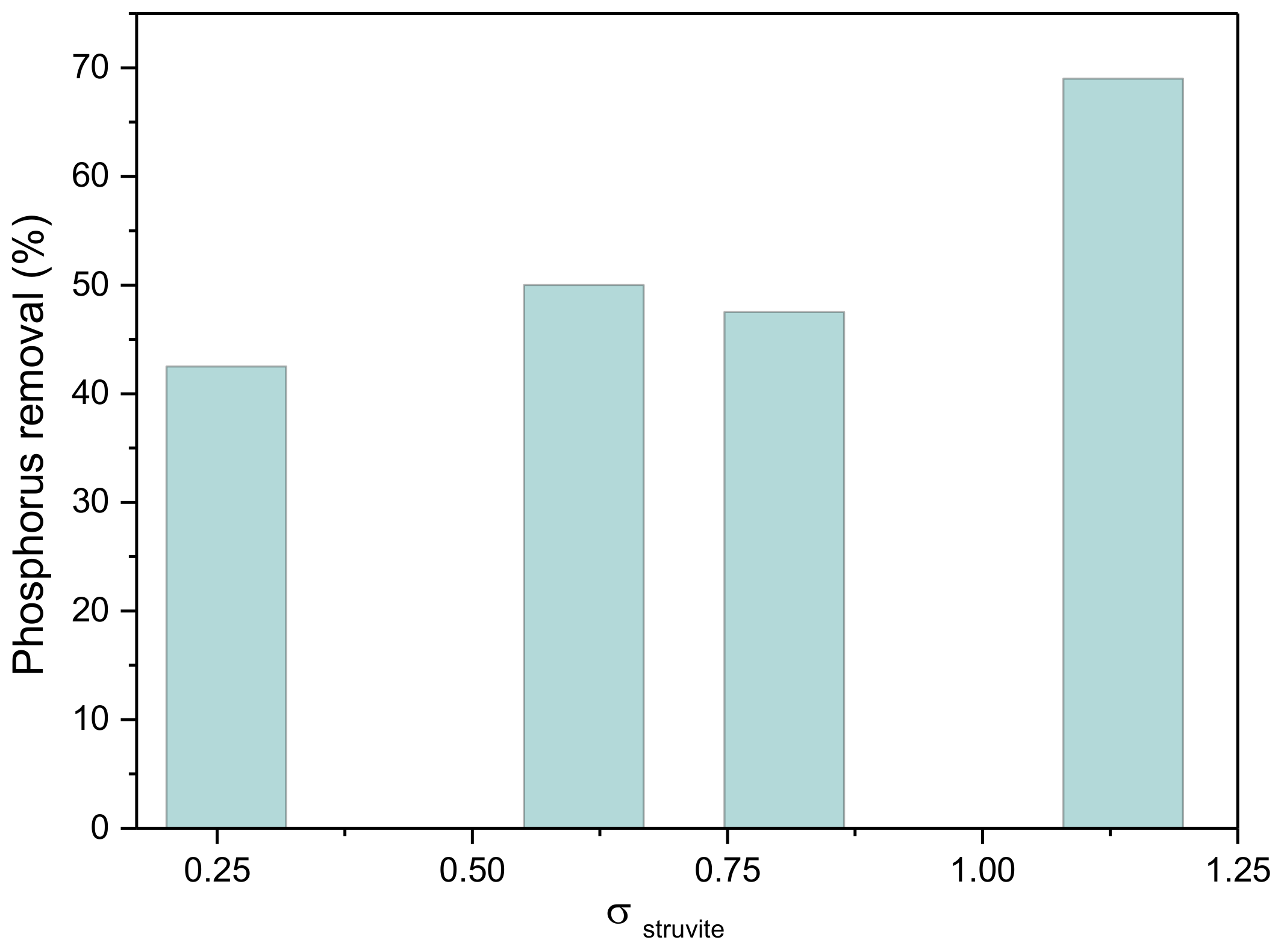
| Experiment Number | [N-NH3]tot = [P]tot /×10−3 mol·L−1 | Relative Supersaturation, σstruvite | Precipitation Rate, RPS/×10−5 mol·min−1 |
|---|---|---|---|
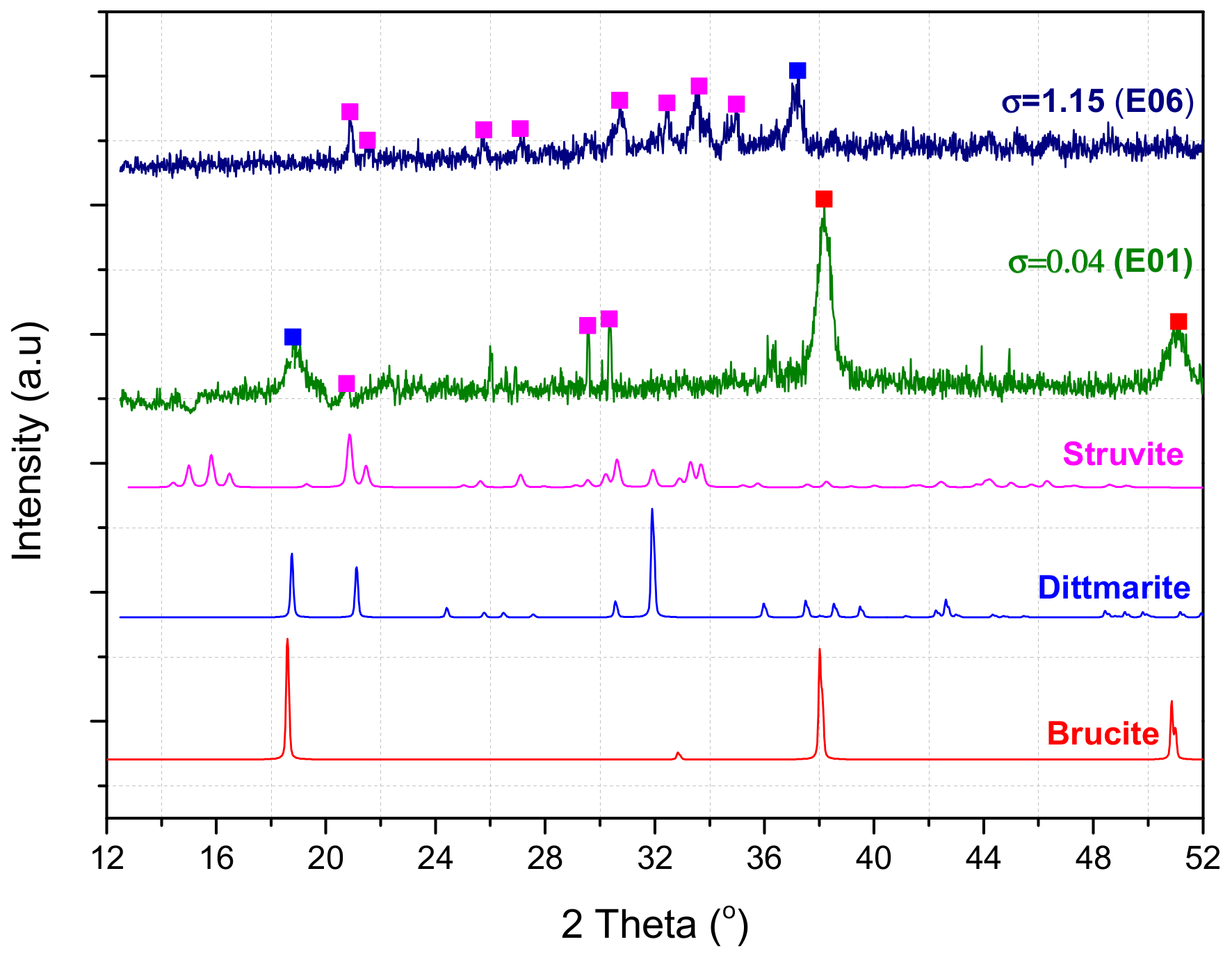
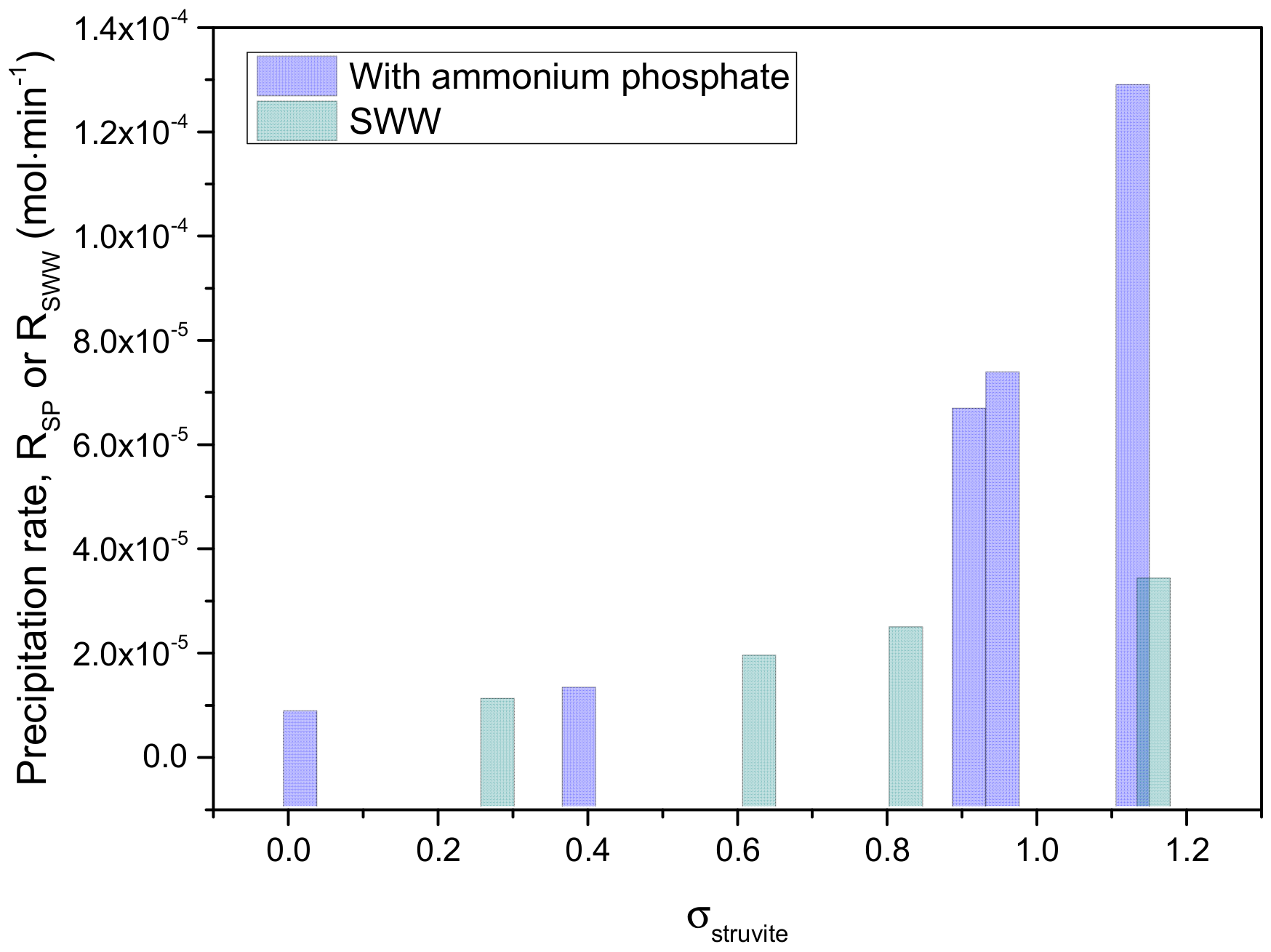
| E01 | 1 | 0.04 | 0.84 |
| E02 | 2 | 0.41 | 1.30 |
| E03 | 4 | 0.93 | 6.67 |
| E04 | 6 | 0.98 | 7.37 |
| E05 | 8 | 1.12 | 5.00 |
| E06 | 10 | 1.15 | 12.9 |
| Magnesium Sources | Relative Supersaturation Range, σstruvite | Apparent Order, n | Rate Constant, ks |
|---|---|---|---|
| Mg-anode (This work) | 0.04 < σ < 1.15 | 3 | 8.19 × 10−5 |
| Mg(OH)2 [23] | 0.3 < σ < 36.6 | 4 | 5.00 × 10−10 |
| MgSO4 [26] | 0.43 < σ < 0.82 | 4 | 1.29 × 10−5 |
| MgCl2 [27] | 0.27 < σ < 0.54 | 3 | 3.40 × 10−5 |
| Experiment Number | [N-NH3]tot = [P]tot /×10−3 mol·L−1 | Relative Supersaturation, σstruvite | Precipitation Rate, RPS/×10−5 mol·min−1 |
|---|---|---|---|
| E07 | 1 | - | - |
| E08 | 2 | - | - |
| E09 | 4 | 0.26 | 1.08 |
| E10 | 6 | 0.61 | 1.91 |
| E11 | 8 | 0.81 | 2.46 |
| E12 | 10 | 1.14 | 3.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natsi, P.D.; Koutsoukos, P.G. Electrochemical Recovery of N and P from Municipal Wastewater. Crystals 2024, 14, 675. https://doi.org/10.3390/cryst14080675
Natsi PD, Koutsoukos PG. Electrochemical Recovery of N and P from Municipal Wastewater. Crystals. 2024; 14(8):675. https://doi.org/10.3390/cryst14080675
Chicago/Turabian StyleNatsi, Panagiota D., and Petros G. Koutsoukos. 2024. "Electrochemical Recovery of N and P from Municipal Wastewater" Crystals 14, no. 8: 675. https://doi.org/10.3390/cryst14080675






