High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
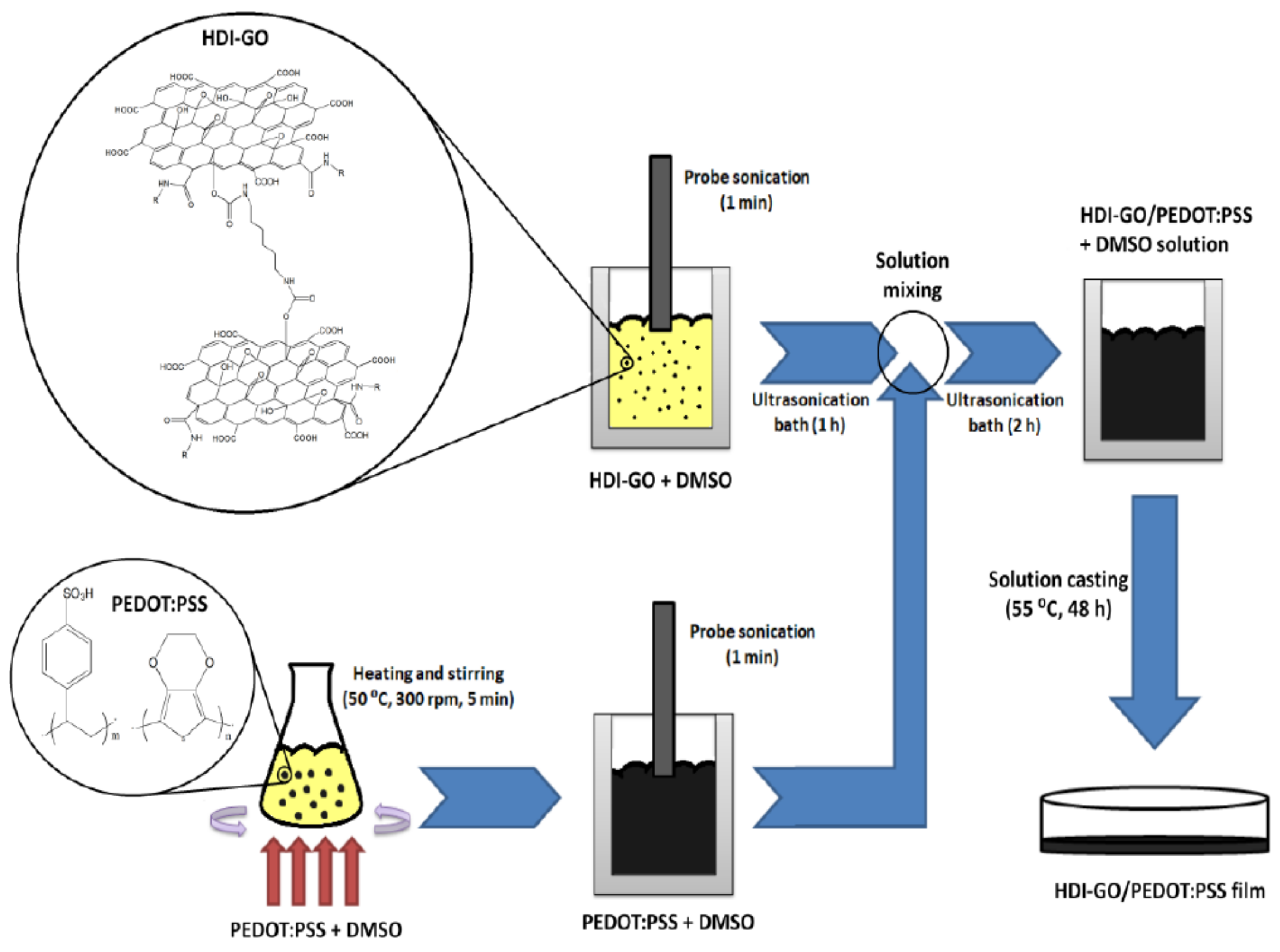
2.2. Synthesis of Hexamethylene Diisocyanate-Functionalized Graphene Oxide (HDI-GO)
2.3. Synthesis of PEDOT:PSS/HDI-Functionalized GO Nanocomposites
2.4. Intrumentation
3. Results and Discussion
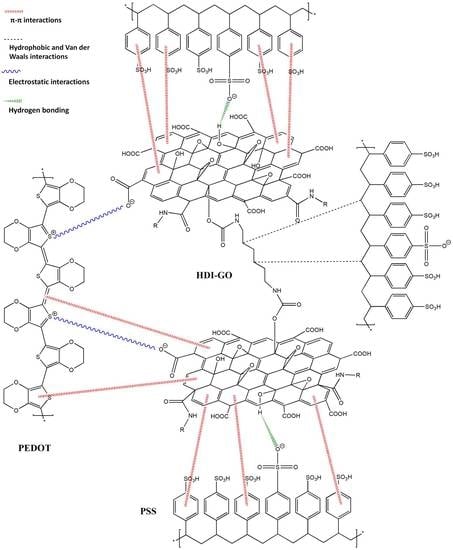
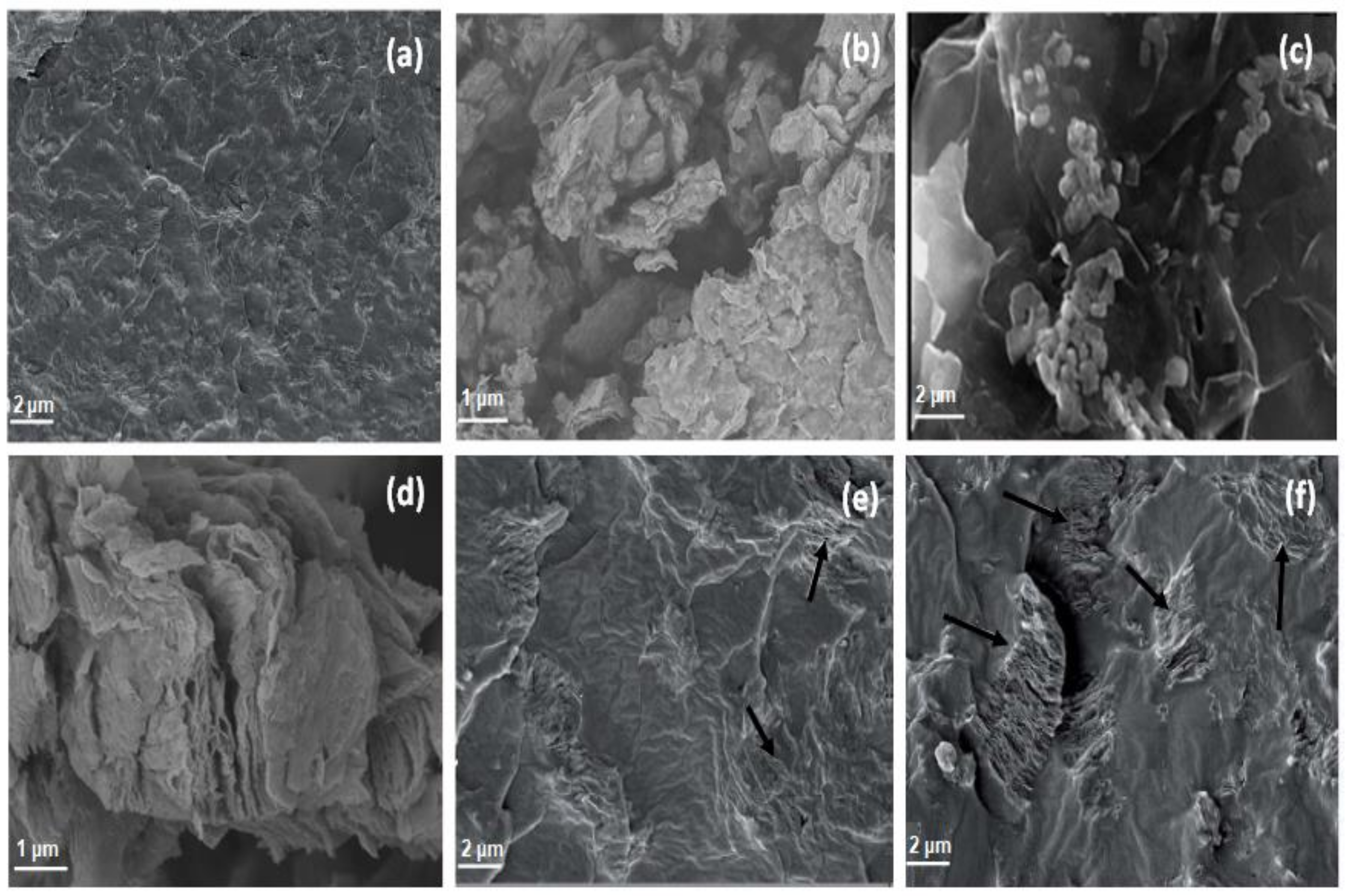
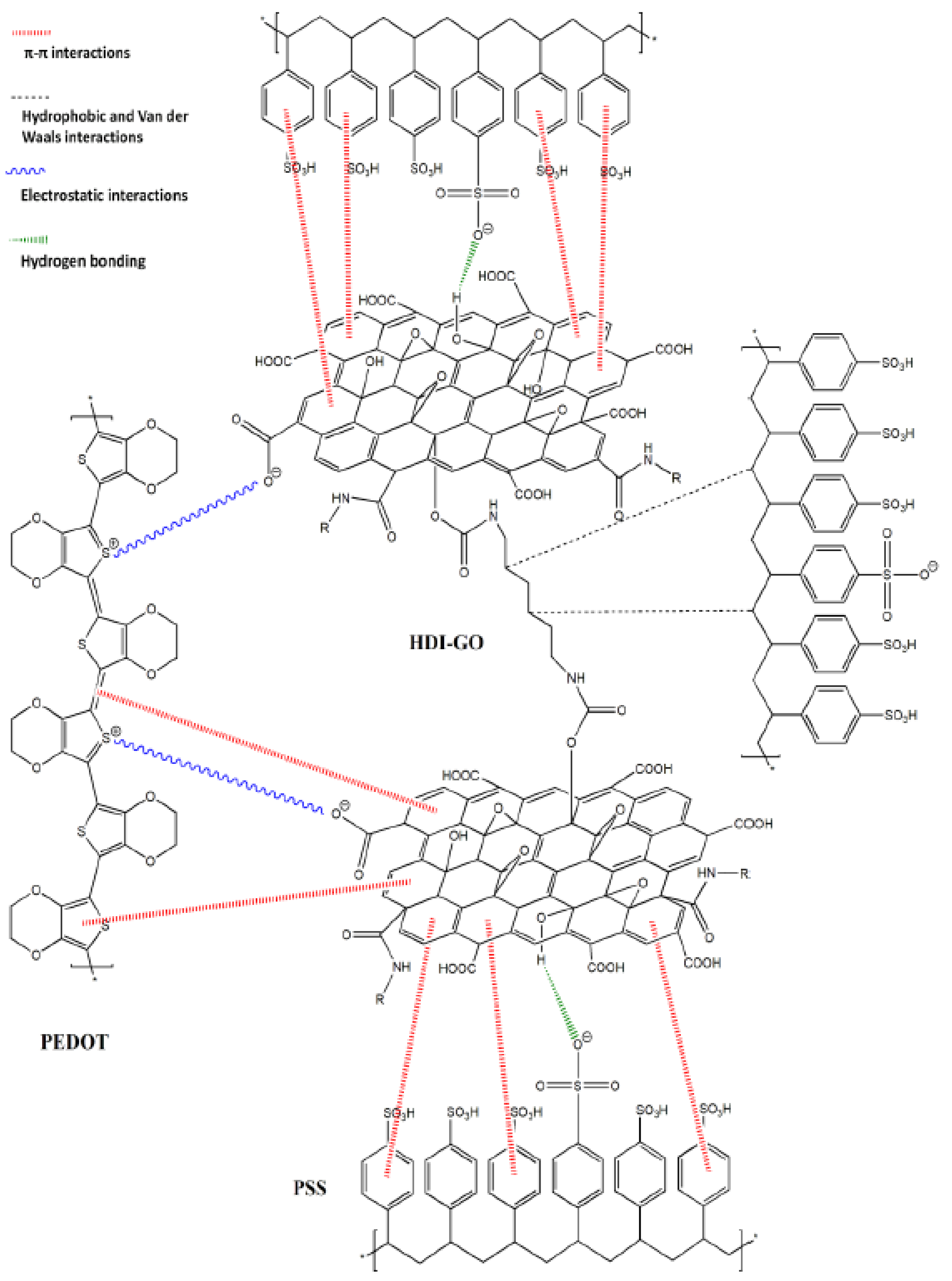
3.1. Morphology of PEDOT:PSS/HDI-GO Nanocomposites
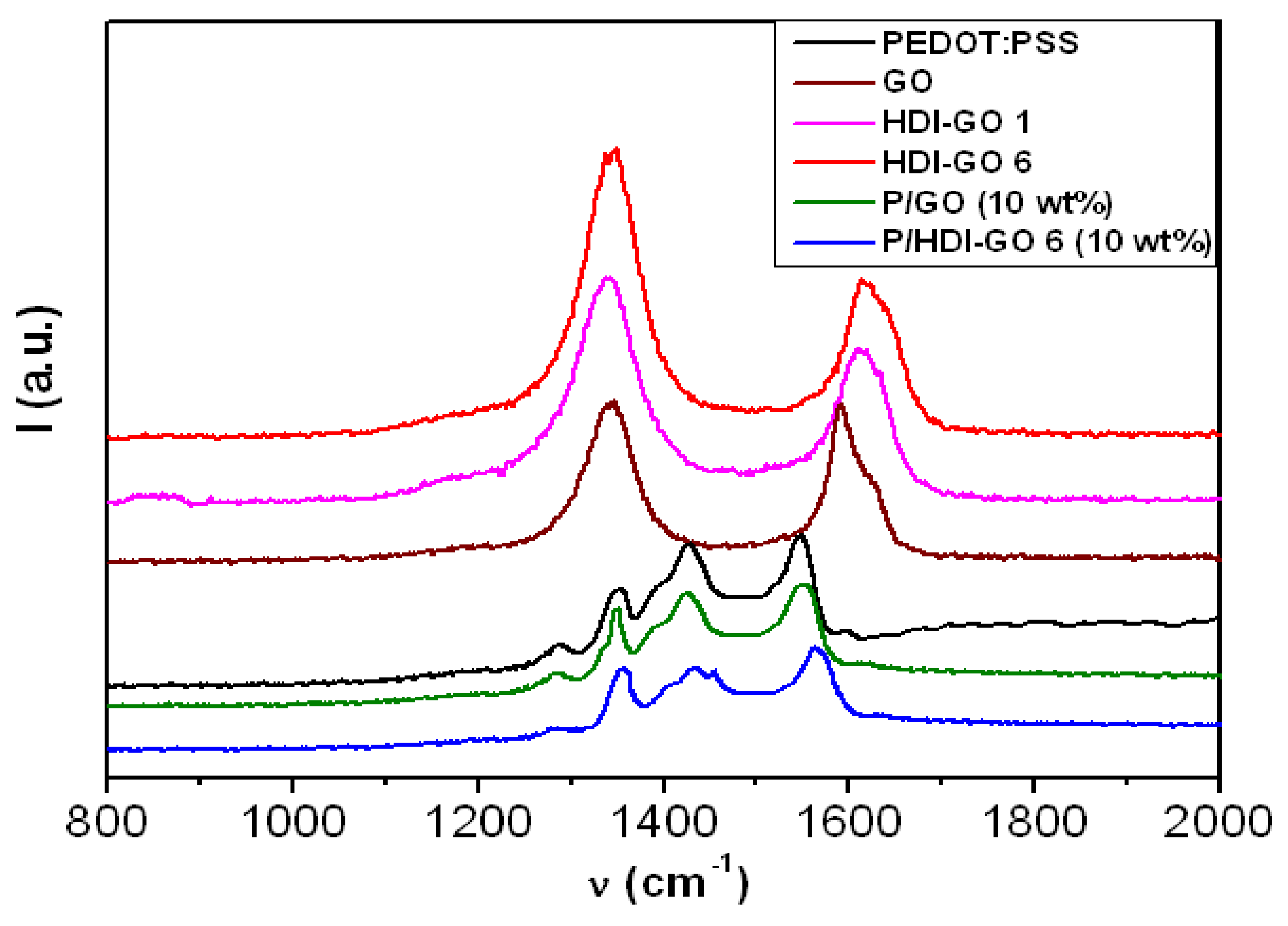
3.2. Raman Spectra of PEDOT:PSS/HDI-GO
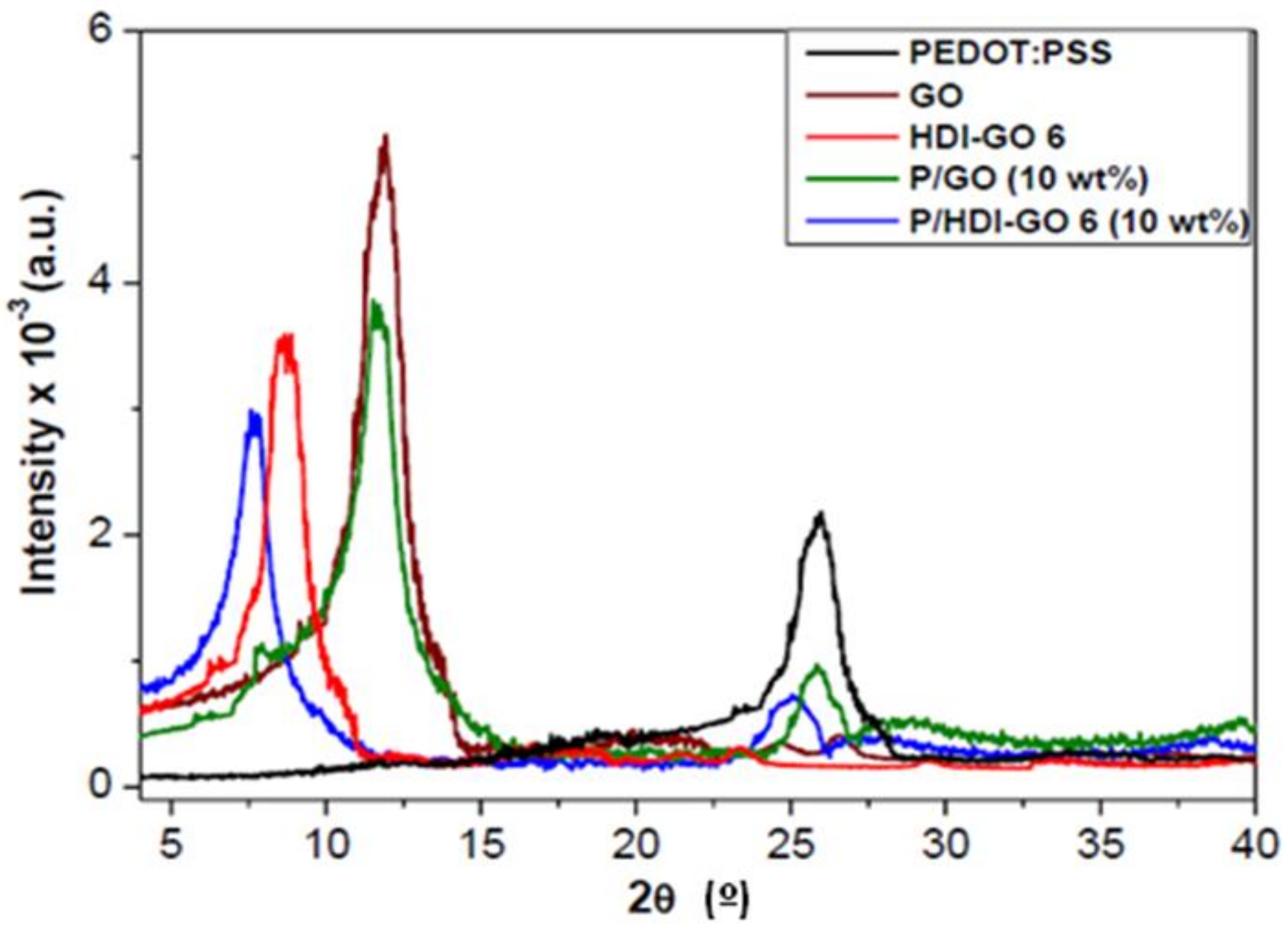
3.3. XRD Analysis of the Nanocomposites
3.4. Thermal Stability of the Nanocomposites
3.5. Optical Transmittance of the Nanocomposites
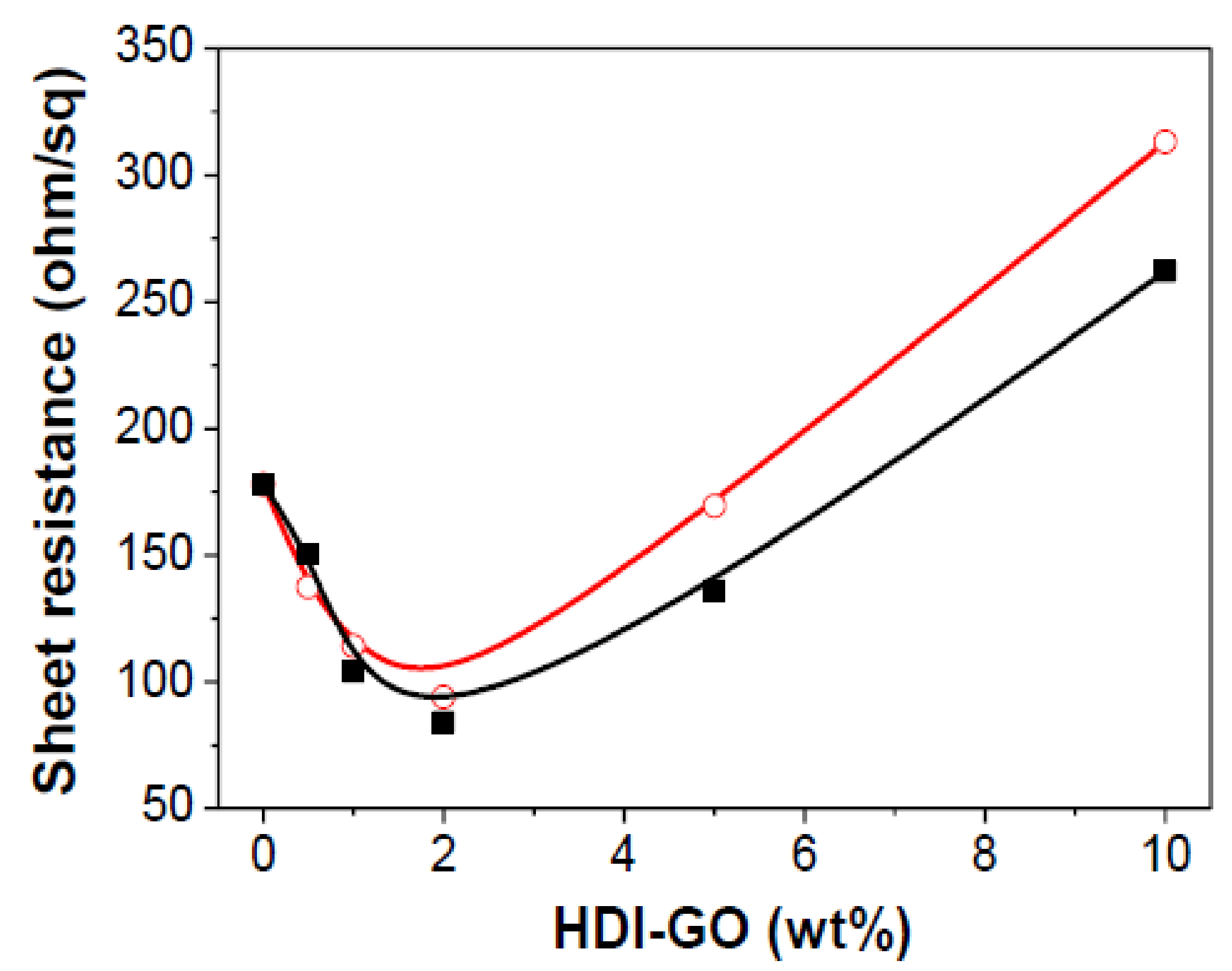
3.6. Sheet Resistance of the Nanocomposites
3.7. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dangqiang, Z.; Renqiang, Y. Conjugated Polymers and Their Applications in Organic Solar Cells. J. Nano Energy Power Res. 2013, 2, 73–91. [Google Scholar] [CrossRef]
- Yun, J.-M.; Yeo, J.-S.; Kim, J.; Jeong, H.-G.; Kim, D.-Y.; Noh, Y.-J.; Kim, S.-S.; Ku, B.-C.; Na, S.-I. Solution-processable reduced graphene oxide as a novel alternative to PEDOT:PSS hole transport layers for highly efficient and stable polymer solar cells. Adv. Mater. 2011, 23, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Diez-Pascual, A.M. Thermoplastic Polymer Nanocomposites Based on Inorganic Fullerene-like Nanoparticles and Inorganic Nanotubes. Inorganics 2014, 2, 291–312. [Google Scholar] [CrossRef] [Green Version]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbarch, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, H. Graphene-Based Nanomaterials: Synthesis, Properties, and Optical and Optoelectronic Applications. Adv. Funct. Mater. 2013, 23, 1984–1997. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Luceño-Sanchez, J.A.; Peña-Capilla, R.; Garcia-Diaz, P. Recent Developments in Graphene/Polymer Nanocomposites for Application in Polymer Solar Cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef]
- Lian, H.T.; Zhenyu, T.; Hongen, G.; Zheng, Z.; Jian, W.; Qingchen, D.; Furong, Z.; Bin, W.; Wong, W.-Y. Magnetic nanoparticles/PEDOT:PSS composite hole-injection layer for efficient organic light-emitting diodes. J. Mater. Chem. C 2018, 6, 4903–4911. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The Chemistry of Graphene Oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Poly(Propylene Fumarate)/Polyethylene Glycol-Modified Graphene Oxide Biocomposites for Tissue Engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Hermosa-Ferreira, C.; San Andres, M.P.; Valiente, M.; Vera, S. Effect of Graphene and Graphene Oxide Dispersions in Poloxamer-407 on the Fluorescence of Riboflavin: A Comparative Study. J. Phys. Chem. C 2017, 121, 830–843. [Google Scholar] [CrossRef]
- Zheng, Q.; Kim, J.K. Synthesis, Structure and Properties of Graphene and Graphene Oxide. In Graphene for Transparent Conductors. Synthesis, Properties and Applications; Springer: New York, NY, USA, 2015; pp. 29–94. ISBN 978-1493927685. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Diez-Pascual, A.M.; Gomez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation in polymer nanocomposites. Prog. Mater. Sci. 2015, 67, 1–94. [Google Scholar] [CrossRef] [Green Version]
- Salavagione, H.J.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gómez-Fatou, M.A. Chemical sensors based on polymer composites with carbon nanotubes and graphene: The role of the polymer. J. Mater. Chem. A 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Garcia-Garcia, D.; San Andres, M.P.; Vera, S. Determination of riboflavin based on fluorescence quenching by graphene dispersions in polyethylene glycol. RSC Adv. 2016, 6, 19686–19699. [Google Scholar] [CrossRef]
- Wang, H.L.; Hao, Q.L.; Xia, X.F.; Wang, Z.J.; Tian, J.; Zhu, J.H.; Tang, C.; Wang, X. In Situ Fabrication of Nanoscale Graphene Oxide/Polyaniline Composite and its Electrochemical Properties. Adv. Mat. Res. 2010, 148–149, 1547–1550. [Google Scholar] [CrossRef]
- Yu, D.; Yang, Y.; Durstock, M.; Baek, J.-B.; Dai, L. Soluble P3HT-Grafted Graphene for Efficient Bilayer−Heterojunction Photovoltaic Devices. ACS Nano 2010, 4, 5633–5640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, K.; Lee, T.; Choi, H.J.; Park, J.H.; Lee, D.J.; Lee, D.W.; Kim, B.-S. Stable Aqueous Dispersion of Reduced Graphene Nanosheets via Non-Covalent Functionalization with Conducting Polymers and Application in Transparent Electrodes. Langmuir 2011, 27, 2014–2018. [Google Scholar] [CrossRef] [PubMed]
- Trang, L.K.H.; Thanh Tung, T.; Young Kim, T.; Yang, W.S.; Kim, H.; Suh, K.S. Preparation and characterization of graphene composites with conducting polymers. Polym. Int. 2012, 61, 93–98. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Chen, X.; Wu, N.; Nguyen, S.T.; Ruoff, R.S. Stable aqueous dispersions of graphitic nanoplatelets via the reduction of exfoliated graphite oxide in the presence of poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 16, 155–158. [Google Scholar] [CrossRef]
- Kim, G.H.; Hwang, D.H.; Woo, S.I. Thermoelectric properties of nanocomposite thin films prepared with poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) and graphene. Phys. Chem. Chem. Phys. 2012, 14, 3530–3536. [Google Scholar] [CrossRef] [PubMed]
- Luceño, J.A.; Maties, G.; Gonzalez-Arellano, C.; Díez-Pascual, A.M. Synthesis and characterization of graphene oxide derivatives via functionalization reaction with hexamethylene diisocyanate. In Proceedings of the 1st International Online-Conference on Nanomaterials, 1–15 September 2018. [Google Scholar] [CrossRef]
- Miyamoto, S. Hydrophobic interaction between alkyl side chains of polyions and alkyl quaternary ammonium ions. Macromolecules 1981, 14, 1054–1058. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, J.; Zhu, K.; Li, J.; Wang, J.; Li, S.; Wang, X. Ultra high permittivity and significantly enhanced electric field induced strain in PEDOT:PSS–RGO@PU intelligent shape-changing electro-active polymers. RSC Adv. 2014, 4, 64061–64067. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Valles, C.; Mateos, R.; Vera-López, S.; Kinloch, I.A.; San Andrés, M.P. Influence of surfactants of different nature and chain length on the morphology, thermal stability and sheet resistance of graphene. Soft Matter 2018, 14, 6013–6023. [Google Scholar] [CrossRef] [PubMed]
- Dresselahaus, M.S.; Jorio, A.; Souza, A.G.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, D.; Kim, J.; Lee, S.H.; Cho, W.; Choi, H.H.; Kin, F.S.; Kim, J.H. Effects of one- and two-dimensional carbon hybridization of PEDOT:PSS on the power factor of polymer thermoelectric energy conversion devices. J. Mater. Chem. A 2015, 3, 6526–6533. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, D.; Liu, J.; Luo, Y. Conducting polymer aerogels from supercritical CO2 drying PEDOT-PSS hydrogels. J. Mater. Chem. 2010, 20, 5080–5085. [Google Scholar] [CrossRef]
- Lee, C.-P.; Lai, K.-Y.; Lin, C.-A.; Li, C.-T.; Ho, K.-C.; Wu, C.-I.; Lau, S.-P.; He, J.-H. A paper based electrode using a graphene dot/PEDOT:PSS composite for flexible solar cells. Nano Energy 2017, 36, 260–267. [Google Scholar] [CrossRef]
- Kou, L.; He, H.; Gao, C. Click Chemistry Approach to Functionalize Two-Dimensional Macromolecules of Graphene Oxide Nanosheets. Nano-Micro Lett. 2010, 2, 177–183. [Google Scholar] [CrossRef]
- Alexander, L.E.; Krieger, R.E. X-ray Diffraction Methods in Polymer Science; Wiley: New York, NY, USA, 1969. [Google Scholar]
- Wang, C.; Feng, L.; Yang, H.; Xin, G.; Li, W.; Zheng, J.; Tian, W.; Li, X. Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 2012, 14, 13233–13238. [Google Scholar] [CrossRef] [PubMed]
- Friedel, B.; Keivanidis, P.E.; Brenner, T.J.K.; Abrusci, A.; McNeill, C.R.; Friend, R.H.; Greenham, N.C. Effects of Layer Thickness and Annealing of PEDOT:PSS Layers in Organic Photodetectors. Macromolecules 2009, 42, 6741–6747. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Liang, J.; Huang, Y.; Ma, Y.; Wan, X.; Chen, Y. A hybrid material of graphene and poly(3,4-ethyldioxythiophene) with high conductivity, flexibility, and transparency. Nano Res. 2009, 2, 343–348. [Google Scholar] [CrossRef]
- Hu, W.; Zhan, G.; Wang, X.; Hong, N.; Wang, B.; Song, L.; Stec, A.A.; Hull, R.; Wang, J.; Hu, Y. Effect of Functionalized Graphene Oxide with Hyper-Branched Flame Retardant on Flammability and Thermal Stability of Cross-Linked Polyethylene. Ind. Eng. Chem. Res. 2014, 53, 3073–3083. [Google Scholar] [CrossRef] [Green Version]
- Neto, A.H.C.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109. [Google Scholar] [CrossRef]
- Byrne, M.T.; Gunko, Y.K. Recent Advances in Research on Carbon Nanotube-Polymer Composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.S.; Singh, J.P.; Singh, R. A novel composite material of graphene and PEDOT:PSS. AIP Conf. Proc. 2016, 1731, 140021. [Google Scholar] [CrossRef]
- Chopra, K.L.; Major, S.; Pandya, D.K. Transparent conductors—A status review. Thin Solid Films 1983, 102, 1–46. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Ouyang, J. Solution-Processed Metallic Conducting Polymer Films as Transparent Electrode of Optoelectronic Devices. Adv. Mater. 2012, 24, 2436–2440. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Xu, Q.; Chu, C.-W.; Yang, Y.; Li, G.; Shinar, J. On the mechanism of conductivity enhancement in poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) film through solvent treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of electrical conductivity of poly(3,4-ethylenedioxythiophene)/poly(4-styrenesulfonate) by a change of solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Lee, J.R.; Lee, D.Y.; Kim, D.G.; Lee, G.H.; Kim, Y.D.; Song, P.K. Characteristics of ITO films deposited on a PET substrate under various deposition conditions. Met. Mater. Int. 2008, 14, 745. [Google Scholar] [CrossRef]
- Wöbkenberg, P.H.; Eda, G.; Leem, D.-S.; Mello, J.C.; Bradley, D.D.C.; Chhowalla, M.; Anthopoulos, T.D. Reduced Graphene Oxide Electrodes for Large Area Organic Electronics. Adv. Mater. 2011, 23, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.H.; Cuong, T.V.; Hur, S.H.; Shin, E.W.; Kim, J.S.; Chung, J.S.; Kim, E.J. Fast and simple fabrication of a large transparent chemically-converted graphene film by spray-coating. Carbon 2010, 48, 1945–1951. [Google Scholar] [CrossRef]
- Okuzaki, H.; Ishihara, M. Spinning and Characterization of Conducting Microfibers. Macromol. Rapid Commun. 2003, 24, 261–264. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Monolayer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Layek, R.K.; Nandi, A.K. A review on synthesis and properties of polymer functionalized graphene. Polymer 2013, 54, 5087–5103. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M.; González-Domínguez, J.M.; Ansón, A.; Martínez-Rubi, Y.; Martínez, M.T.; Simard, B.; Gómez, M.A. High performance PEEK/carbon nanotube composites compatibilized with polysulfones-II. Mechanical and electrical properties. Carbon 2010, 48, 3500–3511. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Naffakh, M.; Marco, C.; Ellis, G.; Gomez-Fatou, M.A. High-performance nanocomposites based on polyetherketones. Prog. Mater. Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luceño Sánchez, J.A.; Peña Capilla, R.; Díez-Pascual, A.M. High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties. Polymers 2018, 10, 1169. https://doi.org/10.3390/polym10101169
Luceño Sánchez JA, Peña Capilla R, Díez-Pascual AM. High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties. Polymers. 2018; 10(10):1169. https://doi.org/10.3390/polym10101169
Chicago/Turabian StyleLuceño Sánchez, José Antonio, Rafael Peña Capilla, and Ana Maria Díez-Pascual. 2018. "High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties" Polymers 10, no. 10: 1169. https://doi.org/10.3390/polym10101169
APA StyleLuceño Sánchez, J. A., Peña Capilla, R., & Díez-Pascual, A. M. (2018). High-Performance PEDOT:PSS/Hexamethylene Diisocyanate-Functionalized Graphene Oxide Nanocomposites: Preparation and Properties. Polymers, 10(10), 1169. https://doi.org/10.3390/polym10101169