Defect Passivation for Highly Efficient and Stable Sn-Pb Perovskite Solar Cells
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Device Fabrication
2.2.1. ITO Conductive Glass Treatment
2.2.2. Hole Transport Layer (ETL) Fabrication
2.2.3. Precursors Preparation and Perovskite Film Fabrication
2.2.4. Electron Transport Layer Fabrication
2.2.5. BCP Layer Fabrication
2.2.6. Metal Electrode Fabrication
2.3. Characterizations
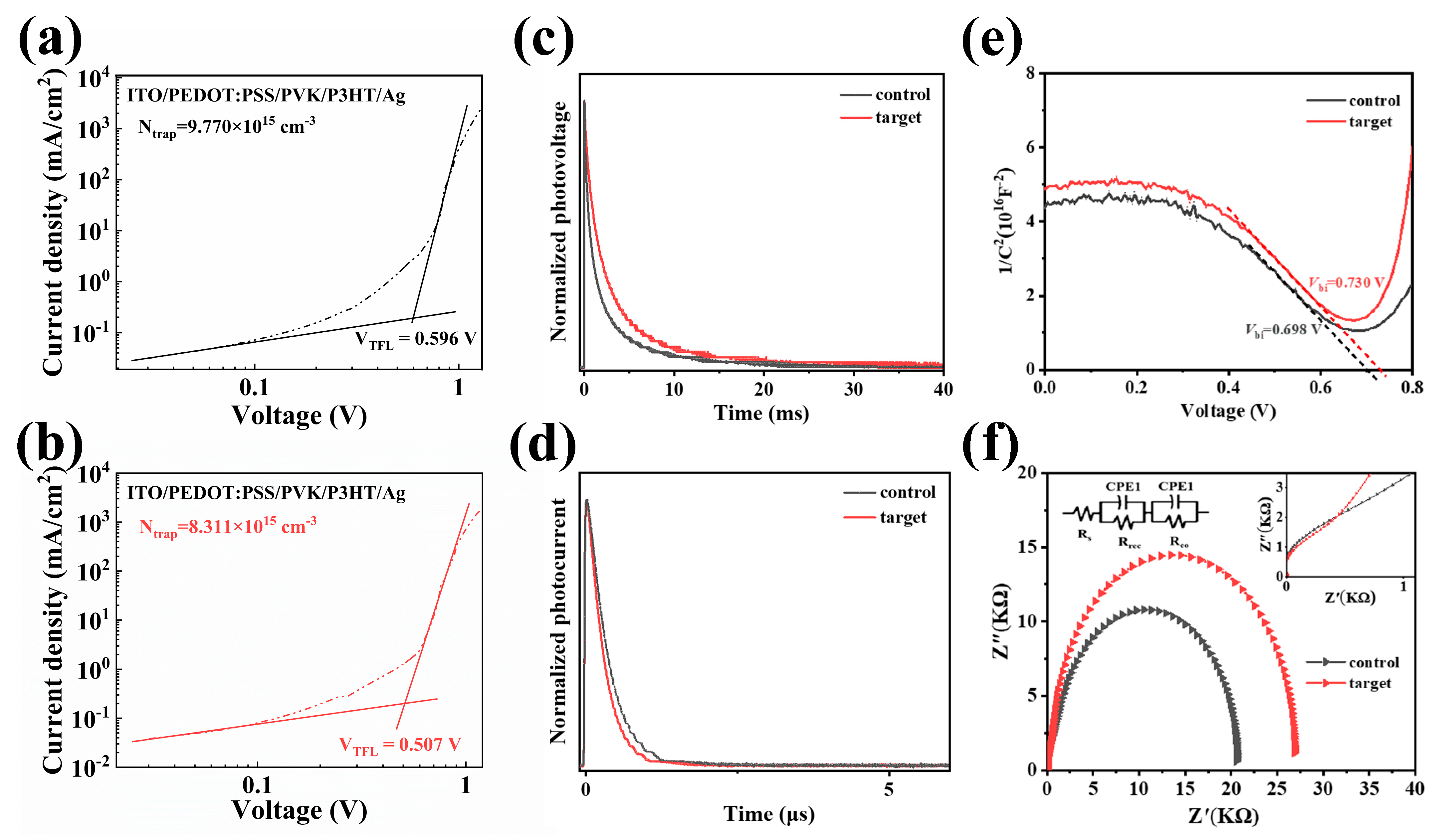
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-Hole Diffusion Lengths > 175 μm in Solution-Grown CH3NH3PbI3 Single Crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Jain, A.; Voznxy, O.; Lan, X.; De, A.F.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and Stable Solution-Processed Planar Perovskite Solar Cells via Contact Passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, B.; Song, Z.; Yu, Y.; Chen, C.; Zhao, X.; Zhu, K.; Yan, Y. Four-Terminal All-Perovskite Tandem Solar Cells Achieving Power Conversion Efficiencies Exceeding 23%. ACS Energy Lett. 2018, 3, 305–306. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.B.; et al. Conformal Quantum Dot-SnO2 Layers as Electron Transporters for Efficient Perovskite Solar Cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef]
- Zhou, J.; Tan, L.; Liu, Y.; Li, H.; Liu, X.; Li, M.; Wang, S.; Zhang, Y.; Jiang, C.; Hua, R.; et al. Highly Efficient and Stable Perovskite Solar Cells via A Multifunctional Hole Transporting Material. Joule 2024, 8, 1691–1706. [Google Scholar] [CrossRef]
- Yang, S.; Fu, W.; Zhang, Z.; Chen, H.; Li, C. Recent Advances in Perovskite Solar Cells: Efficiency, Stability and Lead-Free Perovskite. J. Mater. Chem. A 2017, 5, 11462–11482. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-Free Organic-Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives. Adv. Mater. 2017, 29, 1605005. [Google Scholar] [CrossRef]
- Umari, P.; Mosconi, E.; De Angelis, F. Relativistic GW Calculations on CH3NH3PbI3 and CH3NH3SnI3 Perovskites for Solar Cell Applications. Sci. Rep. 2014, 4, 4467. [Google Scholar] [CrossRef]
- Rajagopal, A.; Stoddard, R.J.; Hillhouse, H.W.; Jen, A.K. On Understanding Bandgap Bowing and Optoelectronic Quality in Pb-Sn Alloy Hybrid Perovskites. J. Mater. Chem. A 2019, 7, 16285–16293. [Google Scholar] [CrossRef]
- Prasanna, R.; Gold-Parker, A.; Leijtens, T.; Conings, B.; Babayigit, A.; Boyen, H.G.; Toney, M.F.; McGehee, M.D. Band Gap Tuning via Lattice Contraction and Octahedral Tilting in Perovskite Materials for Photovoltaics. J. Am. Chem. Soc. 2017, 139, 11117–11124. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Abdi-Jalebi, M.; Tabachnyk, M.; Glass, H.; Kamboj, V.S.; Nie, W.; Pearson, A.J.; Puttisong, Y.; Gödel, K.C.; Beere, H.E.; et al. High Open-Circuit Voltages in Tin-Rich Low-Bandgap Perovskite-Based Planar Heterojunction Photovoltaics. Adv. Mater. 2017, 29, 1604744. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Yang, B.; Zhang, C.; Huang, Y.; Cui, Y.; Liu, P.; Zhou, C.; Hao, Y.; Gao, Y.; Yang, J. Prominent Efficiency Enhancement in Perovskite Solar Cells Employing Silica-Coated Gold Nanorods. J. Phys. Chem. C 2016, 120, 6996–7004. [Google Scholar] [CrossRef]
- Jang, Y.H.; Jang, Y.; Kim, S.; Quan, L.; Chung, K.; Kim, D.H. Plasmonic Solar Cells: From Rational Design to Mechanism Overview. Chem. Rev. 2016, 116, 14982–15034. [Google Scholar] [CrossRef]
- Mahapatra, A.; Prochowicz, D.; Tavakoli, M.M.; Trivedi, S.; Kumar, P.; Yadav, P. A review of aspects of additive engineering in perovskite solar cells. J. Mater. Chem. A 2020, 8, 27–54. [Google Scholar] [CrossRef]
- Yeom, K.W.; Lee, D.K.; Park, N.G. Hard and Soft Acid and Base (HSAB) Engineering for Efficient and Stable Sn-Pb Perovskite Solar Cells. Adv. Energy Mater. 2022, 12, 2202496. [Google Scholar] [CrossRef]
- Lin, R.; Xu, J.; Wei, M.; Wang, Y.; Qin, Z.; Liu, Z.; Wu, J.; Xiao, K.; Chen, B.; Park, S.M.; et al. All-perovskite tandem solar cells with improved grain surface passivation. Nature 2022, 603, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, C.; MacKenzie, R.C.I.; Zhu, Z.; Chen, Y.; Ruan, S.; Wen, S. Using Ligand Engineering to Produce Efficient and Stable Pb-Sn Perovskite Solar Cells with Antioxidative 2D Capping Layers. ACS Appl. Mater. Interfaces 2022, 14, 14729–14738. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wei, Q.; Li, H.; Xie, J.; Jiang, X.; Pan, T.; Wang, H.; Pan, M.; Zhou, W.; Liu, W.; et al. Quasi-2D Bilayer Surface Passivation for High Efficiency Narrow Bandgap Perovskite Solar Cells. Angew. Chem. Int. Ed. 2022, 61, e202202346. [Google Scholar] [CrossRef]
- Sahamir, S.R.; Kamarudin, M.A.; Ripolles, T.S.; Baranwal, A.K.; Kapil, G.; Shen, Q.; Segawa, H.; Bisquert, J.; Hayase, S. Enhancing the Electronic Properties and Stability of High-Efficiency Tin–Lead Mixed Halide Perovskite Solar Cells via Doping Engineering. J. Phys. Chem. Lett. 2022, 13, 3130–3137. [Google Scholar] [CrossRef]
- Yuce, H.; Perini, C.A.R.; Hidalgo, J.; Castro-Ménde, Z.A.F.; Evans, C.; Betancur, P.F.; Vagott, J.N.; An, Y.; Bairley, K.; Demir, M.M.; et al. Understanding the impact of SrI2 additive on the properties of Sn-based halide perovskites. Opt. Mater. 2022, 123, 111806. [Google Scholar] [CrossRef]
- Lin, R.; Xiao, K.; Qin, Z.; Han, Q.; Zhang, C.; Wei, M.; Saidaminov, M.I.; Gao, Y.; Xu, J.; Xiao, M.; et al. Monolithic all-perovskite tandem solar cells with 24.8% efficiency exploiting comproportionation to suppress Sn(ii) oxidation in precursor ink. Nat. Energy 2019, 4, 864–873. [Google Scholar] [CrossRef]
- Liu, H.; Wang, L.; Li, R.; Shi, B.; Wang, P.; Zhao, Y.; Zhang, X. Modulated Crystallization and Reduced VOC Deficit of Mixed Lead-Tin Perovskite Solar Cells with Antioxidant Caffeic Acid. ACS Energy Lett. 2021, 6, 2907–2916. [Google Scholar] [CrossRef]
- Kapil, G.; Bessho, T.; Maekawa, T.; Baranwal, A.K.; Zhang, Y.; Kamarudin, M.A.; Hirotani, D.; Shen, Q.; Segawa, H.; Hayase, S. Tin-Lead Perovskite Fabricated via Ethylenediamine Interlayer Guides to the Solar Cell Efficiency of 21.74%. Adv. Energy Mater. 2021, 11, 2101069. [Google Scholar] [CrossRef]
- Wei, M.; Xiao, K.; Walters, G.; Lin, R.; Zhao, Y.; Saidaminov, M.I.; Todorovic, P.; Johnston, A.; Huang, Z.; Chen, H.; et al. Combining Efficiency and Stability in Mixed Tin-Lead Perovskite Solar Cells by Capping Grains with an Ultrathin 2D Layer. Adv. Mater. 2020, 32, 1907058. [Google Scholar] [CrossRef]
- Zhang, Z.; Liang, J.; Zheng, Y.; Wu, X.; Wang, J.; Huang, Y.; Yang, Y.; Zhou, Z.; Wang, L.; Kong, L.; et al. Balancing Crystallization Rate in A Mixed Sn-Pb Perovskite Film for Efficient and Stable Perovskite Solar Cells of more than 20% Efficiency. J. Mater. Chem. A 2021, 9, 17830–17840. [Google Scholar] [CrossRef]
- Luo, J.; He, R.; Lai, H.; Chen, C.; Zhu, J.; Xu, Y.; Yao, F.; Ma, T.; Luo, Y.; Yi, Z.; et al. Improved Carrier Management via a Multifunctional Modifier for High-Quality Low-Bandgap Sn-Pb Perovskites and Efficient All-Perovskite Tandem Solar Cells. Adv. Mater. 2023, 35, 2300352. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Gao, W.; Xing, G.; Chao, L.; Song, L.; Li, M.; Fu, L.; Chen, Y.; Ran, C. Improving the Air Resistance of the Precursor Solution for Ambient-Air Coating of an Sn-Pb Perovskite Film with Superior Photovoltaic Performance. ACS Appl. Mater. Interfaces 2022, 14, 43362–43371. [Google Scholar] [CrossRef]
- Hu, S.; Otsuka, K.; Murdey, R.; Nakamura, T.; Truong, M.A.; Yamada, T.; Handa, T.; Matsuda, K.; Nakano, K.; Sato, A.; et al. Optimized Carrier Extraction at Interfaces for 23.6% Efficient Tin-Lead Perovskite Solar Cells. Energy Environ. Sci. 2022, 15, 2096–2107. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Wang, C.; Liao, W.; Shrestha, N.; Grice, C.R.; Cimaroli, A.J.; Guan, L.; Ellingson, R.J.; Zhu, K.; et al. Low-Bandgap Mixed Tin-Lead Iodide Perovskite Absorbers with Long Carrier Lifetimes for All-Perovskite Tandem Solar Cells. Nat. Energy 2017, 2, 17018. [Google Scholar] [CrossRef]
- Wang, J.; Uddin, M.A.; Chen, B.; Ying, X.; Ni, Z.; Zhou, Y.; Li, M.; Wang, M.; Yu, Z.; Huang, J. Enhancing Photostability of Sn-Pb Perovskite Solar Cells by an Alkylammonium Pseudo-Halogen Additive. Adv. Energy. Mater. 2023, 13, 2204115. [Google Scholar] [CrossRef]
- Lian, X.; Chen, J.; Zhang, Y.; Qin, M.; Li, J.; Tian, S.; Yang, W.; Lu, X.; Wu, G.; Chen, H. Highly Efficient Sn/Pb Binary Perovskite Solar Cell via Precursor Engineering: A Two-Step Fabrication Process. Adv. Funct. Mater. 2019, 29, 1807024. [Google Scholar] [CrossRef]
- Wang, J.; Datta, K.; Li, J.; Verheijen, M.A.; Zhang, D.; Wienk, M.M.; Janssen Rene, A.J. Understanding the Film Formation Kinetics of Sequential Deposited Narrow-Bandgap Pb-Sn Hybrid Perovskite Films. Adv. Energy Mater. 2020, 10, 2000566. [Google Scholar] [CrossRef]
- Xing, Y.; Deng, Z.; Wang, Q.; Xiong, J.; Liu, X.; Huang, L.; Zhu, Y.; Zhang, J. Polymer Lewis Base for Improving the Charge Transfer in Tin-Lead Mixed Perovskite Solar Cells. Nanomaterials 2024, 14, 437. [Google Scholar] [CrossRef]
- Xu, Z.; Lu, D.; Dong, X.; Chen, M.; Fu, Q.; Liu, Y. Highly Efficient and Stable Dion-Jacobson Perovskite Solar Cells Enabled by Extended π-Conjugation of Organic Spacer. Adv. Mater. 2021, 33, 2105083. [Google Scholar] [CrossRef]
- Garai, R.; Gupta, R.K.; Tanwar, A.S.; Hossain, M.; Iyer, P.K. Conjugated Polyelectrolyte-Passivated Stable Perovskite Solar Cells for Efficiency Beyond 20%. Chem. Mater. 2021, 33, 5709–5717. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, F.; Li, S.; Liu, Y.; Ren, J.; Wu, Y.; Sun, Q.; Hao, Y. Regulating the Crystallization Growth of Sn-Pb Mixed Perovskites Using the 2D Perovskite (4-AMP)PbI4 Substrate for High-Efficiency and Stable Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 34862–34873. [Google Scholar] [CrossRef]
- Yang, D.; Yang, R.; Ren, X.; Zhu, X.; Yang, Z.; Li, C.; Liu, S. Hysteresis-Suppressed High-Efficiency Flexible Perovskite Solar Cells Using Solid-State Ionic-Liquids for Effective Electron Transport. Adv. Mater. 2016, 28, 5206–5213. [Google Scholar] [CrossRef]

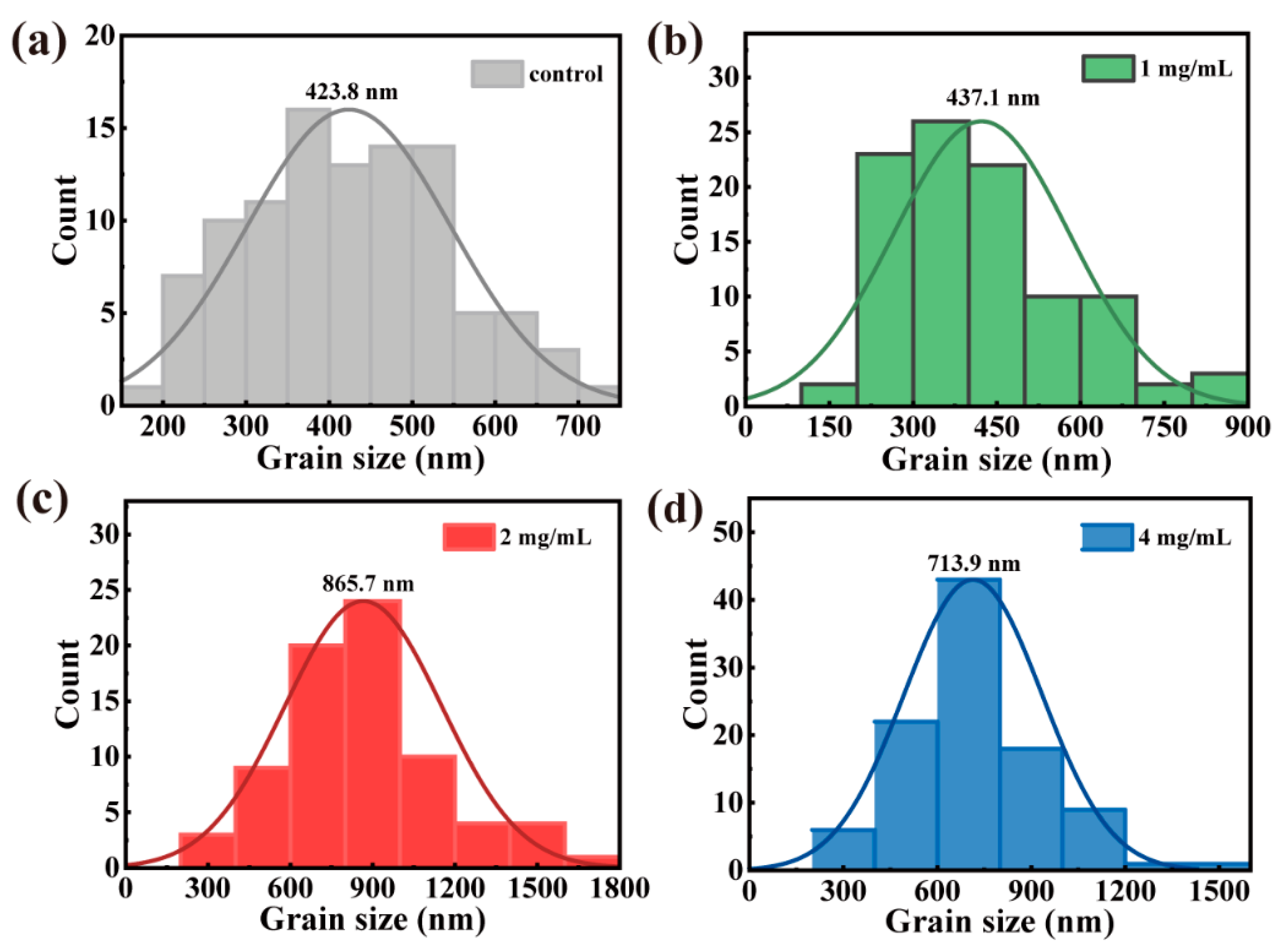
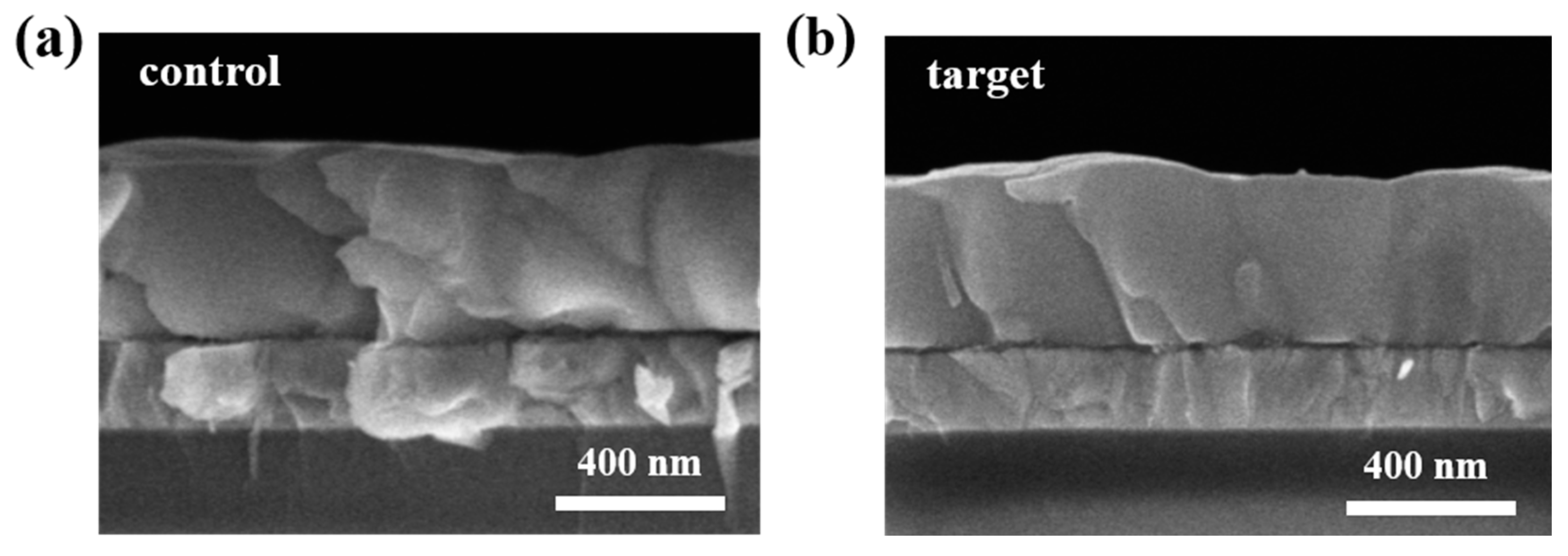
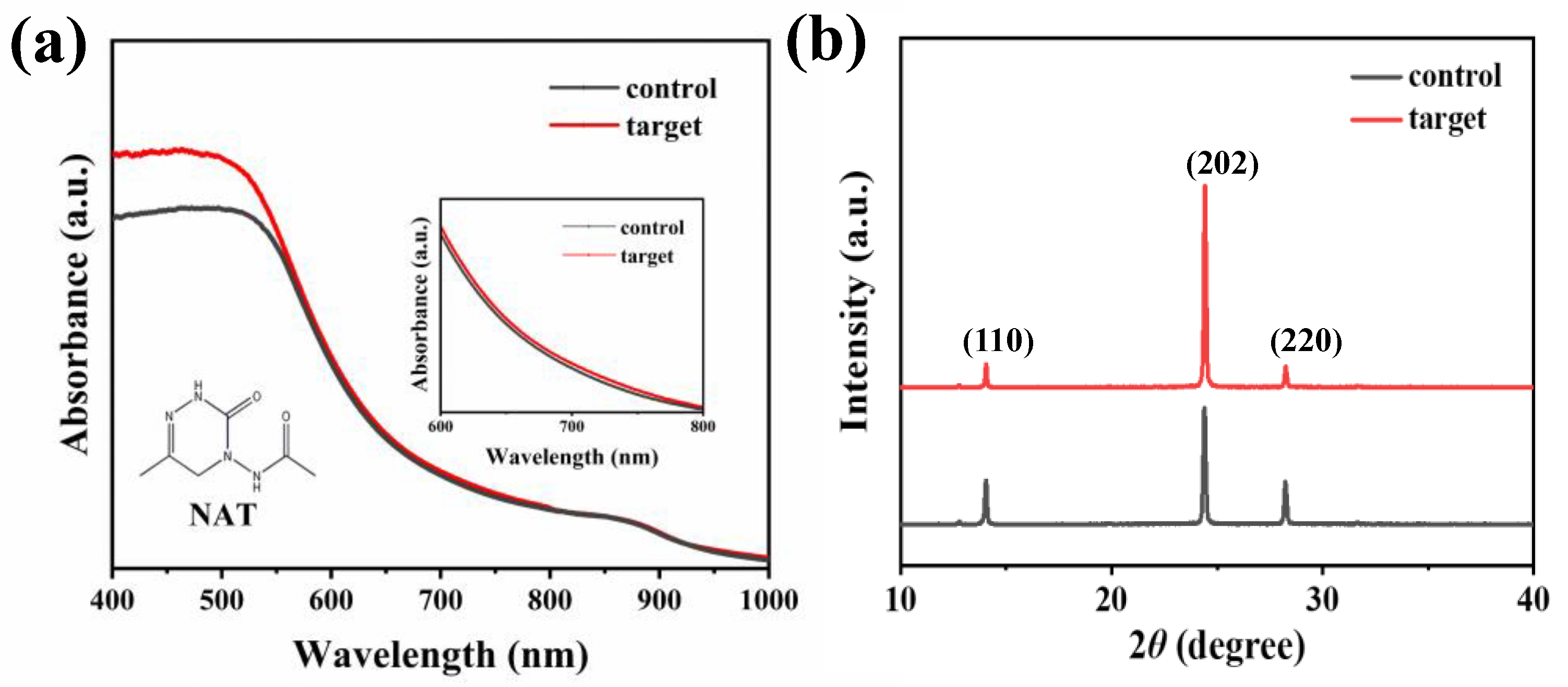
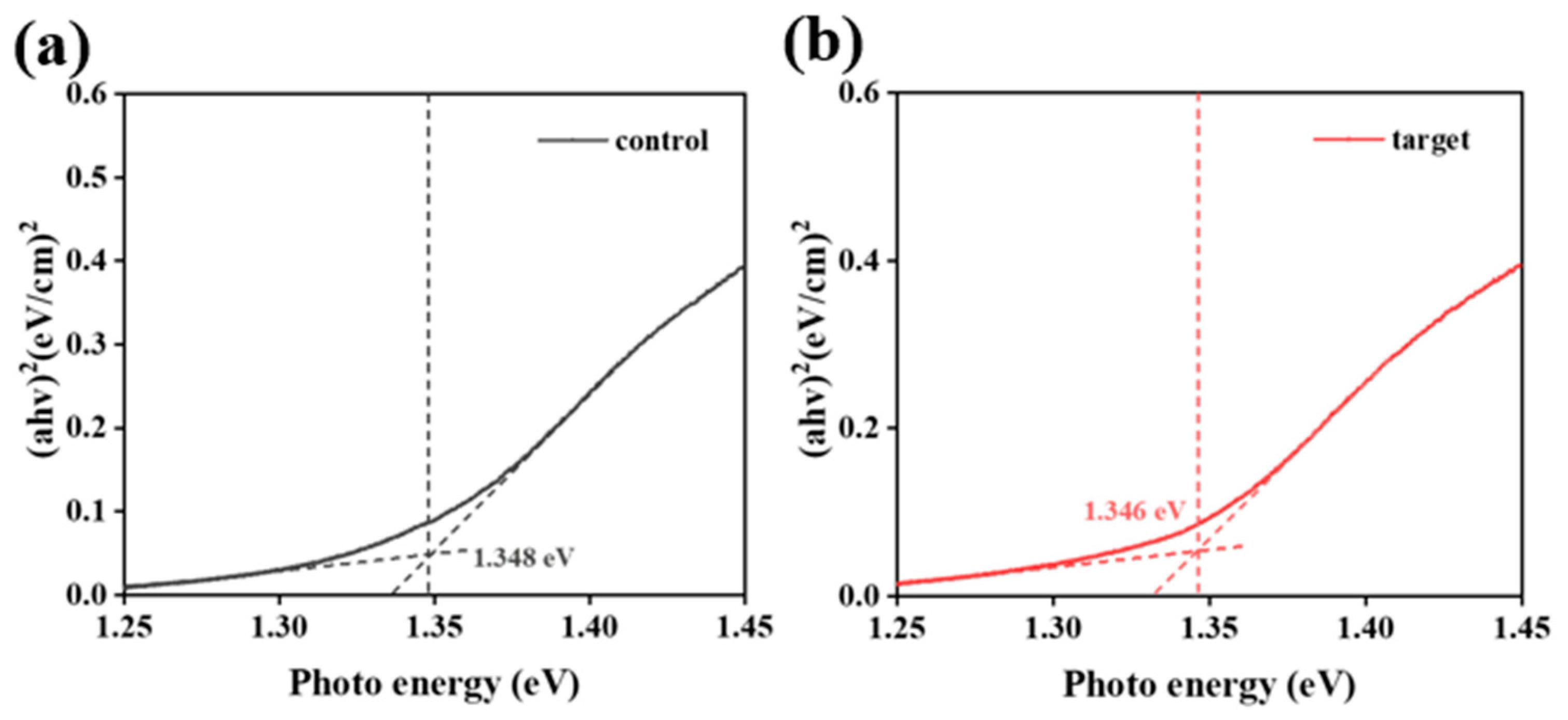
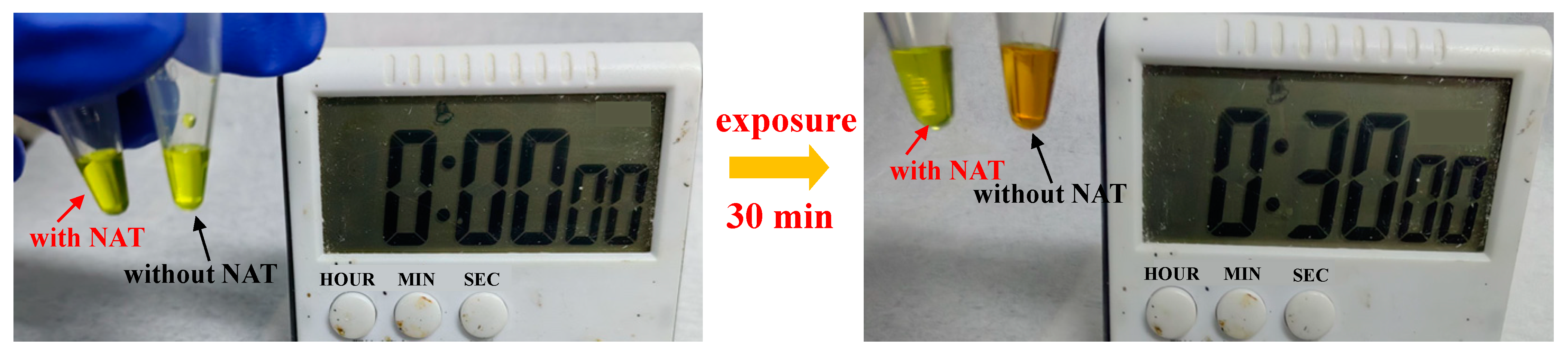
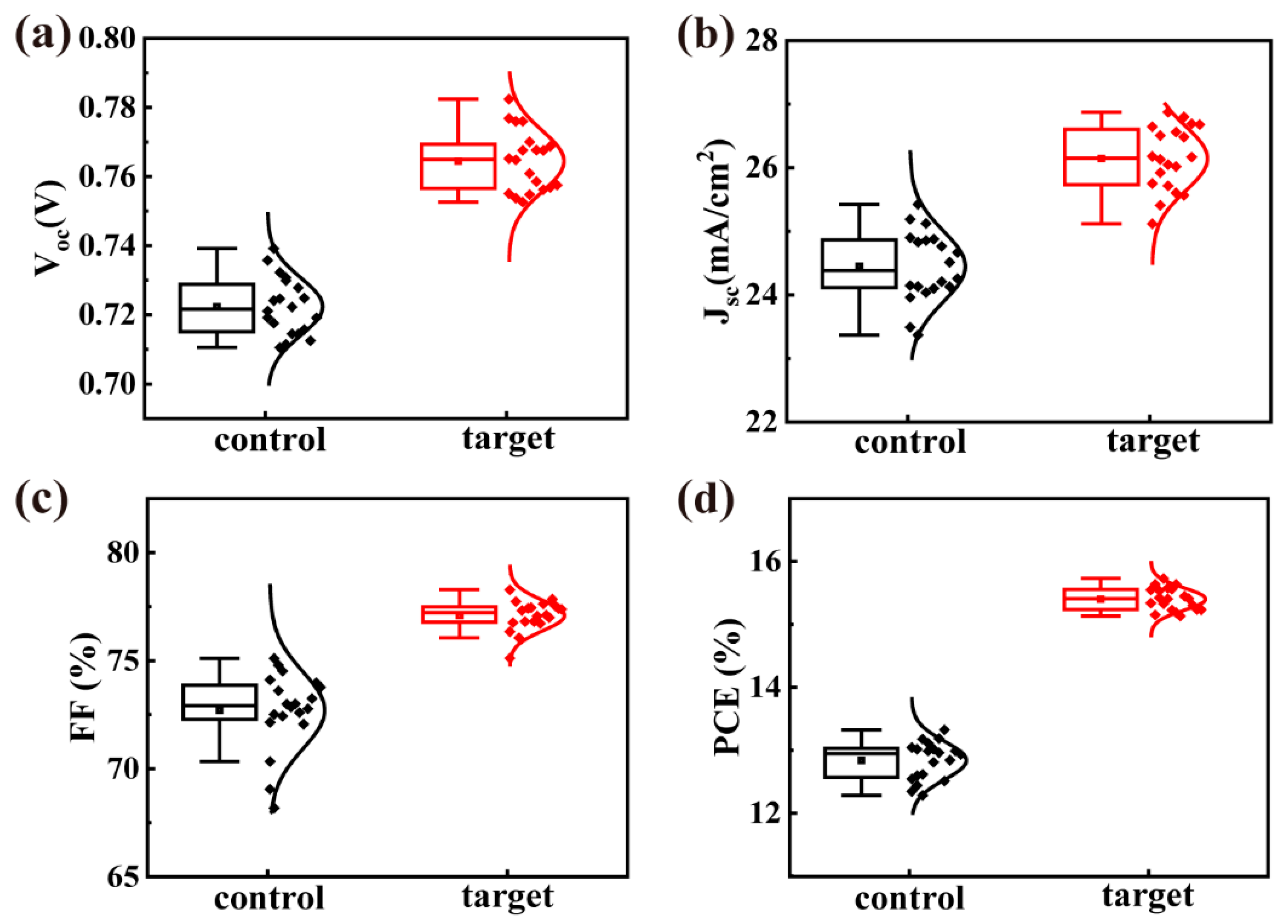

| Sample | JSC (mA/cm2) | VOC (V) | FF | PCE (%) |
|---|---|---|---|---|
| Control | 24.67 24.45 ± 0.55 | 0.72 0.72 ± 0.01 | 0.75 0.73 ± 0.02 | 13.32 12.83 ± 0.30 |
| 1 mg/mL | 24.77 24.74 ± 0.28 | 0.74 0.72 ± 0.01 | 0.76 0.75 ± 0.01 | 13.97 13.50 ± 0.20 |
| 2 mg/mL | 26.80 26.14 ± 0.51 | 0.76 0.76 ± 0.01 | 0.78 0.77 ± 0.01 | 15.73 15.40 ± 0.18 |
| 4 mg/mL | 25.85 25.67 ± 0.52 | 0.76 0.76 ± 0.01 | 0.77 0.76 ± 0.01 | 15.18 14.80 ± 0.27 |
| Sample | JSC (mA/cm2) | VOC (V) | FF | PCE (%) | HI (%) |
|---|---|---|---|---|---|
| Control (Forward) | 24.46 | 0.72 | 0.72 | 12.73 | 4.43 |
| Control (Reverse) | 24.67 | 0.72 | 0.75 | 13.32 | |
| Target (Forward) | 26.69 | 0.76 | 0.77 | 15.57 | 1.01 |
| Target (Reverse) | 26.80 | 0.76 | 0.78 | 15.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Ma, F.; Hao, Y.; Wu, H.; Zhu, P.; Li, Z.; Li, F.; Yu, J.; Liu, M.; Lei, C.; et al. Defect Passivation for Highly Efficient and Stable Sn-Pb Perovskite Solar Cells. Crystals 2024, 14, 802. https://doi.org/10.3390/cryst14090802
Li T, Ma F, Hao Y, Wu H, Zhu P, Li Z, Li F, Yu J, Liu M, Lei C, et al. Defect Passivation for Highly Efficient and Stable Sn-Pb Perovskite Solar Cells. Crystals. 2024; 14(9):802. https://doi.org/10.3390/cryst14090802
Chicago/Turabian StyleLi, Tengteng, Fupeng Ma, Yafeng Hao, Huijia Wu, Pu Zhu, Ziwei Li, Fengchao Li, Jiangang Yu, Meihong Liu, Cheng Lei, and et al. 2024. "Defect Passivation for Highly Efficient and Stable Sn-Pb Perovskite Solar Cells" Crystals 14, no. 9: 802. https://doi.org/10.3390/cryst14090802
APA StyleLi, T., Ma, F., Hao, Y., Wu, H., Zhu, P., Li, Z., Li, F., Yu, J., Liu, M., Lei, C., & Liang, T. (2024). Defect Passivation for Highly Efficient and Stable Sn-Pb Perovskite Solar Cells. Crystals, 14(9), 802. https://doi.org/10.3390/cryst14090802







