Synthesis and Evaluation of 8-Aminoquinoline-Grafted Poly(glycidyl methacrylate) for the Recovery of Pd(II) from Highly Acidic Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
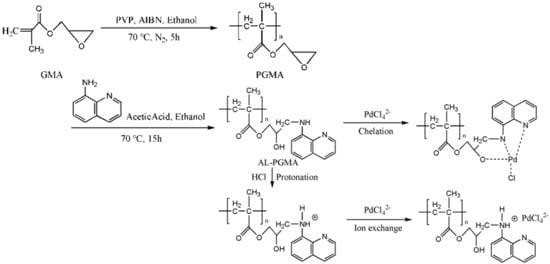
2.2. Synthesis of 8-Aminoquinoline-Grafted Poly(Glycidyl Methacrylate) (AQ-PGMA)
2.3. Adsorption Experiments
2.4. Characterization
3. Results and Discussion
3.1. Characterization of AQ-PGMA
3.2. Effect of Experimental Parameters on Pd(II) Adsorption by AQ-PGMA
3.2.1. Effect of the HCL Concentration on Pd(II) Adsorption
3.2.2. Effect of Adsorption Time on Pd(II) Adsorption and Adsorption Kinetics
3.2.3. Effect of Initial Pd(II) Concentration on Pd(II) Adsorption and Adsorption Isotherms
3.2.4. Effect of Coexisting Ions on Pd(II) Adsorption
3.2.5. Effect of Reused Cycles Of AQ-PGMA on Pd(II) Adsorption
3.3. Adsorption Mechanism of Pd(II) Onto AQ-PGMA
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Morcali, M.H.; Zeytuncu, B.; Akman, S.; Yucel, O. Preparation and sorption behavior of DEAE-cellulose-thiourea-glutaraldehyde sorbent for Pt(IV) and Pd(II) from leaching solutions. Desalin. Water Treat. 2015, 57, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y. The enrichment and separation of race gold, Pt and Pd from the ores based on co-precipitation. Gold 2006, 27, 42–45. [Google Scholar]
- Gomes, C.P.; Almeida, M.F.; Loureiro, J.M. Gold recovery with ion exchange used resins. Sep. Purif. Technol. 2001, 24, 35–57. [Google Scholar] [CrossRef]
- Al-Merey, R.; Hariri, Z.; Hilal, J.A. Selective separation of gold from iron ore samples using ion exchange resin. Microchem. J. 2003, 75, 169–177. [Google Scholar] [CrossRef]
- Akita, S.; Yang, L.; Takeuchi, H. Solvent extraction of gold(III) from hydrochloric acid media by nonionic surfactants. Hydrometallurgy 1996, 43, 37–46. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Separation of copper(II) from palladium(II) and platinum(IV) by di(2-ethylhexyl) phosphoric acid-based liquid membranes during electrodialysis. J. Membr. Sci. 2006, 275, 195–201. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chen, D.H. Recovery of gold(III) ions by a chitosancoated magnetic nano-adsorbent. Gold Bull. 2006, 39, 98–102. [Google Scholar] [CrossRef]
- Lam, K.F.; Chi, M.F.; Yeung, K.L. Separation of precious metals using selective mesoporous adsorbents. Gold Bull. 2007, 40, 192–198. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal-organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Xing-Min, S.; Guan-Jun, Z.; Yu-Kang, Y.; Yue, M.; Gui-Min, X.; Yun, Y. Recovery of N and P from human urine by freezing, struvite precipitation and adsorption to zeolite and active carbon. Bioresour. Technol. 2007, 98, 3112–3121. [Google Scholar]
- Kula, I.; Uğurlu, M.; Karaoğlu, H.; Çelik, A. Adsorption of Cd(II) ions from aqueous solutions using activated carbon prepared from olive stone by ZnCl2 activation. Bioresour. Technol. 2008, 99, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; He, X.W.; Wang, C.R.; Li, J.W.; Zhang, C.H. Cadmium adsorption characteristic of alkali modified sewage sludge. Bioresour. Technol. 2012, 121, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Pehlivan, E. Removal of Cr(VI) from aqueous solution by two Lewatit-anion exchange resins. J. Hazard. Mater. 2005, 119, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Wu, C.M.; Koodali, R.T.; Rajesh, N. An ionic liquid-mesoporous silica blend as a novel adsorbent for the adsorption and recovery of palladium ions, and its applications in continuous flow study and as an industrial catalyst. Rsc. Adv. 2016, 6, 26668–26678. [Google Scholar] [CrossRef]
- Kim, H.C.; Yu, M.J. Characterization of natural organic matter in conventional water treatment processes for selection of treatment processes focused on DBPs control. Water Res. 2005, 39, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, H.; Fujikubo, K.; Uemura, Y.; Kawano, Y.; Kondo, K.; Hatate, Y. Preparation of divinylbenzene homopolymeric microcapsules with highly porous membranes by in situ polymerization with solvent evaporation. J. Chem. Eng. Jpn. 2005, 28, 78–84. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, L.; Wang, S.; Zhang, G.; Peng, J. Selective recovery of Au(III) from aqueous solutions by nano-silica grafted with 4-(aminomethyl) pyridine. J. Sol-Gel Sci. Techn. 2017, 83, 467–477. [Google Scholar] [CrossRef]
- Sakakibara, K.; Kagata, H.; Ishizuka, N.; Sato, T.; Tsujii, Y. Fabrication of surface skinless membranes of epoxy resin-based mesoporous monoliths toward advanced separators for lithium ion batteries. J. Mater. Chem. A. 2017, 5, 6866–6873. [Google Scholar] [CrossRef]
- Yang, W.; Yun, Z.; Chen, H.; He, X.; Liu, M. Preparation of a novel TETA functionalized magnetic PGMA nano-absorbent by ATRP method and used for highly effective adsorption of Hg(II). J. Taiwan Inst. Chem. Eng. 2015, 58, 283–289. [Google Scholar]
- Wang, L.; Li, F.; Yao, M.; Qiu, T.; Jiang, W.; Fan, L.J. Atom transfer radical polymerization of glycidyl methacrylate followed by amination on the surface of monodispersed highly crosslinked polymer microspheres and the study of cation adsorption. React. Funct. Polym. 2014, 82, 66–71. [Google Scholar] [CrossRef]
- Tsarevsky, N.V.; Jakubowski, W. Atom transfer radical polymerization of functional monomers employing Cu-based catalysts at low concentration: Polymerization of glycidyl methacrylate. J. Polym. Sci. Polym. Chem. 2011, 49, 918–925. [Google Scholar] [CrossRef]
- Mowafy, E.A.; Aly, H.F. Extraction and separation of Pd(II), Pt(IV), Fe(III), Zn(II), Cu(II) and Ag(I) from hydrochloric acid solutions with selected cyanamides as novel extractants. J. Hazard. Mater. 2007, 149, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Seung, L.M.; Young, L.J.; Rajesh, K.J.; Soo, K.J.; Soo, S.J. Solvent Extraction of PtCl4- from Hydrochloric Acid Solution with Alamine336. Mater. Trans. 2008, 49, 2823–2828. [Google Scholar]
- Zhang, L.; Liu, Y.; Wang, S.; Liu, B.; Peng, J. Selective removal of cationic dyes from aqueous solutions by an activated carbon-based multicarboxyl adsorbent. Rsc. Adv. 2015, 5, 99618–99626. [Google Scholar] [CrossRef]
- Huang, J.; Huang, K.; Liu, S.; Luo, Q.; Shi, S. Synthesis, characterization, and adsorption behavior of aniline modified polystyrene resin for phenol in hexane and in aqueous solution. J. Colloid Interface Sci. 2008, 317, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Qu, R.; Ji, C.; Wang, C.; Sun, Y.; Yue, Z.; Cheng, G. Preparation and adsorption properties of crosslinked polystyrene-supported low-generation diethanolamine-typed dendrimer for metal ions. Talanta 2005, 70, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Manzoori, J.L.; Amjadi, M.; Hallaj, T.; Namiesnik, J. Preconcentration of trace cadmium and manganese using 1-(2-pyridylazo)-2-naphthol-modified TiO2 nanoparticles and their determination by flame atomic absorption spectrometry. Int. J. Environ. Anal. Chem. 2009, 89, 749–758. [Google Scholar] [CrossRef]
- Zhou, L.M.; Liu, J.H.; Liu, Z.R. Adsorption of platinum(IV) and palladium(II) from aqueous solution by thiourea-modified chitosan microspheres. J. Hazard. Mater. 2009, 172, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Morcali, M.H.; Zeytuncu, B. Investigation of adsorption parameters for platinum and palladium onto a modified polyacrylonitrile-based sorbent. Int. J. Miner. Process. 2015, 137, 52–58. [Google Scholar] [CrossRef]
- Nagireddi, S.; Katiyar, V.; Uppaluri, R. Pd(II) adsorption characteristics of glutaraldehyde cross-linked chitosan copolymer resin. Int. J. Biol. Macromol. 2017, 94, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.Y.; Nakano, Y. Adsorption mechanism of palladium by redox within condensed-tannin gel. Water Res. 2005, 39, 1324–1330. [Google Scholar]
- Ho, Y.S.; Mckay, G. The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res. 2000, 34, 735–742. [Google Scholar] [CrossRef]
- Yurdakoç, M.; Seki, Y.; Karahan, S.; Yurdakoç, K. Kinetic and thermodynamic studies of boron removal by Siral 5, Siral 40, and Siral 80. J. Colloid Interface Sci. 2005, 286, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.L.; Juang, R.S. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 2001, 35, 613–618. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, L.; An, Z.; Ye, H.; Feng, X. Hydrothermal synthesis of silico-manganese nanohybrid for Cu(II) adsorption from aqueous solution. Appl. Surf. Sci. 2016, 371, 102–111. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Mittal, A.; Kaur, D.; Mittal, J. Batch and bulk removal of a triarylmethane dye, Fast Green FCF, from wastewater by adsorption over waste materials. J. Hazard. Mater. 2009, 163, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, J.; Yuan, W.; Yi, Y.; Zhao, L. Recovery of Au(III) by radiation synthesized aminomethyl pyridine functionalized adsorbents based on cellulose. Chem. Eng. J. 2016, 283, 504–513. [Google Scholar] [CrossRef]
- Sellaoui, L.; Franco, D.S.P.; Dotto, G.L.; Lima, É.C.; Lamine, A.B. Single and binary adsorption of cobalt and methylene blue on modified chitin: Application of the Hill and exclusive extended Hill models. J. Mol. Liq. 2017, 233, 543–550. [Google Scholar] [CrossRef]
- Sellaoui, L.; Dotto, G.L.; Lamine, A.B.; Erto, A. Interpretation of single and competitive adsorption of cadmium and zinc on activated carbon using monolayer and exclusive extended monolayer models. Environ. Sci. Pollut. Res. 2017, 24, 19902–19908. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.J.; Li, R.; Hu, J.T.; Zhang, L.J.; Zhang, M.X.; Yang, C.G.; Wu, G.Z. Functionalized polyethylene fibers for the selective capture of palladium ions from aqueous solution. Appl. Surf. Sci. 2018, 433, 116–124. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.К.; Artyushin, O.I.; Sharova, E.V.; Genkina, G.K. Adsorption of palladium(II) from hydrochloric acid solutions using polymeric resins impregnated with novel n-substituted 2-(diphenylthiophosphoryl)acetamides. Sep. Purif. Technol. 2017, 187, 355–364. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzen, M.; Soylak, M. Biosorption of palladium(II) from aqueous solution by moss (racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2009, 162, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Sharififard, H.; Soleimani, M.; Ashtiani, F.Z. Evaluation of activated carbon and bio-polymer modified activated carbon performance for palladium and platinum removal. J. Taiwan Inst. Chem. Eng. 2012, 43, 696–703. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Sharma, S.; Reddy, R.S.; Barathi, M.; Rajesh, N. Comprehending the interaction between chitosan and ionic liquid for the adsorption of palladium. Int. J. Biol. Macromol. 2015, 72, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Kensuke, F.; Attinti, R.; Teruya, M.; Hiroshi, H.; Kazumasa, U. Adsorption of platinum(IV), palladium(II) and gold(III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. Hazard. Mater. 2007, 146, 39–50. [Google Scholar]











| Kinetic models | Equations |
|---|---|
| Pseudo-first-order | |
| Pseudo-second-order | |
| Intra-particle diffusion |
| Pseudo-first-order | k1 (g/(mg·min)) | qe,1 (mg/g) | R2 | |
| 0.01 | 14.88 | 0.5125 | ||
| Pseudo-second-order | k2 (g/(mg·min)) | qe,2 (mg/g) | R2 | |
| 1.54 × 10−3 | 151.29 | 0.9993 | ||
| Intra-particle diffusion | Stage 1 | ki1 (mg/(g·min1/2)) | C1 | R12 |
| 12.71 | 52.69 | 0.9495 | ||
| Stage 2 | ki2 (mg/(g·min1/2)) | C2 | R22 | |
| −0.19 | 151.26 | 0.7828 | ||
| Experimental data | qe (mg/g) | |||
| 148.60 | ||||
| Adsorbents | Equilibrium time/h | Kinetic rate constant (k2)/g/(mg·min) | References |
|---|---|---|---|
| TCS | 5 | 5.05 × 10−4 | [30] |
| PAN-TU-GA | 6 | 2.10 × 10−6 | [31] |
| GCC | 5 | 1.364 × 10−5 | [32] |
| Diaion WA21J | 10 | 1.17 × 10−5 | [34] |
| AQ-PGMA | 1 | 1.54 × 10−3 | This work |
| Freundlich | KF | nF | R2 | ||
| 79.24 | 5.06 | 0.829 | |||
| Langmuir | KL | qm (mg/g) | R2 | ||
| 0.02 | 292.14 | 0.9807 | |||
| Hill | C1/2 (mg/L) | NM (mg/g) | n | q0 (mg/g) | R2 |
| 45.88 | 188.469 | 1.45 | 273.28 | 0.9988 | |
| Experimental data | q0 (mg/g) | ||||
| 267.90 | |||||
| Adsorbents | qm (mg/g) | References |
|---|---|---|
| polyethylene fibers | 221.8 | [43] |
| macroporous resins XAD-7 HP | 52.77 | [44] |
| Racomitrium lanuginosum biomass | 37.2 | [45] |
| Biopolymer modified activated carbon | 43.5 | [46] |
| Chitosan | 5.88 | [47] |
| Cross-linked chitosan modified with l-lysine | 109.47 | [48] |
| AQ-PGMA | 267.90 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Wang, S.; Fu, L.; Zhang, L. Synthesis and Evaluation of 8-Aminoquinoline-Grafted Poly(glycidyl methacrylate) for the Recovery of Pd(II) from Highly Acidic Aqueous Solutions. Polymers 2018, 10, 437. https://doi.org/10.3390/polym10040437
Zhang B, Wang S, Fu L, Zhang L. Synthesis and Evaluation of 8-Aminoquinoline-Grafted Poly(glycidyl methacrylate) for the Recovery of Pd(II) from Highly Acidic Aqueous Solutions. Polymers. 2018; 10(4):437. https://doi.org/10.3390/polym10040437
Chicago/Turabian StyleZhang, Bing, Shixing Wang, Likang Fu, and Libo Zhang. 2018. "Synthesis and Evaluation of 8-Aminoquinoline-Grafted Poly(glycidyl methacrylate) for the Recovery of Pd(II) from Highly Acidic Aqueous Solutions" Polymers 10, no. 4: 437. https://doi.org/10.3390/polym10040437
APA StyleZhang, B., Wang, S., Fu, L., & Zhang, L. (2018). Synthesis and Evaluation of 8-Aminoquinoline-Grafted Poly(glycidyl methacrylate) for the Recovery of Pd(II) from Highly Acidic Aqueous Solutions. Polymers, 10(4), 437. https://doi.org/10.3390/polym10040437