A Review on the Synthesis of Carbon Dots and Their Applications in Environmental Analysis
Abstract
1. Introduction
2. Synthesis of CDs
2.1. Top-Down
| Methods | Advantages | Disadvantages | Refs. | |
|---|---|---|---|---|
| Bottom-up | Thermal decomposition | More time-saving, easy to operate, low cost, large-scale production | Wide size distribution | [38] |
| Hydrothermal treatment | Cheap, eco-friendly, lack of toxicity, low cost | Low yield | [39] | |
| Microwave synthesis | Fast, low cost, eco-friendly | Poor size control | [40] | |
| Top-down | Electrochemical/ chemical oxidation | High yield, high purity, low cost, control over size | Few small molecule precursors | [41] |
| Arc discharge | Fabricate carbon NPs in various gases | Require complex purification | [36] | |
| Laser ablation | convenient size control and photolumicense property | Costly, sophisticated process | [42] | |
| Ultrasonic treatment | Convenient to break large carbon materials, good dispersion, low crystallinity | High energy cost | [1] | |
2.2. Bottom-Up
3. Properties of CDs
4. Application of CDs
4.1. Sensing Metal Ions
4.2. Sensing of Emerging Contaminants
4.3. Photocatalysts
4.4. Application of CDs in Other Aspects
5. Conclusions and Future Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; He, X.; Lv, Y.; Wang, J.; Wang, L.; Yang, S.; Yan, A.; Wang, S.; Guo, P.; Wang, M. A review of carbon dots in synthesis, property and application. Mater. Today Commun. 2025, 44, 111824. [Google Scholar] [CrossRef]
- Kadamannil, N.N.; Shames, A.I.; Bisht, R.; Biswas, S.; Shauloff, N.; Lee, H.; Kim, J.-M.; Jelinek, R. Light-Induced Self-Assembled Polydiacetylene/Carbon Dot Functional “Honeycomb”. ACS Appl. Mater. Inter. 2024, 16, 22593–22603. [Google Scholar] [CrossRef] [PubMed]
- Shauloff, N.; Bisht, R.; Turkulets, Y.; Manikandan, R.; Morag, A.; Lehrer, A.; Baraban, J.H.; Shalish, I.; Jelinek, R. Multispectral and Circular Polarization-Sensitive Carbon Dot-Polydiacetylene Capacitive Photodetector. Small 2023, 19, 2206519. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Phatake, R.S.; Nabha Barnea, S.; Zerby, N.; Zhu, J.-J.; Shikler, R.; Lemcoff, N.G.; Jelinek, R. Fluorescent self-healing carbon dot/polymer gels. ACS Nano 2019, 13, 1433–1442. [Google Scholar] [CrossRef]
- Arad, E.; Bhunia, S.K.; Jopp, J.; Kolusheva, S.; Rapaport, H.; Jelinek, R. Lysine-derived carbon dots for chiral inhibition of prion peptide fibril assembly. Adv. Ther. 2018, 1, 1800006. [Google Scholar] [CrossRef]
- Bhunia, S.K.; Maity, A.R.; Nandi, S.; Stepensky, D.; Jelinek, R. Imaging cancer cells expressing the folate receptor with carbon dots produced from folic acid. ChemBioChem 2016, 17, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Liu, Y.; Opoku, H.; Gregorsson, M.; Zhang, P.; Auroux, E.; Dang, D.; Mudring, A.-V.; Wågberg, T.; Edman, L. Correction: Fluorescent carbon dots from birch leaves for sustainable electroluminescent devices. Green Chem. 2025, 27, 2776–2777. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Nguyen, D.H.; El-Ramady, H.; Prokisch, J. Food safety aspects of carbon dots: A review. Environ. Chem. Lett. 2025, 23, 337–360. [Google Scholar] [CrossRef]
- Kong, J.; Wei, Y.; Zhou, F.; Shi, L.; Zhao, S.; Wan, M.; Zhang, X. Carbon quantum dots: Properties, preparation, and applications. Molecules 2024, 29, 2002. [Google Scholar] [CrossRef]
- Krishnaiah, P.; Atchudan, R.; Gangadaran, P.; Perumal, S.; Rajendran, R.L.; Sundramoorthy, A.K.; Kumar, R.S.; Ramalingam, S.; Ahn, B.-C.; Lee, S.W. A sustainable synthesis and applications of biomass waste-derived tunable fluorescent carbon dots: In Vitro and in vivo fluorescent imaging. J. Photochem. Photobiol. A Chem. 2025, 458, 115944. [Google Scholar] [CrossRef]
- Mohandoss, S.; Velu, K.S.; Wahab, R.; Ahmad, N.; Palanisamy, S.; You, S.; Roy, P.; Lee, Y.R. One-step hydrothermal synthesis of F, N, S-doped photoluminescent carbon dots with yellowish-orange emission for sensitive Cu2+ ion detection: Environmental and biomedical applications. J. Photochem. Photobiol. A Chem. 2025, 459, 116037. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Lin, C.; Hou, Y.; Cheng, S.; Gao, B. Green synthesis of red-emitting carbon dots for bioimaging, sensing, and antibacterial applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 328, 125458. [Google Scholar] [CrossRef]
- Vadia, F.Y.; Jha, S.; Mehta, V.N.; Park, T.J.; Malek, N.I.; Kailasa, S.K. Development of sustainable fluorescence approach with red emissive carbon dots derived from Grewia asiatica fruit for the detection of quinalphos. J. Photochem. Photobiol. A Chem. 2025, 458, 115948. [Google Scholar] [CrossRef]
- Wang, Q.; He, X.; Mao, J.; Wang, J.; Wang, L.; Zhang, Z.; Li, Y.; Huang, F.; Zhao, B.; Chen, G. Carbon Dots: A Versatile Platform for Cu2+ Detection, Anti-Counterfeiting, and Bioimaging. Molecules 2024, 29, 4211. [Google Scholar] [CrossRef]
- Jiang, M.; Sun, Y.; Chen, M.; Ji, H.; Liu, Y.; Qin, R.; Li, X.; Gao, H.; Zhang, R.; Zhang, L. Multicolor luminescence of carbon Dots: From mechanisms to applications. Chem. Eng. J. 2024, 496, 153761. [Google Scholar] [CrossRef]
- Liu, M. Optical properties of carbon dots: A review. Nanoarchitectonics 2020, 1, 1–12. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, Y.; Sun, X.; Bai, Z.; Zhang, Y.; Zhou, X. Surface modification and chemical functionalization of carbon dots: A review. Microchim. Acta 2018, 185, 424. [Google Scholar] [CrossRef]
- Ren, J.; Opoku, H.; Tang, S.; Edman, L.; Wang, J. Carbon dots: A review with focus on sustainability. Adv. Sci. 2024, 11, 2405472. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2021, 406, 126848. [Google Scholar] [CrossRef]
- Ren, J.; Liu, J.; Wei, B.; Zhang, W.; Edman, L.; Wang, J. Deep-Blue and Narrowband-Emitting Carbon Dots from a Sustainable Precursor for Random Lasing. ACS Appl. Nano Mater. 2025, 8, 2472–2480. [Google Scholar] [CrossRef]
- Hebbar, A.; Selvaraj, R.; Vinayagam, R.; Varadavenkatesan, T.; Kumar, P.S.; Duc, P.A.; Rangasamy, G. A critical review on the environmental applications of carbon dots. Chemosphere 2023, 313, 137308. [Google Scholar] [CrossRef]
- Johny, A.; Pinto da Silva, L.; Pereira, C.M.; Esteves da Silva, J.C. Sustainability assessment of highly fluorescent carbon dots derived from eucalyptus leaves. Environments 2024, 11, 6. [Google Scholar] [CrossRef]
- Rani, M.; Shanker, U.; Kaith, B.S.; Sillanpää, M. Green fabrication of fluorescent N-doped carbon quantum dots from Aegle marmelos leaves for highly selective detection of Fe3+ metal ions. Inorg. Chem. Commun. 2024, 159, 111878. [Google Scholar]
- Singh, J.; Kaur, S.; Lee, J.; Mehta, A.; Kumar, S.; Kim, K.-H.; Basu, S.; Rawat, M. Highly fluorescent carbon dots derived from Mangifera indica leaves for selective detection of metal ions. Sci. Total Environ. 2020, 720, 137604. [Google Scholar] [CrossRef]
- Sharma, V.; Vishal, V.; Chandan, G.; Bhatia, A.; Chakrabarti, S.; Bera, M. Green, sustainable, and economical synthesis of fluorescent nitrogen-doped carbon quantum dots for applications in optical displays and light-emitting diodes. Mater. Today Sustain. 2022, 19, 100184. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Chakradhar, D.; Perumal, S.; Shim, J.-J.; Lee, Y.R. Facile green synthesis of nitrogen-doped carbon dots using Chionanthus retusus fruit extract and investigation of their suitability for metal ion sensing and biological applications. Sens. Actuators B Chem. 2017, 246, 497–509. [Google Scholar] [CrossRef]
- Mohandoss, S.; Velu, K.S.; Wahab, R.; Ahmad, N.; Palanisamy, S.; You, S.; Aslam, M.; Lee, Y.R.; Kim, S.-C. Highly selective and sensitive ratiometric detection of Hg2+ ions with NFS co-doped carbon dots: Real sample analysis, antibacterial properties, and cellular imaging applications. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 326, 125300. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Z.; Fu, J.; Zhang, J.; He, Q.; Lu, H.; Zhou, Q.; Wang, H. Recent Advances in the Synthesis, Characterization, and Application of Carbon Dots in the Field of Wastewater Treatment: A Comprehensive Review. Water 2025, 17, 210. [Google Scholar] [CrossRef]
- Shi, W.; Han, Q.; Wu, J.; Ji, C.; Zhou, Y.; Li, S.; Gao, L.; Leblanc, R.M.; Peng, Z. Synthesis mechanisms, structural models, and photothermal therapy applications of top-down carbon dots from carbon powder, graphite, graphene, and carbon nanotubes. Int. J. Mol. Sci. 2022, 23, 1456. [Google Scholar] [CrossRef]
- Pillar-Little, T.J.; Wanninayake, N.; Nease, L.; Heidary, D.K.; Glazer, E.C.; Kim, D.Y. Superior photodynamic effect of carbon quantum dots through both type I and type II pathways: Detailed comparison study of top-down-synthesized and bottom-up-synthesized carbon quantum dots. Carbon 2018, 140, 616–623. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. A review of carbon dots in synthesis strategy. Coord. Chem. Rev. 2024, 498, 215468. [Google Scholar] [CrossRef]
- Domingo-Tafalla, B.; Martínez-Ferrero, E.; Franco, F.; Palomares-Gil, E. Applications of carbon dots for the photocatalytic and electrocatalytic reduction of CO2. Molecules 2022, 27, 1081. [Google Scholar] [CrossRef]
- Nagarajan, D.; Gangadharan, D.; Venkatanarasimhan, S. Synthetic strategies toward developing carbon dots via top-down approach. In Carbon Dots in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–13. [Google Scholar]
- Liu, M.L.; Xu, Y.H.; Niu, F.S.; Gooding, J.J.; Liu, J.Q. Carbon quantum dots directly generated from electrochemical oxidation of graphite electrodes in alkaline alcohols and the applications for specific ferric ion detection and cell imaging. Analyst 2016, 141, 2657–2664. [Google Scholar] [CrossRef]
- Chao-Mujica, F.; Garcia-Hernández, L.; Camacho-López, S.; Camacho-López, M.; Camacho-López, M.; Reyes Contreras, D.; Pérez-Rodríguez, A.; Peña-Caravaca, J.; Páez-Rodríguez, A.; Darias-Gonzalez, J. Carbon quantum dots by submerged arc discharge in water: Synthesis, characterization, and mechanism of formation. J. Appl. Phys. 2021, 129, 163301. [Google Scholar] [CrossRef]
- Torrisi, L.; Cutroneo, M.; Silipigni, L.; Torrisi, A.; Signorile, A.; Manno, D.; Serra, A. Luminescent carbon dots structure by charcoal laser ablation in biocompatible liquid. Fuller. Nanotub. Carbon Nanostruct. 2025, 1–11. [Google Scholar] [CrossRef]
- Shaik, S.A.; Sengupta, S.; Varma, R.S.; Gawande, M.B.; Goswami, A. Syntheses of N-doped carbon quantum dots (NCQDs) from bioderived precursors: A timely update. ACS Sustain. Chem. Eng. 2020, 9, 3–49. [Google Scholar] [CrossRef]
- Khairol Anuar, N.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Abu Bakar, N.F. A review on multifunctional carbon-dots synthesized from biomass waste: Design/fabrication, characterization and applications. Front. Energy Res. 2021, 9, 626549. [Google Scholar] [CrossRef]
- Biswal, M.R.; Bhatia, S. Carbon dot nanoparticles: Exploring the potential use for gene delivery in ophthalmic diseases. Nanomaterials 2021, 11, 935. [Google Scholar] [CrossRef]
- Khayal, A.; Dawane, V.; Amin, M.A.; Tirth, V.; Yadav, V.K.; Algahtani, A.; Khan, S.H.; Islam, S.; Yadav, K.K.; Jeon, B.-H. Advances in the methods for the synthesis of carbon dots and their emerging applications. Polymers 2021, 13, 3190. [Google Scholar] [CrossRef]
- Ozyurt, D.; Al Kobaisi, M.; Hocking, R.K.; Fox, B. Properties, synthesis, and applications of carbon dots: A review. Carbon Trends 2023, 12, 100276. [Google Scholar] [CrossRef]
- Li, T.; Dong, Y.; Yan, Q.; Liu, Z.; Zeng, H. Rapid microwave preparation of nitrogen doped carbon dots and their applications as highly sensitive nanoprobe for hematin. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135634. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, H.; Wang, M.; Yang, X.; Pang, S. N and S doped carbon dots as novel probes with fluorescence enhancement for fast and sensitive detection of Cr (VI). Colloids Surf. A Physicochem. Eng. Asp. 2022, 638, 128164. [Google Scholar] [CrossRef]
- Ye, R.; Li, H.; Zhou, X. Facile preparation of S, N co-doped carbon dots and the application as an “on-off-on” fluorescent sensor for detecting Cr (VI) and ascorbic acid/sulfide. J. Mater. Sci. Mater. Electron. 2024, 35, 378. [Google Scholar] [CrossRef]
- Keerthana, P.; Das, A.K.; Bharath, M.; Ghosh, M.; Varghese, A. A ratiometric fluorescent sensor based on dual-emissive carbon dot for the selective detection of Cd2+. J. Environ. Chem. Eng. 2023, 11, 109325. [Google Scholar] [CrossRef]
- Ma, H.; Guan, L.; Chen, M.; Zhang, Y.; Wu, Y.; Liu, Z.; Wang, D.; Wang, F.; Li, X. Synthesis and enhancement of carbon quantum dots from Mopan persimmons for Fe3+ sensing and anti-counterfeiting applications. Chem. Eng. J. 2023, 453, 139906. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, L.; Cui, C.; Hu, Y.; Chen, Z.; Zhan, Y.; Zhang, Y. Nitrogen-doped carbon dots as fluorescent probes for sensitive and selective determination of Fe3+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 316, 124347. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, F.; Liao, Y.; Wang, F.; Liu, H. Carbon quantum dots from pomelo peel as fluorescence probes for “turn-off–on” high-sensitivity detection of Fe3+ and L-cysteine. Molecules 2022, 27, 4099. [Google Scholar] [CrossRef]
- Mu, M.; Duan, Z.; Fan, S.; Zhao, W.; Gao, W.; Bai, R.; Li, Y.; Kang, Y. Detection of ferric ions by nitrogen and sulfur co-doped potato-derived carbon quantum dots as a fluorescent probe. Mater. Res. Express 2024, 11, 045501. [Google Scholar] [CrossRef]
- Ahmad, A.; Shahraki, H.S.; Khan, N.; Ahmad, M.; Bushra, R. One-pot synthesized fluorescent CDs from Syzygium cumini for metal ion sensing and cell imaging. Inorg. Chem. Commun. 2024, 160, 111883. [Google Scholar]
- Zhu, L.; Shen, D.; Liu, Q.; Wu, C.; Gu, S. Sustainable synthesis of bright green fluorescent carbon quantum dots from lignin for highly sensitive detection of Fe3+ ions. Appl. Surf. Sci. 2021, 565, 150526. [Google Scholar] [CrossRef]
- Patra, S.; Singh, M.; Subudhi, S.; Mandal, M.; Nayak, A.K.; Sahu, B.B.; Mahanandia, P. One-step green synthesis of in–situ functionalized carbon quantum dots from Tagetes patula flowers: Applications as a fluorescent probe for detecting Fe3+ ions and as an antifungal agent. J. Photochem. Photobiol. A Chem. 2023, 442, 114779. [Google Scholar] [CrossRef]
- McEnroe, A.; Brunt, E.; Mosleh, N.; Yu, J.; Hailstone, R.; Sun, X. Bright, green fluorescent carbon dots for sensitive and selective detection of ferrous ions. Talanta Open 2023, 7, 100236. [Google Scholar] [CrossRef]
- Babu, A.M.; Bijoy, G.; Keerthana, P.; Varghese, A. Fluorescent detection of Pb2+ pollutant in water samples with the help of Delonix regia leaf-derived CQDs. Synth. Met. 2022, 291, 117211. [Google Scholar] [CrossRef]
- Zhang, M.; Yin, H.; Wan, L.; Gao, H.; Liu, S.; Liu, Y. The fluorescent and colorimetric dual-response sensor based on carbon dots doped with nitrogen and sulfur for detecting copper ions. Carbon Lett. 2024, 34, 1155–1164. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, X.; Liu, M.; Wang, W. Blue, yellow, and red carbon dots from aromatic precursors for light-emitting diodes. Molecules 2023, 28, 2957. [Google Scholar] [CrossRef]
- Zeng, J.; Liao, L.; Lin, X.; Liu, G.; Luo, X.; Luo, M.; Wu, F. Red-emissive sulfur-doped carbon dots for selective and sensitive detection of mercury (II) ion and glutathione. Int. J. Mol. Sci. 2022, 23, 9213. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, M.; Liu, X.; Feng, S.; Zhu, Q.; Li, S.; Zhang, X. N-Doping CQDs as an efficient fluorescence probe based on dynamic quenching for determination of copper ions and alcohol sensing in baijiu. J. Fluoresc. 2024, 1–13. [Google Scholar] [CrossRef]
- Chaghaghazardi, M.; Kashanian, S.; Nazari, M.; Omidfar, K.; Joseph, Y.; Rahimi, P. Nitrogen and sulfur co-doped carbon quantum dots fluorescence quenching assay for detection of mercury (II). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 293, 122448. [Google Scholar] [CrossRef]
- Jaison, A.M.C.; Vasudevan, D.; Ponmudi, K.; George, A.; Varghese, A. One pot hydrothermal synthesis and application of bright-yellow-emissive carbon quantum dots in Hg2+ detection. J. Fluoresc. 2023, 33, 2281–2294. [Google Scholar] [CrossRef]
- John, B.K.; Korah, B.K.; Mathew, S.; Thara, C.; Chacko, A.R.; Mathew, B. Nitrogen and sulphur co-doped carbon quantum dots as a dual-mode sensor for mercuric ions and as efficient antimicrobial agents. Biomass Convers. Biorefinery 2023, 14, 20171–20187. [Google Scholar] [CrossRef]
- Srivastava, A.; Khan, Z.M.; Akhtar, M.S.; Khan, S.A. Nitrogen self-doped desiccated coconut–derived carbon dots as optical nanoprobe sensor for the detection of heavy metal ion Hg2+. Emergent Mater. 2024, 7, 1819–1829. [Google Scholar] [CrossRef]
- Kumar, R.; Vincy, A.; Rani, K.; Jain, N.; Singh, S.; Agarwal, A.; Vankayala, R. Facile synthesis of multifunctional carbon dots derived from camel milk for Mn7+ sensing and antiamyloid and anticancer activities. ACS Omega 2023, 8, 36521–36533. [Google Scholar] [CrossRef]
- Wang, B.; Lu, S. The light of carbon dots: From mechanism to applications. Matter 2022, 5, 110–149. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, R.; Feng, B.; Zhong, X.; Ostrikov, K. Photoluminescence mechanism of carbon dots: Triggering high-color-purity red fluorescence emission through edge amino protonation. Nat. Commun. 2021, 12, 6856. [Google Scholar] [CrossRef]
- Ai, L.; Yang, Y.; Wang, B.; Chang, J.; Tang, Z.; Yang, B.; Lu, S. Insights into photoluminescence mechanisms of carbon dots: Advances and perspectives. Sci. Bull. 2021, 66, 839–856. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Li, B.; Yang, K.; Lan, M.; Zeng, L. Advances and perspectives in carbon dot-based fluorescent probes: Mechanism, and application. Coord. Chem. Rev. 2021, 431, 213686. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Zattar, A.P.P.; de Mesquita, J.P.; Pereira, F.V. Luminescent carbon dots obtained from cellulose and their applications as sensors for metal ions. Mater. Chem. Phys. 2022, 290, 126633. [Google Scholar] [CrossRef]
- Pinto, T.d.S.; Paula, E.L.d.; Mesquita, J.P.d.; Pereira, F.V. Carbon dots prepared by different bottom-up methods: A study on optical properties and the application as nanoprobes for metal ions detection. Fuller. Nanotub. Carbon Nanostruct. 2023, 31, 641–651. [Google Scholar] [CrossRef]
- Taborda, N.C.; Ferreira, A.H.; Pereira, F.V. Luminescent carbon dots obtained from different precursors and methods and their applications as sensors for metal ions. Fuller. Nanotub. Carbon Nanostruct. 2023, 31, 231–240. [Google Scholar] [CrossRef]
- da Silva Pinto, T.; Ferreira, A.H.; Taborda, N.C.; de Mesquita, J.P.; Freitas, E.T.F.; do Amparo, S.Z.S.; Soares, E.A.; Donnici, C.L.; de Paula, E.L.; Pereira, F.V. Structure-property relationship between different molecular precursors and the final properties of carbon dots: Preparation, characterization and applications as sensors for metal ions. Opt. Mater. 2024, 156, 115999. [Google Scholar] [CrossRef]
- Kanwal, A.; Bibi, N.; Hyder, S.; Muhammad, A.; Ren, H.; Liu, J.; Lei, Z. Recent advances in green carbon dots (2015–2022): Synthesis, metal ion sensing, and biological applications. Beilstein J. Nanotechnol. 2022, 13, 1068–1107. [Google Scholar] [CrossRef]
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.-H. Carbon dots as an effective fluorescent sensing platform for metal ion detection. Nanoscale Res. Lett. 2019, 14, 272. [Google Scholar] [CrossRef]
- Tang, D.; Lu, Y.; Feng, W.; Tian, D.; Li, W. Detection of Cr (VI) and Hg (II) by fluorescent carbon dots based on polyester-cotton blended fabrics. J. Instrum. Anal. 2024, 43, 440–446. [Google Scholar]
- Wang, H.; Han, L.; Gao, Y.; Gao, X.; He, W.; Shan, C.; Fan, W.; Guo, Y.; Gao, M.; Cheng, H. Preparation of Carbon Dots with High Fluorescence Quantum Yields and its Detection of Hg2+ in Simulated Wastewater. J. Fluoresc. 2025, 1–10. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Jiang, T.; Ran, G.; Song, Q. Cadmium induced aggregation of orange–red emissive carbon dots with enhanced fluorescence for intracellular imaging. J. Hazard. Mater. 2022, 427, 128092. [Google Scholar] [CrossRef]
- Raypah, M.E.; Jamlos, M.F.; Giwa, H.S.; Ahmad, H.F.; Abd Hamid, H. Green synthesis of carbon dots derived from biomass of Polyalthia bullata root extract as colorimetric sensor for selective detection of Fe3+ ions. Inorg. Chem. Commun. 2025, 174, 113944. [Google Scholar] [CrossRef]
- Başoğlu, A. One-Step Green Hydrothermal-Assisted Synthesis of Carbon Quantum Dots from Robinia hispida L. Flowers, and Flourimetric Detection of Au3+ Ions in Aqueous Media. Luminescence 2025, 40, e70099. [Google Scholar] [CrossRef] [PubMed]
- Sudan, S.; Kaushal, J.; Singh, T.G.; Mahmoud, M.H.; Alexiou, A.; Papadakis, M.; Fetoh, M.E.A.-E.; Batiha, G.E.-S. Eco-friendly sensing of hexavalent chromium ions via copper-doped carbon quantum dots: A fluorescent probe for water safety. Microchim. Acta 2025, 192, 88. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, M.; Wang, Y.; Mao, C.; Zhang, Y.; Liu, J.; Zhang, X.; Pang, S.; Yang, X. Construction of carbon dot-embedded fluorescent hydrogels based on oxidized gum arabic-gelatin Schiff base for Cr (VI) detection applications. Int. J. Biol. Macromol. 2025, 294, 139466. [Google Scholar] [CrossRef] [PubMed]
- Adotey, E.K.; Amouei Torkmahalleh, M.; Hopke, P.K.; Balanay, M.P. N, Zn-doped fluorescent sensor based on carbon dots for the subnanomolar detection of soluble Cr (VI) ions. Sensors 2023, 23, 1632. [Google Scholar] [CrossRef]
- Xie, J.; Wu, Z.; Sun, J.; Lv, C.; Sun, Q. Green synthesis of carbon quantum dots derived from Lycium barbarum for effective fluorescence detection of Cr (VI) sensing. J. Fluoresc. 2024, 34, 571–578. [Google Scholar] [CrossRef]
- Lu, P.; Liu, B.; Duan, J.; Wei, S.; Zhang, H.; Wang, J.; Guo, H.; Guo, Y.; Jiang, C.; Sun, G. Surface state dominated and carbon core coordinated red-emitting carbon dots for the detection of Cr2O72− and cell imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 283, 121656. [Google Scholar] [CrossRef]
- Jin, X.; Bai, H.; Ma, Y.; Li, Y.; Chen, W. Green synthesis of biomass-based fluorescent carbon dots for the detection and adsorption of Fe (III). ChemistrySelect 2023, 8, e202204852. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, L.; Hou, Y.; Long, N.; Zhu, G.; Liao, X.; Zhou, L.; Lu, J.; Kong, W. A nitrogen-doped carbon dots based fluorescent nanosensor for sensitive assay of Fe3+ ions in Dioscorea opposita Thunb. Ind. Crops Prod. 2022, 177, 114439. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, I.; Kumar, S.; Sharma, A.; Sharma, A.; Gathania, A.K. A dual-mode fluorescent probe for temperature-sensing and metal ions detection based on castor leaves-derived carbon quantum dots. Carbon Lett. 2025, 1–16. [Google Scholar] [CrossRef]
- Pei, S.; Cai, S.; Yan, K.; Zhou, J.; Luo, K.; Chen, X. Synthesis of N-doped Carbon Quantum Dots as an Effective Fluorescent Sensor of Fe3+ Ions and a Potent Antibacterial Agent. J. Fluoresc. 2025, 1–10. [Google Scholar] [CrossRef]
- Wang, M.; Dong, X.; Guo, B.; Wang, D.; Tang, Y. “Turn-on-off” Fluorescent Probes Based on Carbon Nanoparticles for Hypochlorite and Fe2+ Detection. J. Fluoresc. 2025, 1–11. [Google Scholar] [CrossRef]
- Singh, J.; Bhattu, M.; Verma, M.; Bechelany, M.; Brar, S.K.; Jadeja, R. Sustainable Valorization of Rice Straw into Biochar and Carbon Dots Using a Novel One-Pot Approach for Dual Applications in Detection and Removal of Lead Ions. Nanomaterials 2025, 15, 66. [Google Scholar] [CrossRef]
- Kaur, H.; Bhattu, M.; Chakroborty, S.; Aulakh, M.K.; Mutreja, V.; Verma, M.; Tiwari, K.; Chakraborty, C.; Darwish, I.A. Highly Green Fluorescent Carbon Dots from Gallic Acid: A Turn-On Sensor toward Pb2+ Ions. ACS Omega 2025, 10, 2354–2363. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Ramzy, E.; Toubar, S.; Mahmoud, A.M.; Helmy, M.I. Rational Synthesis of Highly Fluorescent N, S Co-Doped Carbon Dots Using Biogenic Creatinine for Cu2+ Analysis in Drinking Water. Luminescence 2025, 40, e70079. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Zhou, X.; Hao, X.; Yang, F.; Zhang, W.; Feng, X.; Yu, H.; Cui, J.; Gao, J.; Xiong, Y. Ultrasensitive and Selective Nitrogen-Doped Fluorescent Carbon Dots Probe for Quantification Analysis of Trace Cu2+ in the Aqueous Environment. J. Fluoresc. 2025, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, S.; Sun, W.; Zhou, L.; Deng, Y.; Zhao, Q. Nitrogen-Doped Carbon Dots Prepared via Microchannel Method for Visual Detection of Copper Ions. Luminescence 2025, 40, e70113. [Google Scholar] [CrossRef]
- Mathew, S.; Mathew, B. Heteroatom doped carbon dots from green sources as metal ion sensor and as fluorescent ink. Diam. Relat. Mater. 2023, 139, 110293. [Google Scholar] [CrossRef]
- Li, Z.; Lin, L.; Shen, J.; Li, C.-D.; Ostrikov, K.K. Microflow synthesis of fluorescent carbon dots for selective Co2+ detection. Ind. Eng. Chem. Res. 2024, 63, 4420–4429. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Ma, C.; Gu, J.; Yang, T.; Li, L.; Gao, H.; Xiong, Y.; Zhu, C.; Hu, A. A fluorescent probe based on carbon quantum dots with spectral selectivity for sensitive detection of Cr (VI) and Hg (II) in environmental waters. Dye. Pigment. 2024, 222, 111845. [Google Scholar] [CrossRef]
- Khare, S.; Sohal, N.; Kaur, M.; Maity, B. Deep eutectic solvent-assisted carbon quantum dots from biomass Triticum aestivum: A fluorescent sensor for nanomolar detection of dual analytes mercury (II) and glutathione. Heliyon 2025, 11, e41853. [Google Scholar] [CrossRef]
- Dutta, A.; Begum, W.; Sarkar, S.; Dam, S.; Mandal, U. Highly Luminescent Nitrogen Doped Carbon Quantum Dots for Mercury Ion Sensing with Antibacterial Activity. J. Fluoresc. 2025, 1–14. [Google Scholar] [CrossRef]
- Liu, H.; Wu, S. Blue Fluorescent Carbon Dots Doped with Nitrogen and Sulfur as a Dual-Functional Fluorescent Probe for the Detection of Hg2+ and Chloramphenicol. J. Mol. Struct. 2025, 1329, 141459. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, X.; Guo, H.; Cai, Y.; Zhang, T. Surface Amide–Mediated Synthesis of Bright Blue Fluorescent Carbon Dots for High-Sensitivity Detection of Hg2+ Ions. Luminescence 2025, 40, e70092. [Google Scholar] [CrossRef]
- Thara, C.R.; Mathew, B. Microwave synthesized N-doped carbon dots for dual mode detection of Hg (II) ion and degradation of malachite green dye. Talanta 2024, 268, 125278. [Google Scholar] [CrossRef]
- Korah, B.K.; Thara, C.R.; John, N.; John, B.K.; Mathew, S.; Mathew, B. Microwave abetted synthesis of carbon dots and its triple mode applications in tartrazine detection, manganese ion sensing and fluorescent ink. Food Control 2023, 147, 109608. [Google Scholar] [CrossRef]
- Liao, S.; Hu, J.; Long, X.; Wu, S. Green synthesis of baby pumpkin derived fluorescent carbon dots–a potent label-free probe for Cd2+ determination. ChemistrySelect 2024, 9, e202305174. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, G.; Ma, C.; Yang, T.; Li, L.; Gu, J.; Zhu, C.; Hu, A.; Li, X.; Guan, W. Carbon quantum dots as a turn-on fluorescent probe for the sensitive detection of Cd2+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 317, 124453. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, C.; Li, X.; Han, Y.; Zhang, Y. Facile synthesis of PEG-modified fluorescent carbon dots for highly sensitive detection of Ag+. Dye. Pigment. 2023, 219, 111534. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, D.; Fan, X.; Luo, B.; Jiang, L.; Qin, Y.; Liao, L.; Wang, Y.; Feng, L.; Li, Z. Dual Photoluminescence Emission Chiral Carbon Quantum Dots for Ratiometric and Visual Fluorescent Ag+ Sensing. ACS Appl. Nano Mater. 2025, 8, 1944–1955. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Wu, Y.; Zhou, A.; Jiang, X.; Zhan, Y.; Sun, Z. Nitrogen and boron co-doped carbon dots as a novel fluorescent probe for fluorogenic sensing of Ce4+ and ratiometric detection of Al3+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 282, 121638. [Google Scholar] [CrossRef]
- Chen, B.; Chai, S.; Liu, J.; Liu, C.; Li, Y.; He, J.; Yu, Z.; Yang, T.; Feng, C.; Huang, C. 2, 4, 6-Trinitrophenol detection by a new portable sensing gadget using carbon dots as a fluorescent probe. Anal. Bioanal. Chem. 2019, 411, 2291–2300. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z. Sewage sludge in microwave oven: A sustainable synthetic approach toward carbon dots for fluorescent sensing of para-Nitrophenol. J. Hazard. Mater. 2020, 382, 121048. [Google Scholar] [CrossRef]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Gul, A.R.; Park, C.Y.; Kim, M.W.; Xu, P.; Baek, S.H.; Bhamore, J.R.; Kailasa, S.K.; Park, T.J. Facile synthesis of carbon dots from Tagetes erecta as a precursor for determination of chlorpyrifos via fluorescence turn-off and quinalphos via fluorescence turn-on mechanisms. Chemosphere 2021, 279, 130515. [Google Scholar] [CrossRef]
- Swain, S.; Jena, A.K. Green Synthesis of N, S-Doped Carbon Dots from the Giloy Stem for Fluorimetry Detection of 4-Nitrophenol, Triple-Mode Detection of Congo Red, and Antioxidant Applications. ACS Omega 2025, 10, 5874–5885. [Google Scholar] [CrossRef]
- Torres Landa, S.D.; Kaur, I.; Agarwal, V. Pithecellobium dulce leaf-derived carbon dots for 4-nitrophenol and Cr (VI) detection. Chemosensors 2022, 10, 532. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.; Li, P.; Yu, H.; Xie, Y.; Guo, Y.; Yao, W.; Qian, H.; Cheng, Y. Crayfish shells-derived carbon dots as a fluorescence sensor for the selective detection of 4-nitrophenol. Food Agric. Immunol. 2023, 34, 36–47. [Google Scholar] [CrossRef]
- Yan, C.; Han, S.; Sun, R.; Zhao, L.; Zhang, X.; Zhang, X.; Wang, Y.; Zhai, M. A smart imprinted fluorescence sensor of biomass carbon dots based on magnetic covalent organic frameworks for real-time and sensitive detection of 4-nitrophenol in food. Microchem. J. 2024, 207, 111949. [Google Scholar] [CrossRef]
- Ambaye, A.D.; Kefeni, K.K.; Kebede, T.G.; Ntsendwana, B.; Mishra, S.B.; Nxumalo, E.N. Cu-MOF/N-doped GO nanocomposites modified screen-printed carbon electrode towards detection of 4-nitrophenol. J. Electroanal. Chem. 2022, 919, 116542. [Google Scholar] [CrossRef]
- Ashokan, I.; Bhunia, S.K. Bright yellow fluorescent carbon dots as selective nanoprobe for detection of 2-nitrophenol and 4-nitrophenol in aqueous medium. Inorganica Chim. Acta 2024, 573, 122312. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, S.; Yuan, X.; Wei, Y.; Qin, K.; Zhang, Q.; Chen, X.; Ji, X. A novel fluorescent nitrogen, phosphorus-doped carbon dots derived from Ganoderma lucidum for bioimaging and high selective two nitrophenols detection. Dye. Pigment. 2020, 178, 108316. [Google Scholar] [CrossRef]
- Siddique, A.B.; Pramanick, A.K.; Chatterjee, S.; Ray, M. Amorphous carbon dots and their remarkable ability to detect 2, 4, 6-trinitrophenol. Sci. Rep. 2018, 8, 9770. [Google Scholar] [CrossRef]
- Kim, K.; Chokradjaroen, C.; Saito, N. Solution plasma: New synthesis method of N-doped carbon dots as ultra-sensitive fluorescence detector for 2, 4, 6-trinitrophenol. Nano Express 2020, 1, 020043. [Google Scholar] [CrossRef]
- Kadian, S.; Kalkal, A.; Jain, V.; Shukla, S.; Narayan, R.J. Pomegranate leaf extract-based carbon dots for the selective detection of 2, 4, 6-trinitrophenol. MRS Commun. 2023, 13, 885–891. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, S.; Li, R.; Pan, L.; Li, L.; Qi, Z.; Li, C. Highly selective detection of nitroaromatic explosive 2, 4, 6-trinitrophenol (TNP) using N-doped carbon dots. Res. Chem. Intermed. 2021, 47, 2421–2431. [Google Scholar] [CrossRef]
- Li, S.; Ouyang, T.; Guo, X.; Dong, W.; Ma, Z.; Fei, T. Tetraphenylethene-Based Cross-Linked Conjugated Polymer Nanoparticles for Efficient Detection of 2, 4, 6-Trinitrophenol in Aqueous Phase. Materials 2023, 16, 6458. [Google Scholar] [CrossRef]
- Qu, Y.; Yu, L.; Zhu, B.; Chai, F.; Su, Z. Green synthesis of carbon dots by celery leaves for use as fluorescent paper sensors for the detection of nitrophenols. New J. Chem. 2020, 44, 1500–1507. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z. Yellow emissive Se, N-codoped carbon dots toward sensitive fluorescence assay of crystal violet. J. Hazard. Mater. 2020, 388, 122073. [Google Scholar] [CrossRef] [PubMed]
- Chatzimarkou, A.; Chatzimitakos, T.G.; Kasouni, A.; Sygellou, L.; Avgeropoulos, A.; Stalikas, C.D. Selective FRET-based sensing of 4-nitrophenol and cell imaging capitalizing on the fluorescent properties of carbon nanodots from apple seeds. Sens. Actuators B Chem. 2018, 258, 1152–1160. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, M.; Zhang, K.; Fu, Q.; Gao, M.; Wang, L.; Xia, Z.; Gao, D. Facile and green synthesis of fluorescent carbon dots from the flowers of Abelmoschus manihot (Linn.) Medicus for sensitive detection of 2, 4, 6-trinitrophenol and cellular imaging. Microchem. J. 2019, 148, 385–396. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, J.; Zhao, L.; Wang, Y.; Zheng, Y.; Wu, Y.; Jiang, L. Synthesis of novel fluorescent carbon quantum dots from Rosa roxburghii for rapid and highly selective detection of o-nitrophenol and cellular imaging. Front. Chem. 2020, 8, 665. [Google Scholar] [CrossRef]
- Soni, H.; Pamidimukkala, P.S. Green synthesis of N, S co-doped carbon quantum dots from triflic acid treated palm shell waste and their application in nitrophenol sensing. Mater. Res. Bull. 2018, 108, 250–254. [Google Scholar] [CrossRef]
- Gao, K.; Guo, Y.; Niu, Q.; Han, L.; Zhou, L.; Wang, L. Quaternary ammonium-functionalized carbon dots for sensitive and selective detection of 2, 4, 6-trinitrophenol in aqueous medium. Sens. Actuators B Chem. 2018, 262, 298–305. [Google Scholar] [CrossRef]
- Qin, K.; Zhang, D.; Ding, Y.; Zheng, X.; Xiang, Y.; Hua, J.; Zhang, Q.; Ji, X.; Li, B.; Wei, Y. Applications of hydrothermal synthesis of Escherichia coli derived carbon dots in in vitro and in vivo imaging and p-nitrophenol detection. Analyst 2020, 145, 177–183. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, S.; Yuan, X.; Song, P.; Wei, Y.; Qin, K.; Zhang, Q.; Ji, X. Hydrothermal synthesis of Auricularia auricula derived nitrogen, phosphorus-doped carbon dots and application in Ag (i) and 4-nitrophenol detection and bioimaging. Anal. Methods 2020, 12, 2237–2243. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Guo, X.; Dong, W.; Wang, R.; Shuang, S.; Gong, X.; Dong, C. Comparative study of Cl, N-Cdots and N-Cdots and application for trinitrophenol and ClO− sensor and cell-imaging. Anal. Chim. Acta 2019, 1091, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, J.; Zhang, R.; Zhao, W. Facile and low-energy-consumption synthesis of dual-functional carbon dots from Cornus walteri leaves for detection of p-nitrophenol and photocatalytic degradation of dyes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128351. [Google Scholar] [CrossRef]
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens. Bioelectron. 2017, 94, 292–297. [Google Scholar] [CrossRef]
- Lin, B.; Yan, Y.; Guo, M.; Cao, Y.; Yu, Y.; Zhang, T.; Huang, Y.; Wu, D. Modification-free carbon dots as turn-on fluorescence probe for detection of organophosphorus pesticides. Food Chem. 2018, 245, 1176–1182. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Lan, S.; Zhang, C.; Hou, C.; He, Q.; Huo, D. A turn-on fluorescent sensor based on carbon dots from Sophora japonica leaves for the detection of glyphosate. Anal. Methods 2020, 12, 4130–4138. [Google Scholar] [CrossRef]
- Kazemifard, N.; Ensafi, A.A.; Rezaei, B. Green synthesized carbon dots embedded in silica molecularly imprinted polymers, characterization and application as a rapid and selective fluorimetric sensor for determination of thiabendazole in juices. Food Chem. 2020, 310, 125812. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Tian, Z.; Lu, J.; Wang, Q.; Ai, S.; Wang, M. A sensitive fluorescent sensor for selective determination of dichlorvos based on the recovered fluorescence of carbon dots-Cu (II) system. Food Chem. 2016, 202, 81–87. [Google Scholar] [CrossRef]
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green synthesis of fluorescent carbon dots from cherry tomatoes for highly effective detection of trifluralin herbicide in soil samples. ChemistrySelect 2020, 5, 1956–1960. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Liang, S.; Hou, S.; Chu, T.; Ma, J.; Chen, X.; Zhou, J.; Sun, R. Preparation of sulfur-doped carbon quantum dots from lignin as a sensor to detect Sudan I in an acidic environment. J. Mater. Chem. B 2020, 8, 10788–10796. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, L.; Wang, J.; He, X.; Wang, S.; Yan, A.; Zhang, Z.; Li, G.; Li, Y. Methods and Prospects for Enhancing Heterogeneous Fenton Catalytic Activity. ChemistrySelect 2025, 10, e202404183. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, S.; Chen, X.; Ni, J.; Du, J.; Li, Y.; Xin, X.; Zhao, B.; Chen, G. Synergistic Catalysis of Water-Soluble Exogenous Catalysts and Reservoir Minerals during the Aquathermolysis of Heavy Oil. Molecules 2024, 29, 3761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Huang, F.; Song, S.; Ai, G.; Xin, X.; Zhao, B.; Zheng, Y.; Zhang, Z. Recent advances in g-C3N4-based materials and their application in energy and environmental sustainability. Molecules 2023, 28, 432. [Google Scholar] [CrossRef]
- Xu, X.Y.; Bao, Z.J.; Zhou, G.; Zeng, H.B.; Hu, J.G. Enriching photoelectrons via three transition channels in amino conjugated carbon quantum dots to boost photocatalytic hydrogen generation. ACS Appl. Mater. Inter. 2016, 8, 14118–14124. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.N. Carbon Dots from Tire Waste for the Photodegradation of Methyl Orange Dye, Antimicrobial Activity, and Molecular Docking Study. Chem. Biodivers. 2023, 20, e202301358. [Google Scholar] [CrossRef]
- Sun, J.; Maimaiti, H.; Xu, B.; Feng, L.; Bao, J.; Zhao, X. Photoelectrocatalytic degradation of wastewater and simultaneous hydrogen production on copper nanorod-supported coal-based N-carbon dot composite nanocatalysts. Appl. Surf. Sci. 2022, 585, 152701. [Google Scholar] [CrossRef]
- Das, G.S.; Shim, J.P.; Bhatnagar, A.; Tripathi, K.M.; Kim, T. Biomass-derived carbon quantum dots for visible-light-induced photocatalysis and label-free detection of Fe (III) and ascorbic acid. Sci. Rep. 2019, 9, 15084. [Google Scholar] [CrossRef]
- Nandi, S.; Ritenberg, M.; Jelinek, R. Bacterial detection with amphiphilic carbon dots. Analyst 2015, 140, 4232–4237. [Google Scholar] [CrossRef]
- Yang, B.; Jelinek, R.; Kang, Z. Current progress in carbon dots: Synthesis, properties and applications. Mater. Chem. Front. 2020, 4, 1287–1288. [Google Scholar] [CrossRef]
- Song, X.; Zhou, Q.; Zhang, T.; Xu, H.; Wang, Z. Pressure-assisted preparation of graphene oxide quantum dot-incorporated reverse osmosis membranes: Antifouling and chlorine resistance potentials. J. Mater. Chem. A 2016, 4, 16896–16905. [Google Scholar] [CrossRef]

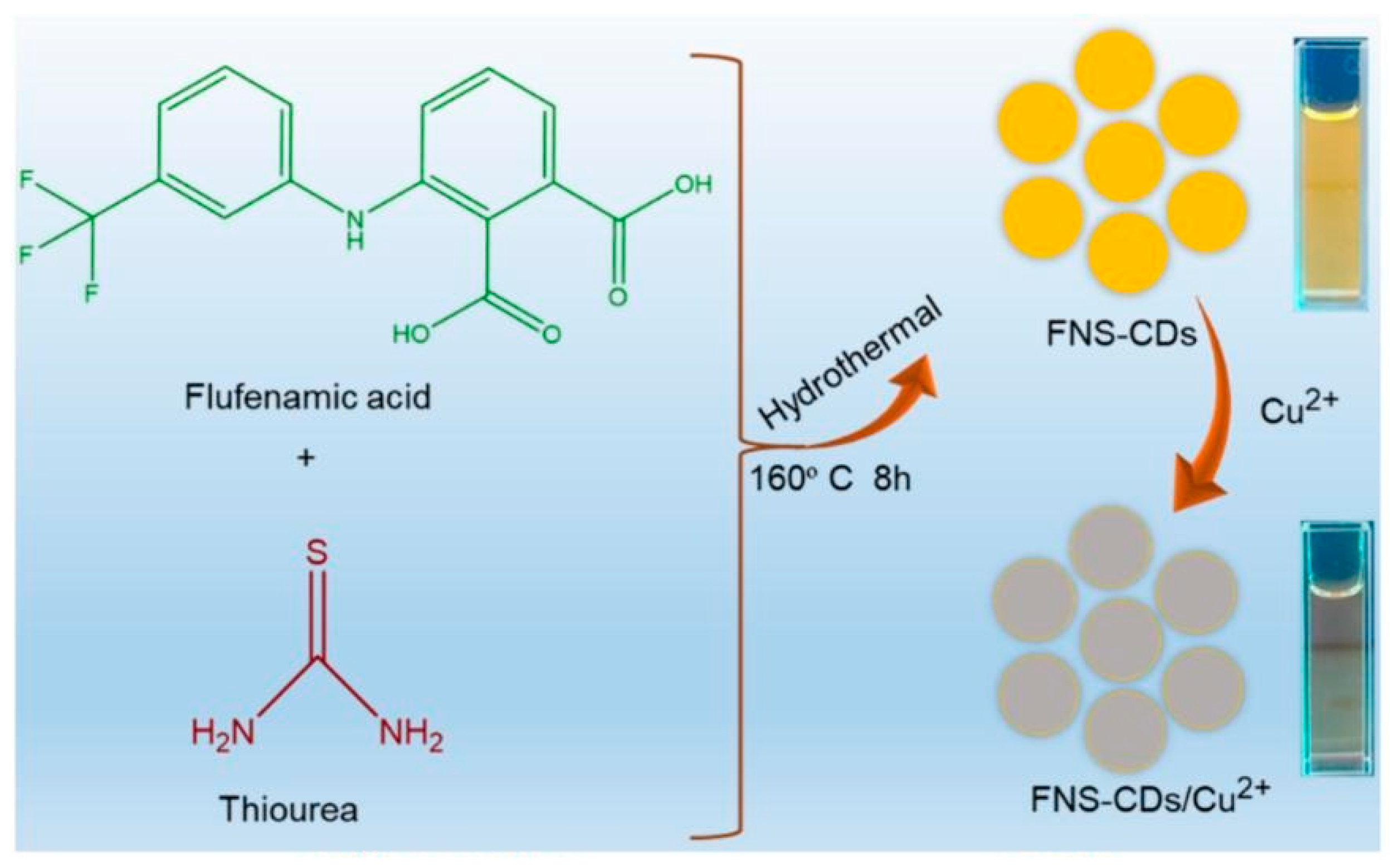

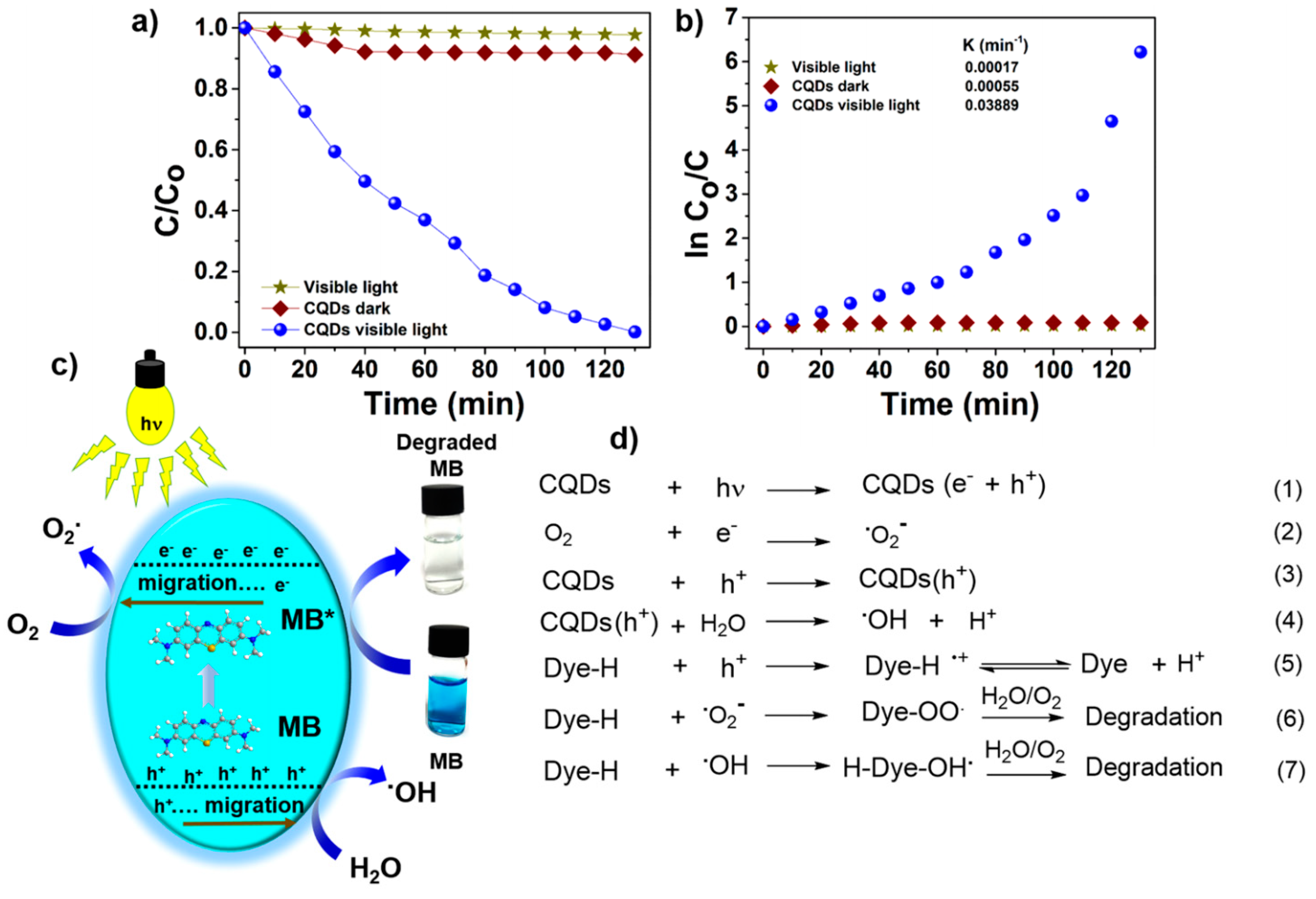
| Chemosensors | Precursors | Size (nm) | QYs (%) | Fluorescence | Refs. |
|---|---|---|---|---|---|
| CDs | o-phenylene-diamine, DL-Thioctic acid | 3.3 ± 0.4 | 21.82 | Yellow | [44] |
| SN-CCDs | Citric acid, thiourea | 4.4 | 30.60 | Bright blue | [45] |
| PC-CD | Water amaranth leaves, 1-pyrenecarboxaldehyde | 7.75 ± 0.51 | 12.10 | Red | [46] |
| N-CDs | C. retusus fruits | 5 ± 2 | 9.00 | Blue | [27] |
| MP-CQDs | Persimmon pulp | 3.18 ± 0.69 | 8.39 | Blue | [47] |
| N-CDs | citric acid, melamine | 2.58 | 45.00 | Blue | [48] |
| CQDs | green pomelo peel via | 5.5 | 17.31 | Blue | [49] |
| NSCQDs | potato, ammonium sulphate | 5 | 16.96 | Blue | [50] |
| CDs | black plum leaves | 0.34 | 15.90 | Greenish | [51] |
| N,S-CQDs | o-amino benzenesulfonic acid, Alkali lignin | 4.86 | 23.68 | Greenish | [52] |
| CQDs | Tagetes patula flowers | 5.15 | 29.88 | Bright blue | [53] |
| CDs | m-phenylenediamine, ethylenediamine | 3.5 ± 0.7 | 25.50 | Greenish | [54] |
| GCDs | Delonix regia leaves | 6.75 ± 2.5 | 7.00 | Red | [55] |
| N/SCDs | p-Phenylene-diamine, 2-mercapto-thiazoline | 8.67 | Bright green | [56] | |
| R-CDs | o-phenylenediamine, 1,8-diamino-naphthalene | 1.97 ± 0.24 | 11 | Red | [57] |
| RCDs | tetra (4-carboxyphenyl) porphyrin, thiourea | 5.6 | 26.7 | Red | [58] |
| N-CQDs | citric acid, urea | 2.74 | 9.60 | Blue | [59] |
| N,S-CQDs | Thiourea, citric acid | 30 | 33.00 | Greenish | [60] |
| CQDs | 5-dimethylamino methyl furfuryl alcohol, o-phenylene diamine | 2.6 ± 0.75 | 12.00 | Yellow | [61] |
| N,S-CQDs | o-Phenylene-diamine, methionine | 4 | 21.00 | Blue | [62] |
| N-DCCDs | desiccated coconut flour | 8 | 32.00 | Blue | [63] |
| CM-CDs | camel milk | <10 | 24.60 | Blue | [64] |
| Metal Ion | Chemosensors | Precursors | Synthesis Technique | QYs (%) | Fluorescence | Linear Range (mM) | LOD (nM) | Refs. |
|---|---|---|---|---|---|---|---|---|
| Au3+ | CQDs | Robinia hispida L. flowers | Hydrothermal | 5.13 | Blue | 500–3500 | 400 | [80] |
| Cr6+ | Cu-CDs | Citric acid, urea, copper chloride | Hydrothermal | 27.3 | Blue | 0–80,000 | 186 | [81] |
| Cr6+ | NCDs | Salicylic acid, o-phenylenediamine | Hydrothermal | Yellow | 0–90,000 | 190 | [82] | |
| Cr6+ | SN-CDs | Citric acid, thiourea | Hydrothermal | 30.60 | Bright blue | 0–0.12 | 428 | [45] |
| Cr6+ | N,Zn- CDs | Zinc nitrate hexahydrate, citric acid monohydrate, 4-pyridinecarboxaldehyde | Microwave-assisted | 13.60 | 0.000005–0.000135 | 0.47 | [83] | |
| Cr6+ | N-CQDs | Natural Goji Berry | Hydrothermal | Greenish | 0–0.04 | 160 | [84] | |
| Cr6+ | R-CDs | o-phenylene-diamine, p-Acetylamino benzene sulfonyl azide | Solvothermal strategy | 8.15 | Red | 0.004–0.04 | 80 | [85] |
| Cd2+ | PC-CD | Water amaranth leaves, 1-pyrenecarboxaldehyde | Hydrothermal | 12.10 | Red | 0–0.07 | 15 | [46] |
| Fe3+ | N-CDs | Aegle Marmelos, urea | Microwave assisted synthesis | 14.21 | Blue | 0.001–0.004 | 148 | [24] |
| Fe3+ | N-CDs | C. retusus fruits | Hydrothermal | 9.00 | Blue | 0–0.002 | 70,000 | [27] |
| Fe3+ | MP-CQDs | Persimmon pulp | Hydrothermal | 8.39 | Blue | 0–0.09 | 324 | [47] |
| Fe3+ | N-CDs | Peony seed residue, o-phenylene-diamine | Hydrothermal | Orange -red | 0–0.05 | 20 | [86] | |
| Fe3+ | N-CDs | citric acid, melamine | Hydrothermal | 45.00 | Blue | 0.02–0.08 | 3180 | [48] |
| Fe3+ | N-CDs | citric acid, urea | Microwave assisted | 18.10 | Greenish | 0.001–0.08 | 1000 | [87] |
| Fe3+ | CQDs | green pomelo peel via | Hydrothermal | 17.31 | Blue | 0.0001–0.16 | 86 | [49] |
| Fe3+ | NSCQDs | potato, ammonium sulphate | Hydrothermal | 16.96 | Blue | 0–0.5 | 260 | [50] |
| Fe3+ | CDs | black plum leaves | Hydrothermal | 15.90 | Greenish | 0–0.08 | 130 | [51] |
| Fe3+ | CQDs | Tagetes patula flowers | Hydrothermal | 29.88 | Bright blue | 0–0.004 | 320 | [53] |
| Fe3+ | CQDs | Castor leaves | Hydrothermal | 0–300,000 | 19,100 | [88] | ||
| Fe3+ | N-CDs | Hedyotis diffusa willd | Hydrothermal | 15.2 | Blue | 10–100 | 6.62 | [89] |
| Fe3+ | CDs | Polyalthia bullata | Hydrothermal | 7.55 | Blue | 0–57,000 | 186 | [84] |
| Fe2+ | CNPs | 1, 3, 5-trimesic acid, o-phenylenediamine | Solvothermal | 15.17 | Bright yellow | 10–160 | 88 | [90] |
| Fe2+ | CDs | m-phenylenediamine, ethylenediamine | Hydrothermal | 25.50 | Greenish | 0.033–1.044 | 16,000 | [54] |
| Fe2+ | CQDs | Mango leaves | Pyrolysis | 18.20 | Blue | 0–0.01 | 620 | [25] |
| Pb2+ | GCDs | Delonix regia leaves | Hydrothermal | 7.00 | Red | 0.01–0.18 | 3.3 | [55] |
| Pb2+ | R-CDs | Rice straw | Thermolysis | 44.29 | Blue | 1000–100,000 | 110 | [91] |
| Pb2+ | GA-DMF CDs | Gallic acid, gallic acid | Hydrothermal | Strong green | 30,000–120,000 | 7.15 × 105 | [92] | |
| Cu2+ | N/SCDs | p-Phenylene-diamine, 2-mercapto-thiazoline | Hydrothermal | Bright green | 0.005–0.4 | 215 | [56] | |
| Cu2+ | N,S-CDs | Creatinine, thiourea, disodium edetate | Direct carbonization | 60.5 | Blue | 400–2400 | 70 | [93] |
| Cu2+ | B-CDs | Citric acid, ethylenediamine | Solvothermal | 10.28 | Blue | 250–10,000 | 180 | [94] |
| Cu2+ | N-CDs | Diethylenetriamine, citric acid | Microchannel | 63.87 | 20–100 | 46 | [95] | |
| Cu2+ | N-CQDs | citric acid, urea | Hydrothermal | 9.60 | Blue | 0.0005–0.005 | 32 | [59] |
| Cu2+ | NCDs | Sida cordifolia root, triethylene tetraamine | Hydrothermal | 17.80 | Blue | 0–0.13 | 110 | [96] |
| Co2+ | 0.00001–0.00009 | 0.03 | ||||||
| Co2+ | L-CDs | emon juice, ethylenediamine | Amicroflow approach | 25.40 | Blue | 0–0.1 | 318 | [97] |
| Cr6+ | CDs | 3,5-dihydroxybenzoic acid, L-Arginine | Hydrothermal | 20.00 | Blue | 0.0001–0.002 | 24 | [98] |
| Hg2+ | 0.0004–0.005 | 84 | ||||||
| Hg2+ | N,S-CQDs | Thiourea, citric acid | Hydrothermal | 33.00 | Greenish | 0.000005–0.00016 | 4 | [60] |
| Hg2+ | CQDs | 5-dimethylamino methyl furfuryl alcohol, o-phenylene diamine | Hydrothermal | 12.00 | Yellow | 0.015–0.1 | 5.2 | [61] |
| Hg2+ | N,Cl-CQDs | Triticum aestivum | Microwave synthesis | 36 | Blue | 0–350 | 39 | [99] |
| Hg2+ | N-CDs | Citric acid, urea, glycerol | Microwave solvent | 60.51 | Blue | 30,000–120,000 | 80 | [77] |
| Hg2+ | NCQDs | N-phenyl orthophenylenediamine, citric acid | Hydrothermal | 50.5 | Blue | 0–2000 | 4.98 | [100] |
| Hg2+ | B-CDs | Tartaric acid, 1-amino-2-naphthol-4-sulfonic acid | Solvothermal method | 34 | Strong blue | 1000–8000 | 175 | [101] |
| Hg2+ | CDs | Urea, citric acid | Hydrothermal | 21.3 | Bright Blue | 0–500,000 | 8.2 | [102] |
| Hg2+ | M-NCDs | Urea, D-glucose | Microwave assisted | 14.90 | Blue | 0.00003–0.0001 | 3.5 | [103] |
| Hg2+ | N,S-CQDs | o-Phenylene-diamine, methionine | Hydrothermal | 21.00 | Blue | 0–0.018 | 120 | [62] |
| Hg2+ | N-DCCDs | desiccated coconut flour | Hydrothermal | 32.00 | Blue | 0.0001–0.0002 | 2.4 | [63] |
| Mn7+ | CM-CDs | camel milk | Hydrothermal | 24.60 | Blue | 0–0.5 | 580 | [64] |
| Mn2+ | CDs | citric acid, tris base | Microwave abetted | 14.14 | Blue | 0.00001–0.00007 | 0.37 | [104] |
| Cd2+ | G-CDs | Pumpkin, acetone | Solvent heat | 17.50 | Greenish | 0–0.0165 | 680 | [105] |
| Cd2+ | CDs | ammonium citrate, glutamic acid | Hydrothermal | Blue | 0.001–0.025 | 13 | [106] | |
| Ag+ | PEG-CDs | 1-pyrenecarboxaldehyde, 0-phenylenediamine and PEG | Solid phase | 73.90 | Blue | 0–0.2 | 0.114 | [107] |
| Ag+ | CQDs | Sugar cane molasses, L-cysteine/D-cysteine (L/D-Cys) and Rhodamine B(RhB) | Hydrothermal | 6.33 | Blue | 22,000–220,000 | 140 | [108] |
| Ce4+ | NB-CDs | p-phenylenediamine, boric acid | Solvothermal | 4.00 | Orange | 0–0.18 | 140 | [109] |
| Al3+ | 0–0.1 | 1070 |
| Target | Chemosensors | Precursors | Synthesis Technique | QYs (%) | Fluorescence | Linear Range (mM) | LOD | Refs. |
|---|---|---|---|---|---|---|---|---|
| 4-nitrophenol | N,S-CDs | Giloy stem | Hydrothermal | 7.2 | Green | 500–6000 | 380 nM | [114] |
| Congo red | 10–1000 | 62 nM | ||||||
| 4-nitrophenol | CDs | Pithecellobium dulce | Single-step carbonization | 24 | Blue | 20–80 | 14 nM | [115] |
| 4-nitrophenol | CDs | Crayfish shells | Hydrothermal method | 10.68 | Bright blue | 0–50,000 | 160 nM | [116] |
| 4-nitrophenol | CDs-MFMIP | Magnetic covalent organic frameworks, green persimmons | Hydrothermal | 10.45 | Blue | 50–50,000 | 17.44 nM | [117] |
| 4-nitrophenol | Cu-MOF/NGO | Cu(NO3)2·3H2O, trimesic acid | Solvothermal | 500–100,000 | 35 nM | [118] | ||
| 2-nitrophenol | C-dots | O-phenylenediamine, oleic acid | One-pot carbonization method. | 19.5 | Bright yellow | 74–134 | 6.4 nM | [119] |
| 4-nitrophenol | 4.8 nM | |||||||
| 2,4-dinitrophenol | Gl N,P-CDs | Diammonium hydrogen phosphate, ethanediamine, ganoderma Lucidum | Hydrothermal | 11.41 | Blue | 0–30,000 | 73.03 nM | [120] |
| 4-nitrophenol | 0–30,000 | 68.09 nM | ||||||
| 2,4,6-trinitrophenol | CDs | Dextrose, HCl | Ultrasonication | 40 | Bluish green | 500–200,000 | 200 nM | [121] |
| 2,4,6-trinitrophenol | NCDs | Pyridine, water | Solution plasma | 61 | Bright blue | 0–50,000 | 10 nM | [122] |
| 2,4,6-trinitrophenol | CDs | Pomegranate leaf | Hydrothermal | 14.64 | Blue | 0–120,000 | 62 nM | [123] |
| 2,4,6-trinitrophenol | CDs | 4-(diethylamino)salicylaldehyde | Hydrothermal | Orange | 1–100,000 | 480 nM | [110] | |
| 2,4,6-trinitrophenol | C-dots | Guanine | hydrotherma | 26.8 | Blue | 0–30,000 | 58.5 nM | [124] |
| 2,4,6-trinitrophenol | PTPEBP | Sodium dodecyl sulfate, potassium carbonate | Suzuki-miniemulsion polymerization | 8.14 | 10,000–30,000 | 1070 nM | [125] | |
| 2,4,6-trinitrophenol | CDs | Sewage sludge | Microwave-assisted | 21.7 | Blue | 200–20,000 | 69 nM | [111] |
| 2-nitrophenol | CDs | Celery leaves glutathione | Hydrothermal | 53 | Blue | 50–500 | 39 nM | [126] |
| 3-nitrophenol | 43 nM | |||||||
| 4-nitrophenol | 26 nM | |||||||
| Crystal violet | Se,N-codoped carbon dots | Selenourea o-phenylenediamine | Hydrothermal | 16.7 | Yellow | 20–1600 | 7.3 nM | [127] |
| 4-nitrophenol | CNDs | Apple seeds | Pyrolysis | 20 | Blue | 50–53,000 | 13 nM | [128] |
| 2,4,6-trinitrophenol | CDs | Abelmoschus Manihot Flowers | Hydrothermal | 30.8 | Blue | 25–40,000 | 5 nM | [129] |
| 2-nitrophenol | CQDs | Rosa roxburghii | Hydrothermal | 24.8 | 80–40,000 | 15.2 nM | [130] | |
| 4-nitrophenol | N,S-codoped | Palm Shell powder triflic acid | Hydrothermal | Green | 200–400 | 79 nM | [131] | |
| DNP | 500–850 | 165 nM | ||||||
| 2,4,6-trinitrophenol | 200–4000 | 82 nM | ||||||
| 2,4,6-trinitrophenol | CTA-CDs | 4,7,10-Trioxa-1,13-tridecanediamine, carboxymethyl cellulose sodium | hydrothermal treatment | 87.3 | Blue | 0–100,000 | 704 nM | [132] |
| 4-nitrophenol | CDs-WT | Escherichia coli | Hydrothermal | 15.8 | Blue | 20–33,000 | 11 nM | [133] |
| 4-nitrophenol | Aa N,P-CDs | Auricularia Auricula | Hydrothermal | 7.25 | Blue | 0–37,500 | 198 nM | [134] |
| 4-nitrophenol | Cl,N-CD | Shaddock peel HCl | High-temperature Carbonization and low-temperature concentrated acid (HCl) acidification | 17.99 | Blue | 900–90,000 | 37.1 nM | [135] |
| 4-nitrophenol | G-CDs | Cornus walteri leaves | Hydrothermal | 18.3 | Green | 0–50,000 | 0.0175 nM | [136] |
| Paraoxon | CQDs | Chlorophyll | Hydrothermal | 0.05–50 μgL−1 | 0.050 μgL−1 | [137] | ||
| Chlorpyrifos | CDs | Burning ash of waste paper | Hydrothermal | 20 | 0.01–1.0 μgL−1 | 3 ng mL−1 | [138] | |
| Glyphosate | CD/Fe3+ | Sophora Japonica leaves | Hydrothermal | 6.51 | Blue | 0.1–16 ppm | 8.75 ppb | [139] |
| Thiabendazole | CDs | Rosemary leaves | Hydrothermal | 0.03–0.73 μgL−1 | 8 ng mL−1 | [140] | ||
| Diazinon | CDs | Cauliflower | Hydrothermal | 43 | Green | 0.25–5000 μgL−1 | 0.25 ng mL−1 | [112] |
| Chlorpyrios | TEF-CDs | Tagetes Erecta flower | Solvo/Hydrothermal | 63.7 | Blue | 50–100,000 | 2.1 ng mL−1 | [113] |
| Quinalphos | 10–50,000 | 1.7 ng mL−1 | ||||||
| Dichlorvos | F-CDs | Feather | Hydrothermal | 2.4 | 6–60 | 3.8 nM | [141] | |
| Trifluralin | CQDs | Cherry Tomatoes | Hydrothermal | 9.7 | Green | 50–200,000 | 0.5 nM | [142] |
| Sudan I | SCQDs | Lignin | Hydrothermal | 13.5 | Green | 0–40,000 | 120 nM | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Q.; Liu, W.; Xin, X.; Zhao, B. A Review on the Synthesis of Carbon Dots and Their Applications in Environmental Analysis. Crystals 2025, 15, 384. https://doi.org/10.3390/cryst15050384
Wang Y, Wang Q, Liu W, Xin X, Zhao B. A Review on the Synthesis of Carbon Dots and Their Applications in Environmental Analysis. Crystals. 2025; 15(5):384. https://doi.org/10.3390/cryst15050384
Chicago/Turabian StyleWang, Yuegang, Qian Wang, Weina Liu, Xin Xin, and Bin Zhao. 2025. "A Review on the Synthesis of Carbon Dots and Their Applications in Environmental Analysis" Crystals 15, no. 5: 384. https://doi.org/10.3390/cryst15050384
APA StyleWang, Y., Wang, Q., Liu, W., Xin, X., & Zhao, B. (2025). A Review on the Synthesis of Carbon Dots and Their Applications in Environmental Analysis. Crystals, 15(5), 384. https://doi.org/10.3390/cryst15050384





