Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Ultrasonication
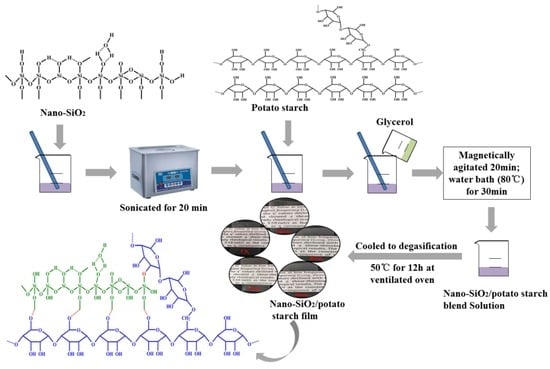
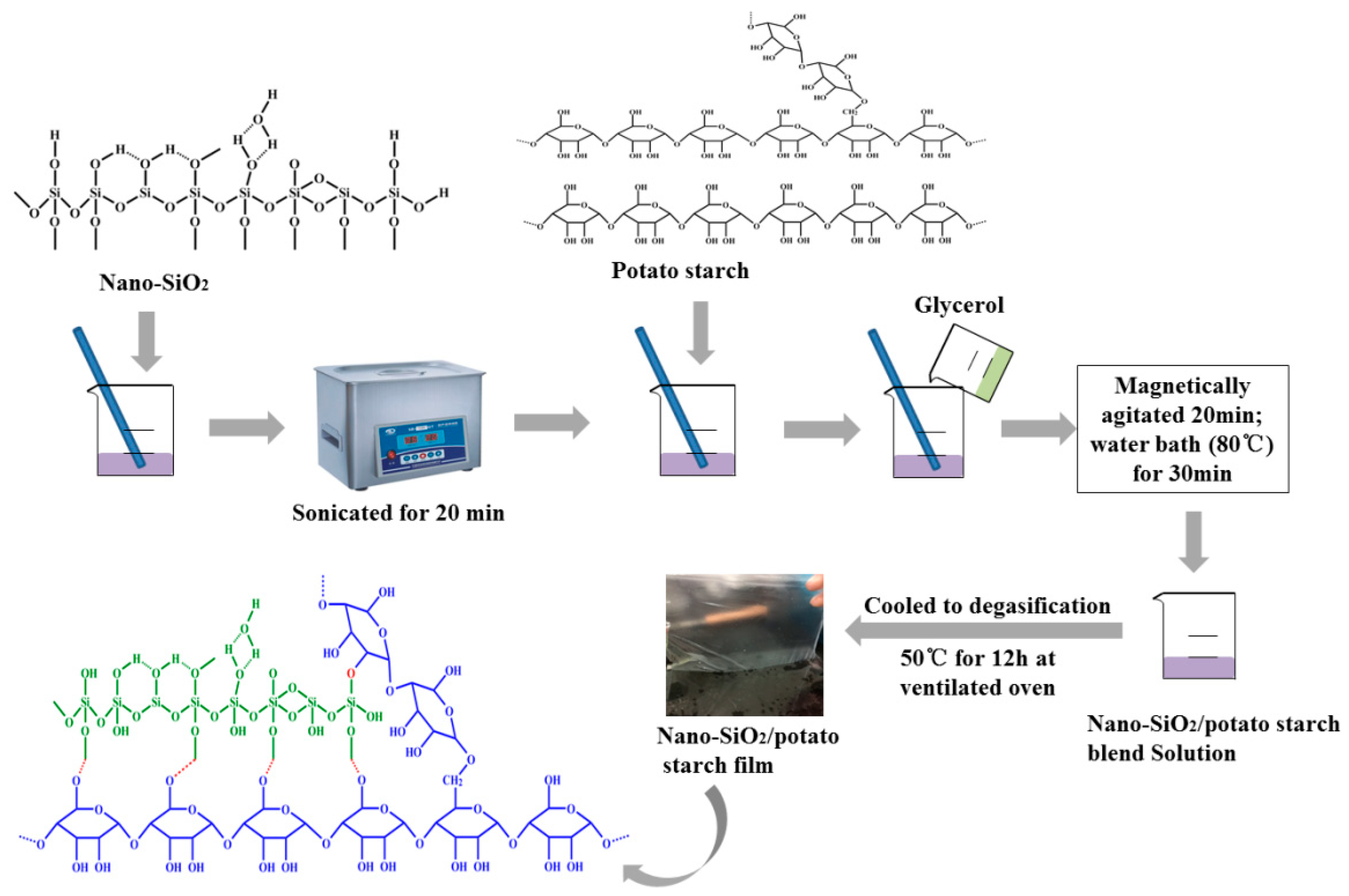
2.3. Preparation of Nano-SiO2/Potato Starch Films
2.4. Physical Properties of the Films
2.4.1. Water Vapor Transmission Rate
2.4.2. Thickness, Oxygen Transmission Rate (OTR), Carbon Dioxide Transmission Rate (CDTR) and Water Resistance of the Films
2.5. Tensile Strength
2.6. Optical Property
2.7. Thermal Properties
2.8. Characterization
2.8.1. Scanning Electron Microscopy (SEM) of Native Starch and Films
2.8.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.8.3. X-ray Diffraction (XRD)
2.9. Antibacterial Property
2.9.1. Scanning Electron Microscope Assay
2.9.2. Agar Disk Diffusion Assay
2.10. Preservation Property
2.11. Statistical Analysis
3. Results and Discussion
3.1. SEM Analysis
3.2. XRD Analysis
3.3. FTIR Analysis
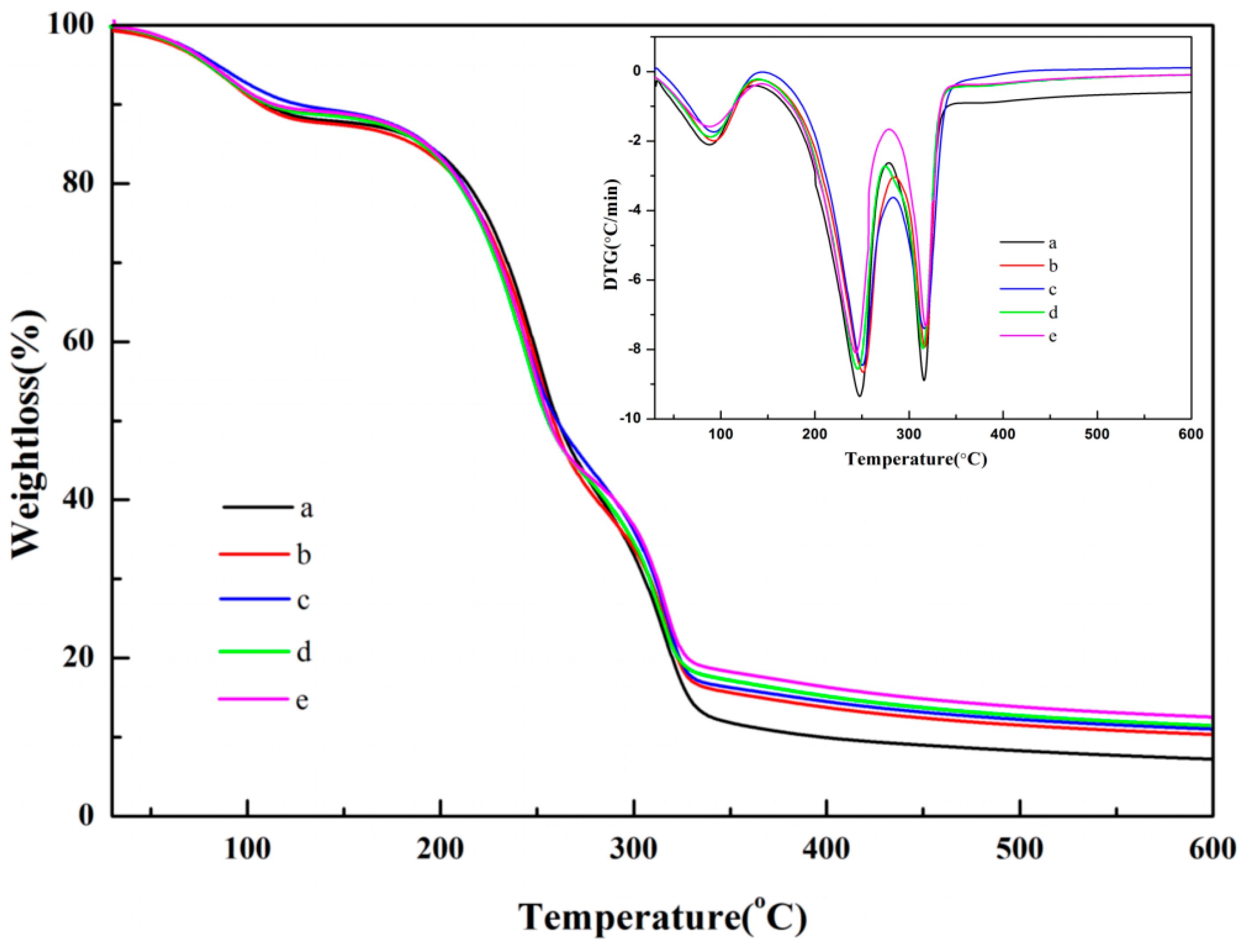
3.4. TG and DTG Analysis
3.5. Physical and Mechanical Properties of the Films
3.5.1. Water Vapor Transmission Rate Analysis
3.5.2. Tensile Strength Analysis
3.5.3. Optical Property Analysis
3.5.4. Thickness
3.5.5. CDTR and OTR Analysis
3.5.6. Water Resistance
3.6. Antibacterial Property Analysis
3.6.1. Scanning Electron Microscopy
3.6.2. Antibacterial Activity of the Films
3.7. Preservation Properties of the Films on White Mushrooms (A. Bisporus)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vargas, C.G.; Costa, T.M.H.; Rios, A.D.O.; Flôres, S.H. Comparative study on the properties of films based on red rice (oryza glaberrima) flour and starch. Food Hydrocoll. 2017, 65, 96–106. [Google Scholar] [CrossRef]
- Pagno, C.H.; Costa, T.M.H.; De Menezes, E.W.; Benvenutti, E.V.; Hertz, P.F.; Matte, C.R.; Tosati, J.V.; Monteiro, A.R.; Rios, A.O.; Flôres, S.H. Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem. 2015, 173, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhang, H.; Jia, R.; Dai, Y.Y.; Dong, H.Z.; Hou, H.X.; Guo, Q.B. High performance extrusion blown starch/polyvinyl alcohol/clay nanocomposite films. Food Hydrocoll. 2018, 79, 534–543. [Google Scholar] [CrossRef]
- Podshivalov, A.; Zakharova, M.; Glazacheva, E.; Uspenskaya, M. Gelatin/potato starch edible biocomposite films: correlation between morphology and physical properties. Carbohydr. Polym. 2017, 157, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Oleyaei, S.A.; Almasi, H.; Ghanbarzadeh, B.; Moayedi, A.A. Synergistic reinforcing effect of TiO2 and montmorillonite on potato starch nanocomposite films: Thermal, mechanical and barrier properties. Carbohydr. Polym. 2016, 152, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Ortega, F.; Giannuzzi, L.; Arce, V.B.; García, M.A. Active composite starch films containing green synthetized silver nanoparticles. Food Hydrocoll. 2017, 70, 152–162. [Google Scholar] [CrossRef]
- Sun, T.; Wu, C.L.; Hao, H.; Dai, Y.; Li, J.R. Preparation and preservation properties of the chitosan coatings modified with the in situ, synthesized nano siox. Food Hydrocoll. 2016, 54, 130–138. [Google Scholar] [CrossRef]
- Yang, M.L.; Shi, J.S.; Xia, Y.Z. Effect of SiO2, PVA and glycerol concentrations on chemical and mechanical properties of alginate-based films. Int. J. Biol. Macromol. 2018, 107, 2686–2694. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiong, H.; Tang, S.; Peng, Z. A starch-based biodegradable film modified by nano silicon dioxide. J. Appl. Polym. Sci. 2009, 113, 34–40. [Google Scholar] [CrossRef]
- Torabi, Z.; Mohammadinafchi, A. The effects of SiO2 nanoparticles on mechanical and physicochemical properties of potato starch films. J. Chem. Health Risks 2013, 3, 33–42. [Google Scholar]
- Rahman, P.M.; Mujeeb, V.M.A.; Muraleedharan, K.; Thomas, S.K. Chitosan/nano-ZnO composite films: Enhanced mechanical, antimicrobial and dielectric properties. Arabian J. Chem. 2018, 11, 120–127. [Google Scholar] [CrossRef]
- Talebian, N.; Zare, E. Structure and antibacterial property of nano-SiO2 supported oxide ceramic. Ceram. Int. 2014, 40, 281–287. [Google Scholar] [CrossRef]
- Song, H.W.; Yuan, W.M.; Jin, P.; Wang, W.; Wang, X.F.; Yang, L.M.; Zhang, Y.F. Effects of chitosan/nano-silica on postharvest quality and antioxidant capacity of loquat fruit during cold storage. Postharvest Biol. Technol. 2016, 119, 41–48. [Google Scholar] [CrossRef]
- Shi, S.Y.; Wang, W.; Liu, L.Q.; Wu, S.J.; Wei, Y.Z.; Li, W.C. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Tabatabaei, R.H.; Jafari, S.M.; Mirzaei, H.; Nafchi, A.M.; Dehnad, D. Preparation and characterization of nano-SiO2 reinforced gelatin-kcarrageenan biocomposites. Int. J. Biol. Macromol. 2018, 111, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, Y.H.; Chen, H.Y. Influence of depositing nano-SiO2 particles on the surface microstructure and properties of jute fibers via in situ synthesis. Compos. Part A 2018, 109, 368–375. [Google Scholar] [CrossRef]
- Ritamäki, M.; Rytöluoto, I.; Lahti, K.; Karttunen, M. Effects of thermal aging on the characteristic breakdown behavior of Nano-SiO2-BOPP and BOPP films. In Proceedings of the 11th International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Sydney, Australia, 19–22 July 2015. [Google Scholar]
- Gong, C.; Yang, L.Q.; Zhou, J.C.; Xiang, G.; Zhuang, Z.X. Possible role of PAPR-1 in protecting human HaCaT cells against cytotoxicity of SiO2 nanoparticles. Toxicol. Lett. 2017, 280, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.C.; Zeng, X.R.; Li, H.Q.; Zhang, J. Preparation and characterization of nano-SiO2/fluorinated polyacrylate composite latex via nano-SiO2/acrylate dispersion. Colloids Surf. A 2012, 396, 328–335. [Google Scholar] [CrossRef]
- Cui, X.; Zhong, S.; Yan, J.; Wang, C.; Zhang, H.; Wang, H. Synthesis and characterization of core–shell SiO2-fluorinated polyacrylate nanocomposite latex particles containing fluorine in the shell. Colloids Surf. A 2010, 360, 41–46. [Google Scholar] [CrossRef]
- Fortuni, B.; Fujita, Y.; Ricci, M.; Inose, T.; Aubert, R.; Lu, G. A novel method for in situ synthesis of sers-active gold nanostars on polydimethylsiloxane film. Chem. Commun. 2017, 53, 5121. [Google Scholar] [CrossRef] [PubMed]
- Hassannia-Kolaee, M.; Khodaiyan, F.; Pourahmad, R.; Shahabi-Ghahfarrokhi, I. Development of ecofriendly bionanocomposite: Whey protein isolate/pullulan films with nano-SiO2. Int. J. Biol. Macromol. 2016, 86, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiao, M.; Dai, S.H.; Song, J.; Ni, X.W.; Fang, Y.P.; Corke, H.; Jiang, F.T. Interactions between carboxymethyl konjac glucomannan and soy protein isolate in blended films. Carbohydr. Polym. 2014, 101, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Moreno, O.; Cárdenas, J.; Atarés, L.; Chiralt, A. Influence of starch oxidation on the functionality of starch-gelatin based active films. Carbohydr. Polym. 2017, 178, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Colussi, R.; Pinto, V.Z.; Slm, E.H.; Biduski, B.; Prietto, L.; Castilhos, D.D.; Zavareze, E.D.R.; Dias, A.R.G. Acetylated rice starches films with different levels of amylose: Mechanical, water vapor barrier, thermal, and biodegradability properties. Food Chem. 2016, 221, 1614–1620. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Yallapu, M.M.; Varaprasad, K.; Reddy, N.N.; Ravindra, S.; Naidu, N.S.; Raju, K.M. Fabrication of curcumin encapsulated chitosan–PVA silver nanocom posite films for improved antimicrobial activity. J. Biomater. Nanobiotechnol. 2011, 2, 55–64. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wang, X.Y.; Zhu, J.Y.; Wang, J. Effect of high oxygen modified atmosphere on post-harvest physiology and sensorial qualities of mushroom. Int. J. Food Sci. Technol. 2010, 45, 1097–1103. [Google Scholar] [CrossRef]
- Lizundia, E.; Vilas, J.L.; Sangroniz, A.; Etxeberria, A. Light and gas barrier properties of PLLA/metallic nanoparticles composite films. Eur. Polym. J. 2017, 91, 10–20. [Google Scholar] [CrossRef]
- Wu, C.H.; Peng, S.H.; Wen, C.R.; Wang, X.M.; Fan, L.L.; Deng, R.H.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Physicochemical and antifungal properties of bio-nanocomposite film based on gelatin-chitin nanoparticles. Int. J. Biol. Macromol. 2017, 97, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, T.; Netravali, A. Cross-linked waxy maize starch-based green composites. ACS Sustain. Chem. Eng. 2013, 1, 1537–1544. [Google Scholar] [CrossRef]
- Yong, C.; Xie, K.; Pan, Y.; Chen, Y.; Xu, J.; Xing, X. Synthesis of Si/C/N ultrafine composite powder by pyrolysis of polynirisilicane. J. Chin. Ceram. Soc. 2000, 28, 491–493. [Google Scholar]
- Zhao, Y.L.; Qi, X.W.; Dong, Y.; Ma, J.; Zhang, Q.L.; Song, L.Z.; Yang, Y.L.; Yang, Q.X. Mechanical, thermal and tribological properties of polyimide/nano-SiO2 composites synthesized using an in-situ polymerization. Tribol. Int. 2016, 103, 599–608. [Google Scholar] [CrossRef]
- Jiang, S.S.; Liu, C.Z.; Wang, X.J.; Xiong, L.; Sun, Q.J. Physicochemical properties of starch nanocomposite films enhanced by self-assembled potato starch nanoparticles. LWT-Food Sci. Technol. 2016, 69, 251–257. [Google Scholar] [CrossRef]
- Dai, L.; Qiu, C.; Xiong, L.; Sun, Q. Characterisation of corn starch-based films reinforced with taro starch nanoparticles. Food Chem. 2015, 174, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Shahabi-Ghahfarrokhi, I.; Khodaiyan, F.; Mousavi, M.; Yousefi, H. Preparation of UV-protective kefiran/Nano-ZnO nanocomposite: physical and mechanical properties. Int. J. Biol. Macromol. 2015, 72, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Kristo, E.; Biliaderis, C.G. Physical properties of starch nanocrystal-reinforced pullulan films. Carbohyd. Polym. 2007, 68, 146–158. [Google Scholar] [CrossRef]
- Almasi, H.; Ghanbarzadeh, B.; Entezami, A.A. Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int. J. Biol. Macromol. 2010, 46, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.G.; Tang, S.W.; Tang, H.L.; Zou, P. The structure and properties of a starch-based biodegradable film. Carbohydr. Polym. 2008, 71, 263–268. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Characterization of edible packaging films based on semi-refined kappa-carrageenan plasticized with glycerol and sorbitol. Food Hydrocoll. 2017, 64, 48–58. [Google Scholar] [CrossRef]
- Luchese, C.L.; Garrido, T.; Spada, J.C.; Tessaro, I.C.; La Caba, K.D. Development and characterization of cassava starch films incorporated with blueberry pomace. Int. J. Biol. Macromol. 2018, 106, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Biduski, B.; Silva, F.T.; Silva, W.M.; Halal, S.L.; Pinto, V.Z.; Dias, A.R. Impact of acid and oxidative modifications, single or dual, of sorghum starch on biodegradable films. Food Chem. 2017, 214, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Hanani, Z.A.N.; Kerry, J.P.; Caba, K.D.L. Characterization of soy protein-based films prepared with acids and oils by compression. J. Food Eng. 2011, 107, 41–49. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Lutfi, Z.; Hasnain, A. Mango kernel starch-gum composite films: Physical, mechanical and barrier properties. Int. J. Biol. Macromol. 2017, 98, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Peng, J.; Yang, Y.; Zhang, L.; Liao, B.; Xiao, F. Effect of nano-SiO2, on the performance of POLY (MMA/BA/MAA)/EP. Mater. Lett. 2007, 61, 725–729. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wang, X.; Cheng, M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers 2018, 10, 1172. https://doi.org/10.3390/polym10101172
Zhang R, Wang X, Cheng M. Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers. 2018; 10(10):1172. https://doi.org/10.3390/polym10101172
Chicago/Turabian StyleZhang, Rongfei, Xiangyou Wang, and Meng Cheng. 2018. "Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2" Polymers 10, no. 10: 1172. https://doi.org/10.3390/polym10101172
APA StyleZhang, R., Wang, X., & Cheng, M. (2018). Preparation and Characterization of Potato Starch Film with Various Size of Nano-SiO2. Polymers, 10(10), 1172. https://doi.org/10.3390/polym10101172