Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Characterisation
2.3.1. Water Sorption and Diffusion
2.3.2. Electron Microscopy
2.3.3. Raman Spectroscopy
2.3.4. Compression Testing
3. Results and Discussion
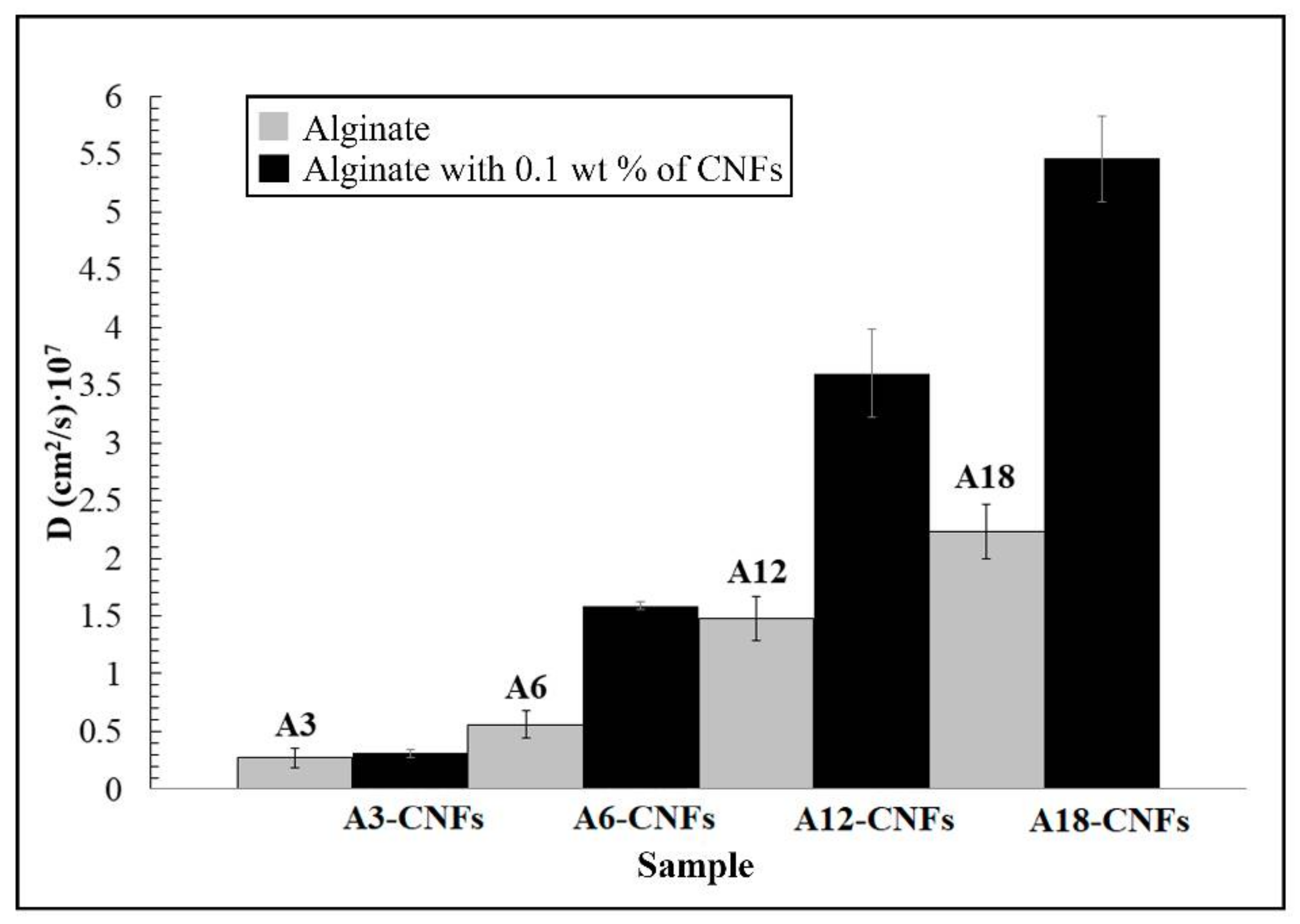
3.1. Water Sorption and Diffusion
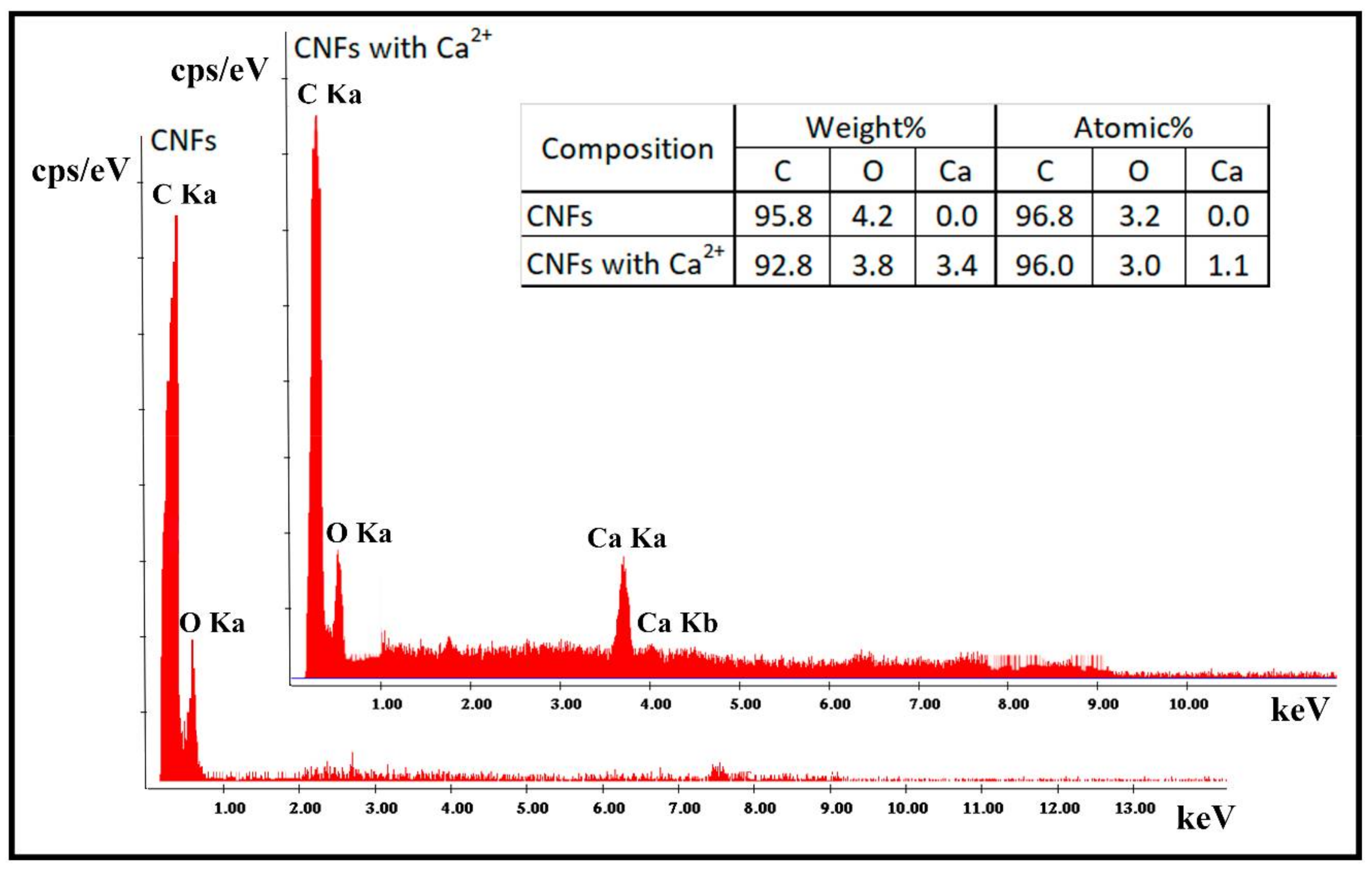
3.2. Electron Microscopy and Raman Spectroscopy
3.3. Compression Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.L. (Ed.) Biopolymers from Renewable Resources; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Vauchel, P.; Kaas, R.; Arhaliass, A.; Baron, R.; Legrand, J. A New Process for Extracting Alginates from Laminaria digitata: Reactive Extrusion. Food Bioprocess Technol. 2008, 1, 297–300. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Godbey, W.T.; Atala, A. In vitro systems for tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Draget, K.; Skjakbrak, G.; Smidsrod, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Zactiti, E.M.; Kieckbusch, T.G. Release of potassium sorbate from active films of sodium alginate crosslinked with calcium chloride. Packag. Technol. Sci. 2009, 22, 349–358. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Academic Press: Toronto, ON, Canada, 2012; ISBN 008087780X. [Google Scholar]
- Doran, P.M. Bioprocess Engineering Principles; Academic Press: Oxford, UK, 2013. [Google Scholar]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Pérez Olmedilla, M.; Garcia-Giralt, N.; Pradas, M.M.; Ruiz, P.B.; Gómez Ribelles, J.L.; Palou, E.C.; García, J.C.M. Response of human chondrocytes to a non-uniform distribution of hydrophilic domains on poly(ethyl acrylate-co-hydroxyethyl methacrylate) copolymers. Biomaterials 2006, 27, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Dou, X.-Q.; Feng, C.-L.; Zhang, D. Mechanical reinforcement of C2-phenyl-derived hydrogels for controlled cell adhesion. Soft Matter 2013, 9, 3750–3757. [Google Scholar] [CrossRef]
- Monleón-Pradas, M.; Gómez-Ribelles, J.L.; Serrano-Aroca, Á.; Gallego-Ferrer, G.; Suay-Antón, J.; Pissis, P. Interaction between water and polymer chains in poly(hydroxyethyl acrylate) hydrogels. Colloid Polym. Sci. 2001, 279, 323–330. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Monleón-Pradas, M.; Gómez-Ribelles, J.L. Effect of crosslinking on porous poly(methyl methacrylate) produced by phase separation. Colloid Polym. Sci. 2008, 286, 209–216. [Google Scholar] [CrossRef]
- Zhao, W.; Han, Z.; Ma, L.; Sun, S.; Zhao, C. Highly hemo-compatible, mechanically strong, and conductive dual cross-linked polymer hydrogels. J. Mater. Chem. B 2016, 4, 8016–8024. [Google Scholar] [CrossRef]
- Khoushabi, A.; Schmocker, A.; Pioletti, D.P.; Moser, C.; Schizas, C.; Manson, J.A.; Bourban, P.E. Photo-polymerization, swelling and mechanical properties of cellulose fibre reinforced poly(ethylene glycol) hydrogels. Compos. Sci. Technol. 2015, 119, 93–99. [Google Scholar] [CrossRef]
- Koenigs, M.M.E.; Pal, A.; Mortazavi, H.; Pawar, G.M.; Storm, C.; Sijbesma, R.P. Tuning cross-link density in a physical hydrogel by supramolecular self-sorting. Macromolecules 2014, 47, 2712–2717. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Gómez-Ribelles, J.L.; Monleón-Pradas, M.; Vidaurre-Garayo, A.; Suay-Antón, J. Characterisation of macroporous poly(methyl methacrylate) coated with plasma-polymerised poly(2-hydroxyethyl acrylate). Eur. Polym. J. 2007, 43, 4552–4564. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Monleón-Pradas, M.; Gómez-Ribelles, J.L.; Rault, J. Thermal analysis of water in reinforced plasma-polymerised poly(2-hydroxyethyl acrylate) hydrogels. Eur. Polym. J. 2015, 72, 523–534. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Monleón-Pradas, M.; Gómez-Ribelles, J.L. Plasma-induced polymerisation of hydrophilic coatings onto macroporous hydrophobic scaffolds. Polymer 2007, 48, 2071–2078. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Ruiz-Pividal, J.F.; Llorens-Gámez, M. Enhancement of water diffusion and compression performance of crosslinked alginate with a minuscule amount of graphene oxide. Sci. Rep. 2017, 7, 11684. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Correa, F.; Vidaurre-Agut, C.; Serrano-Aroca, A.; Campillo-Fernández, A.J. Poly(2-hydroxyethyl acrylate) hydrogels reinforced with graphene oxide: Remarkable improvement of water diffusion and mechanical properties. J. Appl. Polym. Sci. 2018, 135, 46158. [Google Scholar] [CrossRef]
- Wu, X.L.; Wen, T.; Guo, H.L.; Yang, S.; Wang, X.; Xu, A.W. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. ACS Nano 2013, 7, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.; et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci. Rep. 2014, 4, 4271. [Google Scholar] [CrossRef] [PubMed]
- Stout, D.A.; Basu, B.; Webster, T.J. Poly(lactic-co-glycolic acid): Carbon nanofiber composites for myocardial tissue engineering applications. Acta Biomater. 2011, 7, 3101–3112. [Google Scholar] [CrossRef] [PubMed]
- Morales, G.; Barrena, M.I.; de Salazar, J.M.G.; Merino, C.; Rodríguez, D. Conductive CNF-reinforced hybrid composites by injection moulding. Compos. Struct. 2010, 92, 1416–1422. [Google Scholar] [CrossRef]
- Baker, R.T.K. Catalytic growth of carbon filaments. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Hammel, E.; Tang, X.; Trampert, M.; Schmitt, T.; Mauthner, K.; Eder, A.; Pötschke, P. Carbon nanofibers for composite applications. Carbon 2004, 42, 1153–1158. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Morgan, P. Carbon Fibers and Their Composites, 1st ed.; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 9780824709839. [Google Scholar]
- Gardea, F.; Naraghi, M.; Lagoudas, D. Effect of thermal interface on heat flow in carbon nanofiber composites. ACS Appl. Mater. Interfaces 2014, 6, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- De Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Stout, D.A.; Yoo, J.; Santiago-Miranda, A.N.; Webster, T.J. Mechanisms of greater cardiomyocyte functions on conductive nanoengineered composites for cardiovascular applications. Int. J. Nanomed. 2012, 7, 5653–5669. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Duan, B.; Lu, A.; Wang, Y.; Ye, Q.; Zhang, L. Biocompatible chitin/carbon nanotubes composite hydrogels as neuronal growth substrates. Carbohydr. Polym. 2017, 174, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Choi, E.J.; Cho, J.H.; Cho, A.-N.; Jin, Y.; Yang, K.; Song, C.; Cho, S.-W. Three-Dimensional Electroconductive Hyaluronic Acid Hydrogels Incorporated with Carbon Nanotubes and Polypyrrole by Catechol-Mediated Dispersion Enhance Neurogenesis of Human Neural Stem Cells. Biomacromolecules 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Sun, Z.; Xu, L.; Shao, Z. Preparation and characterization of highly aligned carbon nanotubes/polyacrylonitrile composite nanofibers. Polymers 2017, 9, 1. [Google Scholar] [CrossRef]
- Wood, W.; Li, B.; Zhong, W.-H. Influence of Phase Morphology on the Sliding Wear of Polyethylene Blends Filled with Carbon Nanofibers. Polym. Eng. Sci. 2010, 50, 613–623. [Google Scholar] [CrossRef]
- Van Blitterswijk, C.; De Boer, J. Tissue Engineering; Academic Press: Oxford, UK, 2014. [Google Scholar]
- Sun, P.; Liu, H.; Wang, K.; Zhong, M.; Wu, D.; Zhu, H. Ultrafast liquid water transport through graphene-based nanochannels measured by isotope labelling. Chem. Commun. 2015, 51, 3251–3254. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.K. Fast Mass Transport through Sub-2-Nanometer Carbon Nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B.J. Nanoscale hydrodynamics: Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalov, D.W.; Katsnelson, M.I.; Son, Y.W. Origin of anomalous water permeation through graphene oxide membrane. Nano Lett. 2013, 13, 3930–3935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water through Helium-Leak-Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Aroca, Á.; Deb, S. Synthesis of irregular graphene oxide tubes using green chemistry and their potential use as reinforcement materials for biomedical applications. PLoS ONE 2017, 12, e0185235. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Li, C.; Wang, X.; Shi, G. On the Gelation of Graphene Oxide. J. Phys. Chem. C 2011, 115, 5545–5551. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.H.; Kim, S.; Han, D.S.; Kim, G.; Lee, K.R. Improved binding between copper and carbon nanotubes in a composite using oxygen-containing functional groups. Carbon 2011, 49, 811–818. [Google Scholar] [CrossRef]
- Liu, Y.T.; Feng, Q.P.; Xie, X.M.; Ye, X.Y. The production of flexible and transparent conductive films of carbon nanotube/graphene networks coordinated by divalent metal (Cu, Ca or Mg) ions. Carbon 2011, 49, 3371–3375. [Google Scholar] [CrossRef]
- Rhim, J.W. Physical and mechanical properties of water resistant sodium alginate films. LWT Food Sci. Technol. 2004, 37, 323–330. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Oxford University Press: Bristol, UK, 1975. [Google Scholar]
- Şolpan, D.; Torun, M. Synthesis and characterization of sodium alginate/acrylamide semi-interpenetrating polymer networks. J. Appl. Polym. Sci. 2006, 100, 335–342. [Google Scholar] [CrossRef]
- Dhanapal, V.; Subramanian, K. Recycling of reactive dye using semi-interpenetrating polymer network from sodium alginate and isopropyl acrylamide. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Andreopoulos, A.G. Diffusion characteristics of alginate membranes. Biomaterials 1987, 8, 397–400. [Google Scholar] [CrossRef]
- Hirai, A.; Odani, H. Sorption and transport of water vapor in alginic acid, sodium alginate, and alginate-cobalt complex films. J. Polym. Sci. Part B 1994, 32, 2329–2337. [Google Scholar] [CrossRef]
- Vilcinskas, K.; Zlopasa, J.; Jansen, K.M.B.; Mulder, F.M.; Picken, S.J.; Koper, G.J.M. Water Sorption and Diffusion in (Reduced) Graphene Oxide-Alginate Biopolymer Nanocomposites. Macromol. Mater. Eng. 2016, 301, 1049–1063. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Zorky, P.M. New applications of van der Waals radii in chemistry. Russ. Chem. Rev. 1995, 64, 415–428. [Google Scholar] [CrossRef]
- Xie, Q.; Alibakhshi, M.A.; Jiao, S.; Xu, Z.; Hempel, M.; Kong, J.; Park, H.G.; Duan, C. Fast water transport in graphene nanofluidic channels. Nat. Nanotechnol. 2018, 13, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, C.; Wang, J. Raman spectra of carbon nanotubes and nanofibers prepared by ethanol flames. J. Mater. Sci. 2004, 39, 1091–1094. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying defects in graphene via Raman spectroscopy at different excitation energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llorens-Gámez, M.; Serrano-Aroca, Á. Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties. Polymers 2018, 10, 405. https://doi.org/10.3390/polym10040405
Llorens-Gámez M, Serrano-Aroca Á. Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties. Polymers. 2018; 10(4):405. https://doi.org/10.3390/polym10040405
Chicago/Turabian StyleLlorens-Gámez, Mar, and Ángel Serrano-Aroca. 2018. "Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties" Polymers 10, no. 4: 405. https://doi.org/10.3390/polym10040405
APA StyleLlorens-Gámez, M., & Serrano-Aroca, Á. (2018). Low-Cost Advanced Hydrogels of Calcium Alginate/Carbon Nanofibers with Enhanced Water Diffusion and Compression Properties. Polymers, 10(4), 405. https://doi.org/10.3390/polym10040405






