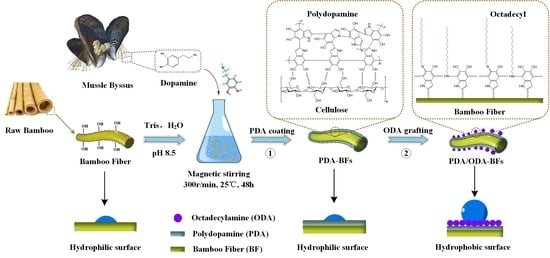
Mussel-Inspired Polydopamine as a Green, Efficient, and Stable Platform to Functionalize Bamboo Fiber with Amino-Terminated Alkyl for High Performance Poly(butylene succinate) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Functionalization of BFs
2.3. Preparation of BFs Reinforced PBS Composites
2.4. Characterization and Measurement of BF and PBS Composites
3. Results and Discussion
3.1. Reaction Mechanism of Depositing Mussel-Inspired Functional Coatings on Bamboo Fibers
3.2. Surface Topography of Bamboo Fibers
3.3. Microstructural and Surface Chemical Composition Analysis of Bamboo Fibers
3.4. Surface Wettability of Bamboo Fibers
3.5. Static Mechanical Properties of BF/PBS Composites
3.6. Dynamic Mechanical Analysis of BF/PBS Composites
3.7. Water Absorption Capability of BF/PBS Composites
3.8. Fracture morphology of BF/PBS composites
3.9. Interfacial Adhesion Mechanism
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lambert, S.; Wagner, M. Environmental performance of bio-based and biodegradable plastics: The road ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef] [PubMed]
- Maziere, A.; Prinsen, P.; Garcia, A.; Luque, R.; Len, C. A review of progress in (bio)catalytic routes from/to renewable succinic acid. Biofuels Bioprod. Biorin. 2017, 11, 908–931. [Google Scholar] [CrossRef]
- Negrin, M.; Macerata, E.; Consolati, G.; Quasso, F.; Genovese, L.; Soccio, M.; Giola, M.; Lotti, N.; Munari, A.; Mariani, M. Gamma radiation effects on random copolymers based on poly(butylene succinate) for packaging applications. Radiat. Phys. Chem. 2018, 142, 34–43. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Munari, A.; Lotti, N. Novel random pbs-based copolymers containing aliphatic side chains for sustainable flexible food packaging. Polymers 2017, 9, 724. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(butylene succinate)-based polyesters for biomedical applications: A review. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- The, D.T.; Yoshii, F.; Nagasawa, N.; Kume, T. Synthesis of poly(butylene succinate)/glass fiber composite by irradiation and its biodegradability. J. Appl. Polym. Sci. 2004, 91, 2122–2127. [Google Scholar] [CrossRef]
- Liang, J.C.; Ding, C.; Wei, Z.Y.; Sang, L.; Song, P.; Chen, G.Y.; Chang, Y.; Xu, J.T.; Zhang, W.X. Mechanical, morphology, and thermal properties of carbon fiber reinforced poly(butylene succinate) composites. Polym. Compos. 2015, 36, 1335–1345. [Google Scholar] [CrossRef]
- Haghdan, S.; Smith, G.D. Natural fiber reinforced polyester composites: A literature review. J. Reinf. Plast. Compos. 2015, 34, 1179–1190. [Google Scholar] [CrossRef]
- Liu, W.D.; Xie, T.S.; Qiu, R.H. Bamboo fibers grafted with a soybean-oil-based monomer for its unsaturated polyester composites. Cellulose 2016, 23, 2501–2513. [Google Scholar] [CrossRef]
- Hong, G.H.; Meng, Y.; Yang, Z.X.; Cheng, H.T.; Zhang, S.B.; Song, W. Mussel-inspired polydopamine modification of bamboo fiber and its effect on the properties of bamboo fiber/polybutylene succinate composites. Bioresources 2017, 12, 8419–8442. [Google Scholar] [CrossRef]
- Feng, J.; Chen, J.; Chen, M.J.; Su, X.L.; Shi, Q.S. Effects of biocide treatments on durability of wood and bamboo/high density polyethylene composites against algal and fungal decay. J. Appl. Polym. Sci. 2017, 134, 45148. [Google Scholar] [CrossRef]
- Vu, C.M.; Sinh, L.H.; Choi, H.J.; Pham, T.D. Effect of micro/nano white bamboo fibrils on physical characteristics of epoxy resin reinforced composites. Cellulose 2017, 24, 5475–5486. [Google Scholar] [CrossRef]
- Ying, S.J.; Wang, C.B.; Lin, Q. Effects of heat treatment on the properties of bamboo fiber/polypropylene composites. Fibers Polym. 2013, 14, 1894–1898. [Google Scholar] [CrossRef]
- Zhang, X.P.; Wang, F.; Keer, L.M. Influence of surface modification on the microstructure and thermo-mechanical properties of bamboo fibers. Materials 2015, 8, 6597–6608. [Google Scholar] [CrossRef] [PubMed]
- Song, X.Y.; Wang, M.; Weng, Y.X.; Huang, Z.G. Effect of bamboo flour grafted lactide on the interfacial compatibility of polylactic acid/bamboo flour composites. Polymers 2017, 9, 323. [Google Scholar] [CrossRef]
- Song, W.; Zhao, F.; Yu, X.F.; Wang, C.C.; Wei, W.B.; Zhang, S.B. Interfacial characterization and optimal preparation of novel bamboo plastic composite engineering materials. Bioresources 2015, 10, 5049–5070. [Google Scholar] [CrossRef]
- Forooshani, P.K.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Pol. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.P.; Messersmith, P.B.; Israelachvili, J.N.; Waite, J.H. Mussel-inspired adhesives and coatings. Annu. Rev. Mater. Res. 2011, 41, 99–132. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.Q.; Wang, Y.; Yu, J.R.; Chen, L.; Zhu, J.; Hu, Z.M. Polydopamine particles for next-generation multifunctional biocomposites. J. Mater. Chem. A 2014, 2, 7578–7587. [Google Scholar] [CrossRef]
- Raza, Z.A.; Rehman, A.; Anwar, F.; Usman, A. Development and antibacterial performance of silver nanoparticles incorporated polydopamine-polyester-knitted fabric. Bull. Mater. Sci. 2016, 39, 391–396. [Google Scholar] [CrossRef]
- Posati, T.; Sotgiu, G.; Varchi, G.; Ferroni, C.; Zamboni, R.; Corticelli, F.; Puglia, D.; Torre, L.; Terenzi, A.; Aluigi, A. Developing keratin sponges with tunable morphologies and controlled antioxidant properties induced by doping with polydopamine (PDA) nanoparticles. Mater. Des. 2016, 110, 475–484. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, M.J.; Tang, M.F.; Hong, G.H.; Gao, J.M.; Chen, Y. Preparation of robust superhydrophobic halloysite clay nanotubes via mussel-inspired surface modification. Appl. Sci. 2017, 7, 1129. [Google Scholar] [CrossRef]
- Zhu, Q.; Pan, Q.M. Mussel-inspired direct immobilization of nanoparticles and application for oil-water separation. ACS Nano 2014, 8, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Xu, Y.C.; Liu, Y.Y.; Shao, L. A novel mussel-inspired strategy toward superhydrophobic surfaces for self-driven crude oil spill cleanup. J. Mater. Chem. A 2015, 3, 12171–12178. [Google Scholar] [CrossRef]
- Song, X.P.; Jiang, Y.; Rong, X.J.; Wei, W.; Wang, S.F.; Nie, S.X. Surface characterization and chemical analysis of bamboo substrates pretreated by alkali hydrogen peroxide. Bioresour. Technol. 2016, 216, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Xian, Y.; Smith, L.M.; Wang, G.; Cheng, H.T.; Zhang, S.B. Interfacial properties of bamboo fiber-reinforced high-density polyethylene composites by different methods for adding nano calcium carbonate. Polymers 2017, 9, 587. [Google Scholar] [CrossRef]
- Espert, A.; Vilaplana, F.; Karlsson, S. Comparison of water absorption in natural cellulosic fibres from wood and one-year crops in polypropylene composites and its influence on their mechanical properties. Compos. A 2004, 35, 1267–1276. [Google Scholar] [CrossRef]
- Peng, L.H.; Guo, R.H.; Lan, J.W.; Jiang, S.X.; Lin, S.J. Microwave-assisted deposition of silver nanoparticles on bamboo pulp fabric through dopamine functionalization. Appl. Surf. Sci. 2016, 386, 151–159. [Google Scholar] [CrossRef]
- Chen, F.; Han, G.P.; Li, Q.D.; Gao, X.; Cheng, W.L. High-temperature hot air/silane coupling modification of wood fiber and its effect on properties of wood fiber/HDPE composites. Materials 2017, 10, 286. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Hill, C.A.S.; Xiao, Z.F.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. B 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Luo, R.F.; Tang, L.L.; Wang, J.; Zhao, Y.C.; Tu, Q.F.; Weng, Y.J.; Shen, R.; Huang, N. Improved immobilization of biomolecules to quinone-rich polydopamine for efficient surface functionalization. Colloids Surf. B 2013, 106, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.Q.; Wang, L.H.; Liu, J.L.; Wu, J.Z. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Feng, J.R.; Fan, H.L.; Zha, D.A.; Wang, L.; Jin, Z.X. Characterizations of the formation of polydopamine-coated halloysite nanotubes in various pH environments. Langmuir 2016, 32, 10377–10386. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Shen, Q.; Liu, D.S.; Gao, Y.; Chen, Y. Surface properties of bamboo fiber and a comparison with cotton linter fibers. Colloids Surf. B 2004, 35, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.Y.; Li, Y.B.; He, X.D.; Lv, H.Z.; Hu, P.A.; Shang, Y.Y.; Wang, C.; Wang, R.G.; Sritharan, T.; Du, S.Y. Interfacial enhancement of carbon fiber composites by poly(amido amine) functionalization. Compos. Sci. Technol. 2013, 74, 37–42. [Google Scholar] [CrossRef]
- Zhou, M.; Xu, S.M.; Li, Y.H.; He, C.; Jin, T.X.; Wang, K.; Deng, H.; Zhang, Q.; Chen, F.; Fu, Q. Transcrystalline formation and properties of polypropylene on the surface of ramie fiber as induced by shear or dopamine modification. Polymer 2014, 55, 3045–3053. [Google Scholar] [CrossRef]
- Shahzad, A. Effects of alkalization on tensile, impact, and fatigue properties of hemp fiber composites. Polym. Compos. 2012, 33, 1129–1140. [Google Scholar] [CrossRef]
- Dai, X.Y.; Xiong, Z.; Na, H.N.; Zhu, J. How does epoxidized soybean oil improve the toughness of microcrystalline cellulose filled polylactide acid composites? Compos. Sci. Technol. 2014, 90, 9–15. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, G.X.; Zhang, W.Z.; Guo, W.H.; Wang, J.K. Processing and thermal behaviors of poly (butylene succinate) blends with highly-filled starch and glycerol. J. Polym. Environ. 2013, 21, 46–53. [Google Scholar] [CrossRef]
- Chen, S.Y.; Cheng, L.; Huang, H.M.; Zou, F.Z.; Zhao, H.P. Fabrication and properties of poly(butylene succinate) biocomposites reinforced by waste silkworm silk fabric. Compos. A 2017, 95, 125–131. [Google Scholar] [CrossRef]
- Lee, J.M.; Ishak, Z.A.M.; Taib, R.M.; Law, T.T.; Thirmizir, M.Z.A. Mechanical, thermal and water absorption properties of kenaf-fiber-based polypropylene and poly(butylene succinate) composites. J. Polym. Environ. 2013, 21, 293–302. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Y.H.; He, C.; Jin, T.X.; Wang, K.; Fu, Q. Interfacial crystallization enhanced interfacial interaction of poly (butylene succinate)/ramie fiber biocomposites using dopamine as a modifier. Compos. Sci. Technol. 2014, 91, 22–29. [Google Scholar] [CrossRef]









| No.1 | Labels | DA (mg/mL) | ODA (mM) | BFs (wt %) | PBS (wt %) | Paraffin wax (wt %) |
|---|---|---|---|---|---|---|
| 1 | C–BFs | - | - | 49 | 49 | 2 |
| 2 | PDA–BFs | 2 | - | 49 | 49 | 2 |
| 3 | PDA/ODA–BFs | 2 | 10 | 49 | 49 | 2 |
| 4 | ODA–BFs | - | 10 | 49 | 49 | 2 |
| Test liquids | Surface free energy (mJ/m2) | ||||
|---|---|---|---|---|---|
| () | () | ||||
| Distilled Water | 72.8 | 21.8 | 51.0 | 25.5 | 25.5 |
| Diiodomethane | 50.8 | 50.8 | 0 | 0 | 0 |
| Samples | Composition (At %) | N/C atomic ratio | Ratios of functional group (N 1s) (%) | ||||
|---|---|---|---|---|---|---|---|
| Carbon | Oxygen | Nitrogen | R1–NH2 | R1–NH–R2 | C=NR | ||
| C–BFs | 72.81 | 27.19 | - | - | - | - | - |
| ODA–BFs | 73.36 | 26.64 | - | - | - | - | - |
| PDA–BFs | 69.98 | 23.79 | 6.23 | 0.089 | 13.63 | 79.65 | 6.72 |
| PDA/ODA–BFs | 77.75 | 16.73 | 5.52 | 0.071 | 7.45 | 81.08 | 11.47 |
| Samples | (mJ/m2) | (mJ/m2) | (mJ/m2) | |
|---|---|---|---|---|
| C–BFs | 45.57 | 38.54 | 7.03 | 5.48 |
| PDA–BFs | 49.96 | 37.28 | 12.68 | 2.94 |
| PDA/ODA–BFs | 34.45 | 32.99 | 1.46 | 22.60 |
| Label | Tensile strength (MPa) | Tensile modulus (GPa) | Flexural strength (MPa) | Flexural modulus (GPa) | Impact strength (kJ/m2) |
|---|---|---|---|---|---|
| C–BFs | 13.62(1.15) a | 0.75(0.18) a | 31.38(0.67) a | 2.05(0.13) a | 10.25(1.11) b |
| PDA–BFs | 15.58(0.69) b | 0.79(0.12) a | 33.72(0.43) b | 2.16(0.09) b | 9.02(0.84) a |
| PDA/ODA–BFs | 24.13(0.77) c | 1.16(0.08) b | 44.09(0.51) c | 2.67(0.16) c | 15.16(0.63) c |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, G.; Cheng, H.; Meng, Y.; Lin, J.; Chen, Z.; Zhang, S.; Song, W. Mussel-Inspired Polydopamine as a Green, Efficient, and Stable Platform to Functionalize Bamboo Fiber with Amino-Terminated Alkyl for High Performance Poly(butylene succinate) Composites. Polymers 2018, 10, 461. https://doi.org/10.3390/polym10040461
Hong G, Cheng H, Meng Y, Lin J, Chen Z, Zhang S, Song W. Mussel-Inspired Polydopamine as a Green, Efficient, and Stable Platform to Functionalize Bamboo Fiber with Amino-Terminated Alkyl for High Performance Poly(butylene succinate) Composites. Polymers. 2018; 10(4):461. https://doi.org/10.3390/polym10040461
Chicago/Turabian StyleHong, Gonghua, Haitao Cheng, Yang Meng, Jianyong Lin, Zhenghao Chen, Shuangbao Zhang, and Wei Song. 2018. "Mussel-Inspired Polydopamine as a Green, Efficient, and Stable Platform to Functionalize Bamboo Fiber with Amino-Terminated Alkyl for High Performance Poly(butylene succinate) Composites" Polymers 10, no. 4: 461. https://doi.org/10.3390/polym10040461
APA StyleHong, G., Cheng, H., Meng, Y., Lin, J., Chen, Z., Zhang, S., & Song, W. (2018). Mussel-Inspired Polydopamine as a Green, Efficient, and Stable Platform to Functionalize Bamboo Fiber with Amino-Terminated Alkyl for High Performance Poly(butylene succinate) Composites. Polymers, 10(4), 461. https://doi.org/10.3390/polym10040461