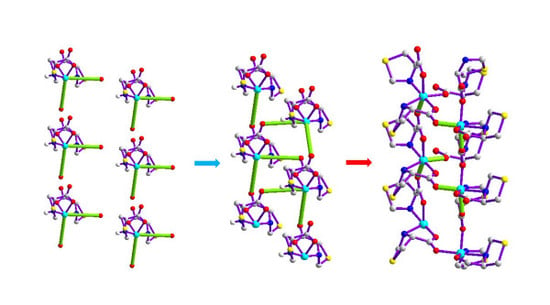
Structural Transformations of Amino-Acid-Based Polymers: Syntheses and Structural Characterization
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Physical Techniques
2.2. Synthesis of [Zn(Tpro)2(H2O)2] (1)
2.3. Synthesis of [Zn(tpro)2]n (2)
2.4. Synthesis of [Zn(tpro)2]n (3)
2.5. Crystallographic Data Collection and Refinement
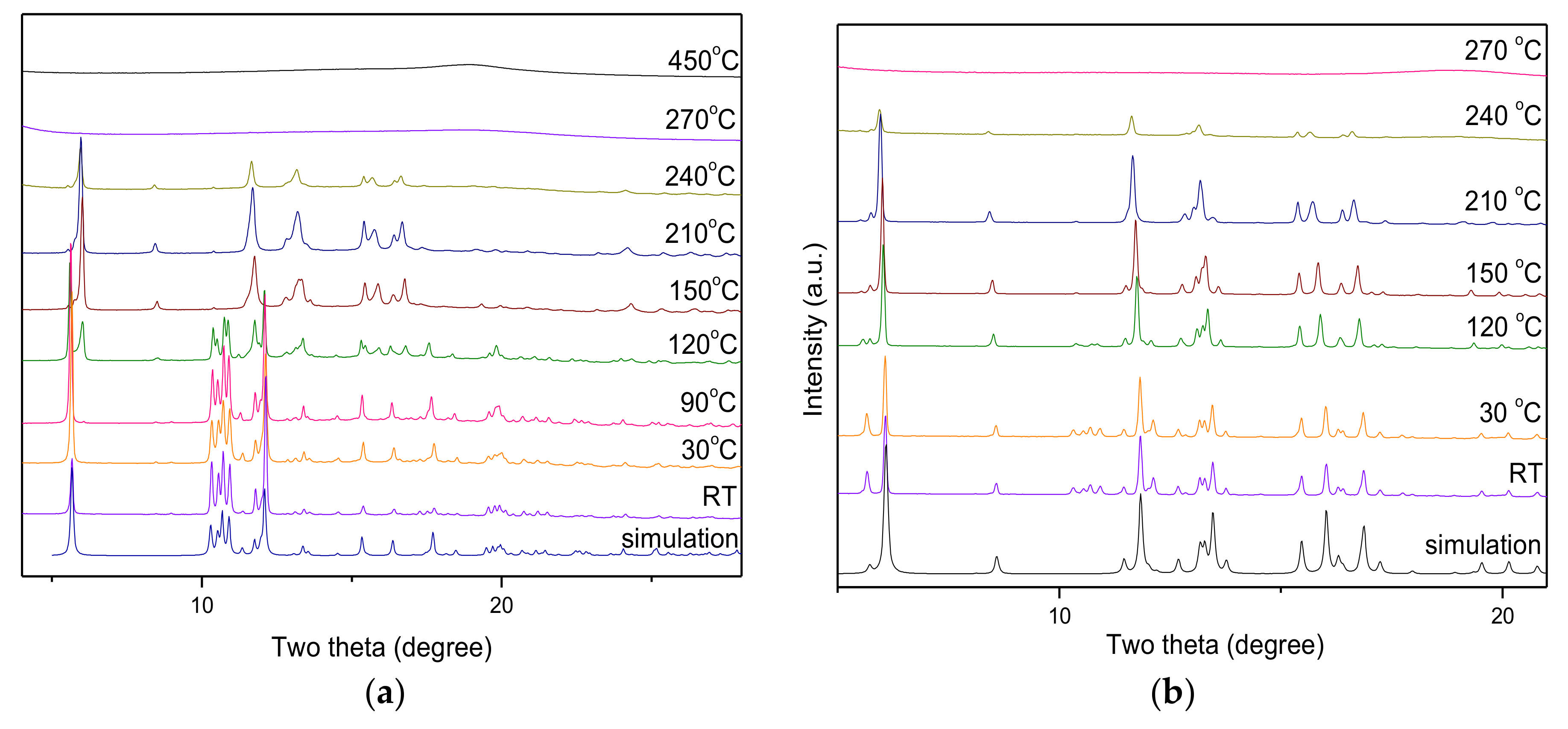
2.6. Powder X-ray Diffraction of Compounds 1–3
3. Results and Discussion
3.1. Syntheses of Compounds 1–3
3.2. Structural Description of [Zn(Tpro)2(H2O)2] (1)
3.3. Structural Description of [Zn(tpro)2]n (2)
3.4. Structural Description of [Zn(tpro)2]n (3)
3.5. The Study of Thermogravimetric Analysis and Powder X-ray Diffraction
3.6. Solid-State Thermal-Driven Structural Transformations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aggarwal, H.; Das, R.K.; Bhatt, P.M.; Barbour, L.J. Isolation of a structural intermediate during switching of degree of interpenetration in a metal-organic framework. Chem. Sci. 2015, 6, 4986–4992. [Google Scholar] [CrossRef]
- Kole, G.K.; Vittal, J.J. Solid-state reactivity and structural transformations involving coordination polymers. Chem. Soc. Rev. 2013, 42, 1755–1775. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Neogi, S.; Rissanen, K.; Bharadwaj, P.K. Solvent induced single-crystal to single-crystal structural transformation and concomitant transmetalation in a 3D cationic Zn(II)-framework. Chem. Commun. 2015, 51, 3173–3176. [Google Scholar] [CrossRef] [PubMed]
- Martí-Rujas, J.; Bonafede, S.; Tushib, D.; Cametti, M. Multiple single-crystal-to-single-crystal guest exchange in a dynamic 1D coordination polymer. Chem. Commun. 2015, 51, 12357–12360. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.X.; Shi, L.L.; Li, K.; Li, B.L.; Li, H.Y. An unusual porous cationic metal-organic framework based on a tetranuclear hydroxyl-copper(II) cluster for fast and highly efficient dichromate trapping through a single-crystal to single-crystal process. Chem. Commun. 2017, 53, 1860–1863. [Google Scholar] [CrossRef] [PubMed]
- Agnew, D.W.; DiMucci, I.M.; Arroyave, A.; Gembicky, M.; Moore, C.E.; MacMillan, S.N.; Rheingold, A.L.; Lancaster, K.M.; Figueroa, J.S. Crystalline coordination networks of zero-valent metal centers: Formation of a 3 dimensional Ni(0) framework with m-terphenyl diisocyanides. J. Am. Chem. Soc. 2017, 139, 17257–17260. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. The new age of MOFs and of their porous-related solids. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Mendecki, L.; Ko, M.; Zhang, X.; Meng, Z.; Mirica, K.A. Porous scaffolds for electrochemically controlled reversible capture and release of ethylene. J. Am. Chem. Soc. 2017, 139, 17229–17232. [Google Scholar] [CrossRef] [PubMed]
- Lochenie, C.; Schötz, K.; Panzer, F.; Kurz, H.; Maier, B.; Puchtler, F.; Agarwal, S.; Köhler, A.; Weber, B. Spin-crossover iron(II) coordination polymer with fluorescent properties: Correlation between emission properties and spin state. J. Am. Chem. Soc. 2018, 140, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Aoki, R.; Toyoda, R.; Kögel, J.F.; Sakamoto, R.; Kumar, J.; Kitagawa, Y.; Harano, K.; Kawai, T.; Nishihara, H. Bis(dipyrrinato)zinc(II) complex chiroptical wires: Exfoliation into single strands and intensification of circularly polarized luminescence. J. Am. Chem. Soc. 2017, 139, 16024–16027. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. functionalities on linking groups. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, L.; Maes, M.; Giebeler, L.; Jacobs, P.A.; Martens, J.A.; Denayer, J.F.M.; Kirschhock, C.E.A.; De Vos, D.E. Selective adsorption and separation of ortho-substituted alkylaromatics with the microporous aluminum terephthalate MIL-53. J. Am. Chem. Soc. 2008, 130, 14170–14178. [Google Scholar]
- Wu, J.Y.; Yang, S.L.; Luo, T.T.; Liu, Y.H.; Cheng, Y.W.; Chen, Y.F.; Wen, Y.S.; Lin, L.G.; Lu, K.L. Time-evolving self-organization and autonomous structural adaptation of cobalt(II)-organic framework materials with scu and pts nets. Chem. Eur. J. 2008, 14, 7136–7139. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Tong, M.L. Solvothermal in situ metal/ligand reactions: A new bridge between coordination chemistry and organic synthetic chemistry. Acc. Chem. Res. 2007, 40, 162–170. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, R.M.; Atwood, J.L. A hydrogen-bonded hexameric nanotoroidal assembly. Angew. Chem. Int. Ed. 2007, 46, 2394–2397. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Cheng, P.; Lin, W. Solvent-induced single-crystal to single-crystal transformation of a 2D coordination network to a 3D metal-organic framework greatly enhances porosity and hydrogen uptake. Chem. Commun. 2012, 48, 2846–2848. [Google Scholar] [CrossRef] [PubMed]
- Burnett, B.J.; Barron, P.M.; Hu, C.; Choe, W. Stepwise synthesis of metal-organic frameworks: Replacement of structural organic linkers. J. Am. Chem. Soc. 2011, 133, 9984–9987. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, H.; Bhatt, P.M.; Bezuidenhout, C.X.; Barbour, L.J. Direct evidence for single-crystal to single-crystal switching of degree of interpenetration in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Wang, S.; Guo, W.; Yan, Y.; Du, M. Dual structure evolution of a Ag(I) supramolecular framework triggered by anion-exchange: Replacement of terminal ligand and switching of network interpenetration degree. Chem. Commun. 2016, 52, 11060–11063. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; Brammer, L. Coordination change, lability and hemilability in metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 5444–5462. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, K.C.; Rabone, J.; Chong, S.Y.; Heck, R.; Armstrong, J.; Wiper, P.V.; Jelfs, K.E.; Zlatogorsky, S.; Bacsa, J.; McLennan, A.G.; et al. Dimensionality transformation through paddlewheel reconfiguration in a flexible and porous Zn-based metal-organic framework. J. Am. Chem. Soc. 2012, 134, 20466–20478. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Kou, H.Z. Solid state reconstructive phase transition from porous supramolecular network to porous coordination polymer. Chem. Commun. 2016, 52, 2952–2955. [Google Scholar] [CrossRef] [PubMed]
- Assi, H.; Mouchaham, G.; Steunou, N.; Devic, T.; Serre, C. Titanium coordination compounds: From discrete metal complexes to metal-organic frameworks. Chem. Soc. Rev. 2017, 46, 3431–3452. [Google Scholar] [CrossRef] [PubMed]
- He, W.W.; Li, S.L.; Zang, H.Y.; Yang, G.S.; Zhang, S.R.; Su, Z.M.; Lan, Y.Q. Entangled structures in polyoxometalate-based coordination polymers. Coord. Chem. Rev. 2014, 279, 141–160. [Google Scholar] [CrossRef]
- Kole, G.K.; Kojima, T.; Kawano, M.; Vittal, J.J. Reversible single-crystal-to-single-crystal photochemical formation and thermal cleavage of a cyclobutane ring. Angew. Chem. Int. Ed. 2014, 53, 2143–2146. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bučar, D.K.; Elacqua, E.; MacGillivray, L.R. Single-crystal-to-single-crystal direct cross-linking and photopolymerisation of a discrete Ag(I) complex to give a 1D polycyclobutane coordination polymer. Chem. Commun. 2013, 49, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zheng, Q.; Chen, Z.; Ling, Y.; Liu, X.; Weng, L.; Zhou, Y. A three-dimensional structure built of paddle-wheel and triazolate-dinuclear metal clusters: Synthesis, deformation and reformation of paddle-wheel unit in the single-crystal-to-single-crystal transformation. CrystEngComm 2013, 15, 7031–7037. [Google Scholar] [CrossRef]
- Girard, J.; Fromm, K. Single crystal to single crystal polymorphic phase transition of a silver nitrate 24-crown-8 complex and its pseudo-polymorphism. CrystEngComm 2012, 14, 6487–6491. [Google Scholar] [CrossRef]
- Tripathi, S.; Srirambalaji, R.; Patra, S.; Anantharaman, G. Anion triggered and solvent assisted structural diversity and reversible single-crystal-to-single crystal (SCSC) transformation between 1D and 2D coordination polymers. CrystEngComm 2015, 17, 8876–8887. [Google Scholar] [CrossRef]
- Ding, B.; Wang, Y.Y.; Liu, S.X.; Wu, X.X.; Zhu, Z.Z.; Huo, J.Z.; Liu, Y.Y. A series of multi-dimensional metal-organic frameworks with trans-4,4′-azo-1,2,4-triazole: Polymorphism, guest induced single-crystal-to single-crystal transformation and solvatochromism. CrystEngComm 2015, 17, 5396–5409. [Google Scholar] [CrossRef]
- Lanza, A.; Germann, L.S.; Fisch, M.; Casati, N.; Macchi, P. Solid-state reversible nucleophilic addition in a highly flexible MOF. J. Am. Chem. Soc. 2015, 137, 13072–13078. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Allan, P.K.; Renouf, C.L.; He, X.; McCormick, L.J.; Morris, R.E. Synthesis and structural characterization of a single-crystal to single-crystal transformable coordination polymer. Dalton Trans. 2014, 43, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Sakurada, K.; Muromoto, M.; Ito, H. Photoinduced single-crystal-to-single-crystal phase transition and photosalient effect of a gold(I) isocyanide complex with shortening of intermolecular aurophilic bonds. Chem. Sci. 2015, 6, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Zhou, H.; Wang, S.; Chen, J.; Wang, Z.L.; Du, M. Highly efficient Cr2O72− removal of a 3D metal-organic framework fabricated by tandem single-crystal to single-crystal transformations from a 1D coordination array. Chem. Commun. 2017, 53, 9206–9209. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Y.; Liu, Y.C.; Chao, T.C. From 1D Helix to 0D Loop: Nitrite anion induced structural transformation associated with unexpected N-nitrosation of amine ligand. Inorg. Chem. 2014, 53, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.; Shi, W.; Cheng, P. A cage-based cationic body-centered tetragonal metal-organic framework: Single-crystal to single-crystal transformation and selective uptake of organic dyes. Chem. Commun. 2015, 51, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Ma, J.G.; Shi, W.; Cheng, P. Water molecule-driven reversible single-crystal to single-crystal transformation of a multi-metallic coordination polymer with controllable metal ion movement. Chem. Commun. 2014, 50, 1839–1841. [Google Scholar] [CrossRef] [PubMed]
- Legrand, Y.M.; van der Lee, A.; Masquelez, N.; Rabu, P.; Barboiu, M. Temperature induced single-crystal-to-single-crystal transformations andstructure directed effects on magnetic properties. Inorg. Chem. 2007, 46, 9083–9089. [Google Scholar] [CrossRef] [PubMed]
- Bardelang, D.; Udachin, K.A.; Anedda, R.; Moudrakovski, I.; Leek, D.M.; Ripmeester, J.A.; Ratcliffe, C.I. Single-crystal to single-crystal phase transition of cucurbit[5]uril hydrochloride hydrates: Large water-filled channels transforming to layers of unusual stability. Chem. Commun. 2008, 4927–4929. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Li, C.P.; Chen, M.; Ge, Z.W.; Wang, X.; Wang, L.; Liu, C.S. Divergent kinetic and thermodynamic hydration of a porous Cu(II) coordination polymer with exclusive CO2 sorption selectivity. J. Am. Chem. Soc. 2014, 136, 10906–10909. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mandal, S.K. Capturing the structural diversification upon thermal desolvation of a robust metal-organic framework via a single-crystal-to-single-crystal transformation. CrystEngComm 2015, 17, 8801–8806. [Google Scholar] [CrossRef]
- Hong, X.J.; Wang, M.F.; Jin, H.G.; Zhan, Q.G.; Liu, Y.T.; Jia, H.Y.; Liu, X.; Cai, Y.P. Single-crystal to single-crystal transformation from a 1-D chain-like structure to a 2-D coordination polymer on heating. CrystEngComm 2013, 15, 5606–5611. [Google Scholar] [CrossRef]
- Wang, G.E.; Xu, G.; Li, P.X.; Wang, S.H.; Wang, M.S.; Guo, G.C.; Huang, J.S. Diplex single-crystal-to-single-crystal transformation by different inducement. CrystEngComm 2013, 15, 2579–2582. [Google Scholar] [CrossRef]
- Siu, C.K.; Ke, Y.; Guo, Y.; Hopkinson, A.C.; Siu, K.W.M. Dissociations of copper(II)-containing complexes of aromatic amino acids: Radical cations of tryptophan, tyrosine, and phenylalanine. Phys. Chem. Chem. Phys. 2008, 10, 5908–5918. [Google Scholar] [CrossRef] [PubMed]
- Rossin, A.; Giambastiani, G. Structural features and applications of metal-organic frameworks containing thiazole- and thiazolidine-based spacers. CrystEngComm 2015, 17, 218–228. [Google Scholar] [CrossRef]
- Rossin, A.; Credico, B.D.; Giambastiani, G.; Gonsalvi, L.; Peruzzini, M.; Reginato, G. Coordination chemistry of thiazole-based ligands: New complexes generating 3D hydrogen-bonded architectures. Eur. J. Inorg. Chem. 2011, 539–548. [Google Scholar] [CrossRef]
- Luo, T.T.; Wu, H.C.; Jao, Y.C.; Huang, S.M.; Tseng, T.W.; Wen, Y.S.; Lee, G.H.; Peng, S.M.; Lu, K.L. Self-assembled arrays of single-walled metal-organic nanotubes. Angew. Chem. Int. Ed. 2009, 48, 9461–9464. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.W.; Luo, T.T.; Tsai, C.H.; Chen, C.C.; Lee, G.H.; Wang, C.C.; Lu, K.L. Benzene absorption in a protuberant-grid-type zinc(II)-organic framework triggered by the migration of guest water molecules. Dalton Trans. 2015, 44, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Mendiratta, S.; Usman, M.; Tseng, T.W.; Luo, T.T.; Lee, S.F.; Zhao, L.; Wu, M.K.; Lee, M.M.; Sun, S.S.; Lin, Y.C.; et al. Low dielectric behavior of a robust, guest-free magnesium(II)-organic framework: A potential application of an alkaline-earth metal compound. Eur. J. Inorg. Chem. 2015, 1669–1674. [Google Scholar] [CrossRef]
- Tseng, T.W.; Luo, T.T.; Liao, S.H.; Lu, K.H.; Lu, K.L. Isorecticular synthesis of dissectible molecular bamboo tubes of hexarhenium(I) benzene-1,2,3,4,5,6-hexaolate complexes. Angew. Chem. Int. Ed. 2016, 55, 8343–8347. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.W.; Lee, L.W.; Luo, T.T.; Chien, P.H.; Liu, Y.H.; Lee, S.L.; Wang, C.M.; Lu, K.L. Gate-opening upon CO2 adsorption on a metal-organic framework that mimics a natural stimuli-response system. Dalton Trans. 2017, 46, 14728–14732. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Chu, W.; Zhu, Q.; Huang, R. Three novel homochiral helical metal-organic frameworks based on amino acid ligand: Syntheses, crystal structures, and properties. Cryst. Growth Des. 2011, 11, 93–99. [Google Scholar] [CrossRef]
- Yang, J.R.; Xie, S.M.; Zhang, J.H.; Chen, L.; Nong, R.Y.; Yuan, L.M. Metal-organic framework [Cd(LTP)2]n for improved enantioseparations on a chiral cyclodextrin stationary phase in GC. J. Chromatogr. Sci. 2016, 54, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Craciunescu, D.G.; Doadrio, A.; Furlani, A.; Scarcia, V. Synthesis and antitmnour properties of complexes with heavy transition metals and thioproline. Inorg. Chim. Acta 1982, 67, Lll–L13. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, H.; Li, X.; Wang, B.; Hu, J.; Yao, F.; Yang, D.; Xiao, S.; Ye, L. Synthesis of thioproline salicylic acid samarium complex and microcalorimetric study on effects of the complex on the growth metabolism of S. pombe cells. Chin. J. Chem. 2011, 29, 2285–2292. [Google Scholar] [CrossRef]
- Wei, Y.S.; Chen, K.J.; Liao, P.Q.; Zhu, B.Y.; Lin, R.B.; Zhou, H.L.; Wang, B.Y.; Xue, W.; Zhang, J.P.; Chen, X.M. Turning on the flexibility of isoreticular porous coordination frameworks for drastically tunable framework breathing and thermal expansion. Chem. Sci. 2013, 4, 1539–1546. [Google Scholar] [CrossRef]







| Compounds | 1 | 2 | 3 |
|---|---|---|---|
| formula | C8H16N2O6S2Zn | C8H12N2O4S2Zn | C8H12N2O4S2Zn |
| Mw | 365.72 | 329.69 | 329.69 |
| crystal system | monoclinic | monoclinic | orthorhombic |
| space group | P21 | P21 | P212121 |
| a (Å) | 6.0254(2) | 9.714(1) | 5.8839(5) |
| b (Å) | 20.8382(9) | 5.7126(6) | 9.2587(8) |
| c (Å) | 6.1614(2) | 10.336(1) | 21.211(2) |
| α (deg) | 90.00 | 90.00 | 90.00 |
| β (deg) | 111.282(2) | 93.187(2) | 90.00 |
| γ (deg) | 90.00 | 90.00 | 90.00 |
| V (Å3) | 720.86(5) | 572.7(1) | 1157.5(2) |
| Z | 2 | 2 | 4 |
| ρcalc/g·cm−3 | 1.685 | 1.912 | 1.892 |
| 2θ range (deg) | 3.91° to 27.50° | 1.97° to 27.49° | 1.92° to 27.50° |
| µ (mm−1) | 2.014 | 2.512 | 2.486 |
| T/K | 295(2) | 295(2) | 295(2) |
| total no. of data collected | 6832 | 4467 | 8130 |
| no. of observed data | 3090 | 2634 | 2655 |
| no. of observed data (I > 2σ(I)) | 2677 | 2366 | 2506 |
| R1, wR2 (I > 2σ(I)) | 0.0670, 0.1757 | 0.0380, 0.0773 | 0.0329, 0.0753 |
| R1, wR2 (all data) | 0.0759, 0.1868 | 0.0442, 0.0805 | 0.0353, 0.0766 |
| no. of variable | 169 | 162 | 162 |
| GOF | 1.038 | 0.990 | 0.988 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, T.-W.; Luo, T.-T.; Chiu, H.-S.; Wang, C.-C.; Lee, G.-H.; Sheu, H.-S.; Lu, K.-L. Structural Transformations of Amino-Acid-Based Polymers: Syntheses and Structural Characterization. Polymers 2018, 10, 360. https://doi.org/10.3390/polym10040360
Tseng T-W, Luo T-T, Chiu H-S, Wang C-C, Lee G-H, Sheu H-S, Lu K-L. Structural Transformations of Amino-Acid-Based Polymers: Syntheses and Structural Characterization. Polymers. 2018; 10(4):360. https://doi.org/10.3390/polym10040360
Chicago/Turabian StyleTseng, Tien-Wen, Tzuoo-Tsair Luo, Hsiao-Shan Chiu, Chih-Chieh Wang, Gene-Hsiang Lee, Hwo-Shuenn Sheu, and Kuang-Lieh Lu. 2018. "Structural Transformations of Amino-Acid-Based Polymers: Syntheses and Structural Characterization" Polymers 10, no. 4: 360. https://doi.org/10.3390/polym10040360
APA StyleTseng, T.-W., Luo, T.-T., Chiu, H.-S., Wang, C.-C., Lee, G.-H., Sheu, H.-S., & Lu, K.-L. (2018). Structural Transformations of Amino-Acid-Based Polymers: Syntheses and Structural Characterization. Polymers, 10(4), 360. https://doi.org/10.3390/polym10040360