Cationic Moieties in Polystyrene Gels Swollen with d-Limonene Improved Transdermal Delivery System
Abstract
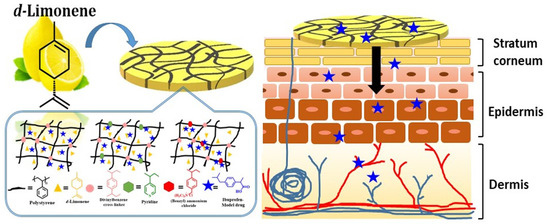
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Organogels
2.3. Swelling Property
2.4. Rheological Study
2.5. Preparation of Rat Skin
2.6. In Vitro Skin Permeation Study
2.7. HPLC Measurements for Drug Assay
2.8. Calculation of Permeation Parameter
3. Results and Discussion
3.1. Synthesis and Characterization of Limonene Organogels
3.2. Swelling Behavior and Rheological Property of PS Gels Swollen in Limonene
3.3. Effect of Limonene as Chemical Enhancer and Density of the Network Structure on Permeability
3.4. Effect of the Cationic Moiety on the Controlled Release of Ibuprofen for Permeation through the Skin
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.; Marks, R. Delivering drugs by the transdermal route: review and comment. Skin Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevc, G.; Vierl, U. Nanotechnology and the transdermal route: A state of the art review and critical appraisal. J. Control. Release 2010, 141, 277–299. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sunoqrot, S.; Stowell, C.; Ji, J.; Lee, C.-W.; Kim, J.W.; Khan, S.A.; Hong, S. Effect of size, surface charge and hydrophobicity of poly(amidoamine) dendrimers on their skin penetration. Biomacromolecules 2012, 13, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Songa, C.; Baika, S.; Kima, D.; Hyeona, T.; Kima, D.-H. Device-assisted transdermal drug delivery. Adv. Drug Deli. Rev. 2017, 127, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Karande, P.; Jain, A.; Ergun, K.; Kispersky, V.; Mitragotri, S. Design principles of chemical penetration enhancers for transdermal drug delivery. Proc. Natl. Acad. Sci. USA 2005, 102, 4688–4693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyagi, T.; Terashima, O.; Suzuki, N.; Matsui, K.; Nagase, Y. Polymerization of benzalkonium chloride-type monomer and application to percutaneous drug absorption enhancer. J. Control. Release 1990, 13, 63–71. [Google Scholar] [CrossRef]
- Vaddi, H.K.; Ho, P.C.; Chan, Y.W.; Chan, S.Y. Terpenes in ethanol: haloperidol permeation and partition through human skin and stratum corneum changes. J. Control. Release 2002, 81, 121–133. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Terpenes and the lipid–protein-partitioning theory of skin penetration enhancement. Pharm. Res. 1991, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, Q.-D.; Chai, Y.-P.; Zhang, H.; Peng, P.; Yang, X.-X. Natural terpenes as penetration enhancers for transdermal drug delivery. Molecule 2016, 12, 1709. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Chetoni, P.; Burgalassi, S.; Najarro, M.; Saettone, M.F.; Boldrini, E. Effect of different terpene-containing essential oils on permeation of estradiol through hairless mouse skin. Int. J. Pharm. 2002, 237, 209–214. [Google Scholar] [CrossRef]
- Yang, Z.; Teng, Y.; Wang, H.; Hou, H. Enhancement of skin permeation of bufalin by limonene via reservoir type transdermal patch: Formulation design and biopharmaceutical evaluation. Int. J. Pharm. 2013, 447, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Okada, J.; Sugibayashi, K. Enhancing effects of six chemical enhancers on the permeation of ketotifen through excised hairless mouse skin from aqueous donor solutions buffered at pH 5 and 10. J Drug Deliv. Sci. Technol. 2008, 18, 335–341. [Google Scholar] [CrossRef]
- Almirall, M.; Montaña, J.; Escribano, E.; Obach, R.; Berrozpe, J.D. Effect of d-limonene, alpha-pinene and cineole on in vitro transdermal human skin penetration of chlorpromazine and haloperidol. Drug Res. 1996, 46, 676–680. [Google Scholar]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar] [PubMed]
- Saroha1, K.; Singh, S.; Aggarwal, A.; Nanda, S. Transdermal gels —An alternative vehicle for drug delivery. IJPCBS 2003, 3, 495–503. [Google Scholar]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: an update of state-of-the-art and recent applications. J. Control. Release 2018, 271, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.F.; Liu, X.Y.; Kang, L.; Ho, P.C.; Chan, Y.W.; Chan, S.Y. Limonene GP1/PG organogel as a vehicle in transdermal delivery of haloperidol. Int. J Pharm. 2006, 311, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Liandong, H.; Qiaofeng, H.; Yang, Y.J. Enhancement of transdermal delivery of ibuprofen using microemulsion vehicle. Iran J. Basic Med. Sci. 2014, 17, 760–766. [Google Scholar]
- Charoensumran, P.; Ajiro, H. The electrostatic advantages on cross-linked polystyrene organogels swollen with limonene for selective adsorbent and hydrophobic drug storage. Polym. J. 2018. [Google Scholar] [CrossRef]
- Monte, W.C.; Landau-West, D. Solubility of polystyrene in certain vegetable oils, essential oils and their constituents. J. Food Sci. 1982, 47, 1832–1835. [Google Scholar] [CrossRef]
- Chandra, S.; Mangalara, H.; Varughese, S. Green recycling approach to obtain nano- and microparticles from expanded polystyrene waste. ACS Sustain. Chem. 2016, 4, 6095–6100. [Google Scholar]
- Monti, D.M.; Guarnieri, D.; Napolitano, G.; Piccoli, R.; Nettic, P.; Fuscoc, S.; Arciello, A. Biocompatibility, uptake and endocytosis pathways of polystyrenenanoparticles in primary human renal epithelial cells. J. Biotechnol. 2015, 193, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Tsyurupa, M.P.; Ilyin, M.M.; Andreeva, A.I.; Davankov, V.A. Use of the hyper-crosslinked polystyrene sorbents “tyrosorb” for solid phase extraction of phenols from water. Fresenius J. Anal. Chem. 1995, 352, 672–675. [Google Scholar] [CrossRef]
- Zhu, H.; Qiu, S.; Jiang, W.; Wu, D.; Zhang, C. Evaluation of electrospun polyvinyl chloride/polystyrene fibers as sorbent materials for oil spill cleanup. Environ. Sci. Technol. 2011, 45, 4527–4531. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, H. Methotrexate-loaded porous polymeric adsorbents as oral sustained release formulations. Mater. Sci. Eng. C 2017, 78, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; Wijngaart, W.; Stemme, G.; Niklaus, F. Flexible and stretchable microneedle patches with integrated rigid stainless steel microneedles for transdermal biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef] [PubMed]
- Coulman, S.A.; Anstey, A.; Gateley, C.; Morrissey, A.; McLoughlin, P.; Allendera, C.; Birchall, J.C. Microneedle mediated delivery of nanoparticles into human skin. Int. J. Pharm. 2009, 366, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: Oxford, UK, 1975; pp. 47–53. [Google Scholar]
- Vaddi, H.K.; Wang, L.Z.; Ho, P.C.; Chan, S.Y. Effect of some enhancers on the permeation of haloperidol through rat skin in vitro. Int. J. Pharm. 2001, 212, 247–255. [Google Scholar] [CrossRef]
- Vaddi, H.K.; Ho, P.C.; Chan, S.Y. Terpenes in propylene glycol as skin-penetration enhancers: Permeation and partition of haloperidol fourier transform infrared spectroscopy and differential scanning calorimetry. J. Pharm. Sci. 2002, 91, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, H. Skin adsorption of solvent mixtures, effect of vehicles on skin adsorption of toluene. Ind. Health 1996, 34, 369–378. [Google Scholar] [CrossRef] [PubMed]






| Entry | Sample | Gel Type | Ratio of PSn-DVBm-SDp (n: m:p) a | Comonomer | Comonomer (mol %) | Conc. (M) | G’ (Pa) |
|---|---|---|---|---|---|---|---|
| 1 | Gel A | PS gel | 95:5:0 | - | - | 4 | 680 |
| 2 | Gel B | 90:10:0 | - | - | 4 | 6800 | |
| 3 | Gel C | 95:5:0 | - | - | 8 | 44,200 | |
| 4 | Gel D | 90:10:0 | - | - | 8 | 98,600 | |
| 5 | Gel E b | 95:5:0 | - | - | 4 | 1600 | |
| 6 | Gel F | PS-4VP gel | 95:5:2 | 4VP | 2 | 4 | 2600 |
| 7 | Gel G | 95:5:2.5 | 4VP | 2.5 | 4 | 10,300 | |
| 8 | Gel H | 95:5:3 | 4VP | 3 | 4 | 11,700 | |
| 9 | Gel I | 95:5:4 | 4VP | 4 | 4 | 21,300 | |
| 10 | Gel J | 95:5:5 | 4VP | 5 | 4 | 14,000 | |
| 11 | Gel K | PS-VBAC gel | 95:5:0.5 | VBAC | 0.5 | 4 | 5000 |
| 12 | Gel L | 95:5:1 | VBAC | 1 | 4 | 1400 |
| Entry | Gel | Flux, Jss (μg/cm2⋅h) | Lag Time (h) | D/L2 (h−1) | KD/L (*10−4 cm/h) |
|---|---|---|---|---|---|
| 1 | Gel A | 43.7 ± 0.3 | 1.72 ± 0.16 | 0.10 ± 1.03 | 53.1 ± 0.03 |
| 2 | Gel B | 29.4 ± 2.5 | 1.31 ± 0.29 | 0.13 ± 0.58 | 35.8 ± 0.30 |
| 3 | Gel C | 29.9 ± 1.0 | 3.15 ± 0.16 | 0.05 ± 1.06 | 36.4 ± 0.12 |
| 4 | Gel D | 14.7 ± 1.6 | 1.18 ± 0.36 | 0.14 ± 0.46 | 17.9 ± 0.20 |
| 5 | Gel E | 50.9 ± 5.5 | 0.91 ± 0.16 | 0.18 ± 1.06 | 61.9 ± 0.67 |
| Entry | Gel | Mol % of Cationic Moiety | Flux, Jss (μg/cm2⋅h) | Lag Time (h) | D/L2 (h-1) | KD/L (*10−4 cm/h) |
|---|---|---|---|---|---|---|
| 1 | Gel F | 2 | 55.2 ± 0.80 | 0.51 ± 0.15 | 0.32 ± 1.14 | 67.1 ± 0.10 |
| 2 | Gel G | 2.5 | 44.2 ± 7.22 | 1.22 ± 0.37 | 0.14 ± 0.45 | 53.7 ± 0.88 |
| 3 | Gel H | 3 | 34.4 ± 1.71 | 1.67 ± 0.48 | 0.10 ± 0.35 | 41.8 ± 0.21 |
| 4 | Gel I | 4 | 18.1 ± 3.13 | 3.77 ± 0.18 | 0.04 ± 0.93 | 22.0 ± 0.38 |
| 5 | Gel J | 5 | 11.6 ± 2.03 | 3.27 ± 0.08 | 0.05 ± 2.00 | 14.1 ± 0.25 |
| 6 | Gel K | 0.5 | 30.8 ± 3.14 | 2.22 ± 0.42 | 0.07 ± 0.40 | 37.4 ± 0.38 |
| 7 | Gel L | 1 | 22.1 ± 2.42 | 3.02 ± 0.90 | 0.06 ± 0.18 | 26.9 ± 0.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charoensumran, P.; Ajiro, H. Cationic Moieties in Polystyrene Gels Swollen with d-Limonene Improved Transdermal Delivery System. Polymers 2018, 10, 1200. https://doi.org/10.3390/polym10111200
Charoensumran P, Ajiro H. Cationic Moieties in Polystyrene Gels Swollen with d-Limonene Improved Transdermal Delivery System. Polymers. 2018; 10(11):1200. https://doi.org/10.3390/polym10111200
Chicago/Turabian StyleCharoensumran, Preeyarad, and Hiroharu Ajiro. 2018. "Cationic Moieties in Polystyrene Gels Swollen with d-Limonene Improved Transdermal Delivery System" Polymers 10, no. 11: 1200. https://doi.org/10.3390/polym10111200
APA StyleCharoensumran, P., & Ajiro, H. (2018). Cationic Moieties in Polystyrene Gels Swollen with d-Limonene Improved Transdermal Delivery System. Polymers, 10(11), 1200. https://doi.org/10.3390/polym10111200