pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems
Abstract
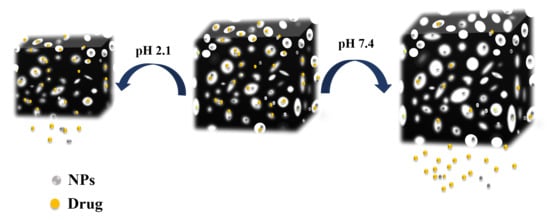
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.3. Preparation of Poly(succinimide) (PSI)
2.4. Synthesis of Graphene Oxide (GO)
2.5. Synthesis of GO-NH2
2.6. Preparation of IPN Hydrogel
2.7. Preparation of Nanoparticle-Embedded IPN Hydrogels
2.8. Swelling Studies
2.9. In Vitro Drug Loading and Release Studies
2.10. Antibacterial Activity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xu, L.; Qiu, L.; Sheng, Y.; Sun, Y.; Deng, L.; Li, X.; Bradley, M.; Zhang, R. Biodegradable pH-responsive hydrogels for controlled dual-drug release. J. Mater. Chem. B 2018. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Kharat, A.N.; Adeli, M. Targeted multifunctional redox-sensitive micelles co-delivery of DNA and doxorubicin for treatment of breast cancer. J. Mater. Chem. B 2015, 3, 3896–3921. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Sahraei, R.; Ghaemy, M. Synthesis of modified gum tragacanth/graphene oxide composite hydrogel for heavy metal ions removal and preparation of silver nanocomposite for antibacterial. Carbohydr. Polym. 2017, 157, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Pathania, D.; Gupta, D.; Agarwal, S.; Asif, M.; Gupta, V.K. Fabrication of chitosan-g-poly(acrylamide)/CuS nanocomposite for controlled drug delivery and antibacterial activity. Mater. Sci. Eng. C 2016, 64, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Lima-Tenório, M.K.; Tenorio-Neto, E.T.; Garcia, F.P.; Nakamura, C.V.; Guilherme, M.R.; Muniz, E.C.; Pineda, E.A.; Rubira, A.F. Hydrogel nanocomposite based on starch and Co-doped zinc ferrite nanoparticles that shows magnetic field-responsive drug release changes. J. Mol. Liq. 2015, 210, 100–105. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: Concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- De Koker, S.; Cui, J.; Vanparijs, N.; Albertazzi, L.; Grooten, J.; Caruso, F.; de Geest, B.G. Engineering Polymer Hydrogel Nanoparticles for Lymph Node-Targeted Delivery. Angew. Chem. Int. Ed. 2016, 55, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Damasceno, P.F.; Somashekar, B.S.; Engel, M.; Tian, F.; Zhu, J.; Huang, R.; Johnson, K.; McIntyre, C.; Sun, K. Unusual multiscale mechanics of biomimetic nanoparticle hydrogels. Nat. Commun. 2018, 9, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumann, B.; Jungst, T.; Stichler, S.; Feineis, S.; Wiltschka, O.; Kuhlmann, M.; Lindén, M.; Groll, J. Control of Nanoparticle Release Kinetics from 3D Printed Hydrogel Scaffolds. Angew. Chem. Int. Ed. 2017, 56, 4623–4628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, X.; Guo, B.; Ma, P.X. Multifunctional interpenetrating polymer network hydrogels based on methacrylated alginate for the delivery of small molecule drugs and sustained release of protein. Biomacromolecules 2014, 15, 3246–3252. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S. Chemical Engineering Journal, Design and applications of interpenetrating polymer network hydrogels. Rapra Rev. Rep. 2014, 243, 572–590. [Google Scholar]
- Wang, J.; Hu, H.; Yang, Z.; Wei, J.; Li, J. IPN hydrogel nanocomposites based on agarose and ZnO with antifouling and bactericidal properties. Mater. Sci. Eng. C 2016, 61, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Samanta, H.S.; Ganguly, J. Green synthesis and swelling behavior of Ag-nanocomposite semi-IPN hydrogels and their drug delivery using Dolichos biflorus Linn. Soft Mater. 2018, 16, 7–19. [Google Scholar] [CrossRef]
- Meng, H.; Zhang, X.; Chen, Q.; Wei, J.; Wang, Y.; Dong, A.; Yang, H.; Tan, T.; Cao, H. Preparation of poly(aspartic acid) superabsorbent hydrogels by solvent-free processes. J. Polym. Eng. 2015, 35, 647–655. [Google Scholar] [CrossRef]
- Gyarmati, B.; Vajna, B.; Némethy, Á.; László, K.; Szilágyi, A. Redox-and pH-Responsive Cysteamine-Modified Poly(aspartic acid) Showing a Reversible Sol–Gel Transition. Macromol. Biosci. 2013, 13, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hou, Y.; Ruan, G.; Pan, M.; Liu, T. Study on the polymerization of aspartic acid catalyzed by phosphoric acid. J. Macromol. Sci. Part A Pure Appl. Chem. 2003, 40, 293–307. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M.; Marcano, D.C.; et al. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Samadaei, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H.; Banaei, M. A structural study on ethylenediamine-and poly(amidoamine)-functionalized graphene oxide: Simultaneous reduction, functionalization, and formation of 3D structure. RSC Adv. 2015, 5, 71835–71843. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Catalytic reduction of 4-nitrophenol by silver nanoparticles stabilized on environmentally benign macroscopic biopolymer hydrogel. Bioresour. Technol. 2013, 132, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Gil, J.; Natesan, S.; Li, J.; Valdes, J.; Harding, A.; Solis, M.; Davis, S.C.; Christy, R.J. A PEGylated fibrin hydrogel-based antimicrobial wound dressing controls infection without impeding wound healing. Int. Wound J. 2017, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Mondal, S.; Das, P.; Bhawal, P.; Maity, P.P.; Ghosh, S.; Dhara, S.; Das, N.C. Design of psyllium-g-poly(acrylic acid-co-sodium acrylate)/cloisite 10A semi-IPN nanocomposite hydrogel and its mechanical, rheological and controlled drug release. Int. J. Biol. Macromol. 2018, 111, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Jokar, S.; Pourjavadi, A.; Adeli, M. Albumin–graphene oxide conjugates; carriers for anticancer drugs. RSC Adv. 2014, 4, 33001–33006. [Google Scholar] [CrossRef]
- Wang, P.; Wu, Q.; Han, L.; Wang, S.; Fang, S.; Zhang, Z.; Sun, S. Synthesis of conjugated covalent organic frameworks/graphene composite for supercapacitor electrodes. RSC Adv. 2015, 5, 27290–27294. [Google Scholar] [CrossRef]
- Gong, C.; Lu, C.; Li, B.; Shan, M.; Wu, G. Injectable dopamine-modified poly(α,β-aspartic acid) nanocomposite hydrogel as bioadhesive drug delivery system. J. Biomed. Mater. Res. Part A 2017, 105, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.L.; Tang, W.N.H.; Ooi, C.W.; Chan, E.S.; Tey, B.T. Rapid swelling and deswelling of semi-interpenetrating network poly(acrylic acid)/poly(aspartic acid) hydrogels prepared by freezing polymerization. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Aldalbahi, A.; Al-Hajji, A.B.; Chaudhary, A.A.; Alhokbany, N.; Ahamad, T. Development of carboxymethyl cellulose-based hydrogel and nanosilver composite as antimicrobial agents for UTI pathogens. Carbohydr. Polym. 2016, 138, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Di, Z.; Shi, Z.; Ullah, M.W.; Li, S.; Yang, G. A transparent wound dressing based on bacterial cellulose whisker and poly(2-hydroxyethyl methacrylate). Int. J. Biol. Macromol. 2017, 105, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Oun, A.A.; Rhim, J.-W. Carrageenan-based hydrogels and films: Effect of ZnO and CuO nanoparticles on the physical, mechanical, and antimicrobial properties. Food Hydrocoll. 2017, 67, 45–53. [Google Scholar] [CrossRef]
- Yang, J.; Tan, W.; Chen, C.; Tao, Y.; Qin, Y.; Kong, Y. Nonenzymatic glucose sensing by CuO nanoparticles decorated nitrogen-doped graphene aerogel. Mater. Sci. Eng. C 2017, 78, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, V.B.; Thétiot, F.; Ritz, S.; Pütz, S.; Choritz, L.; Lappas, A.; Förch, R.; Landfester, K.; Jonas, U. Antibacterial surface coatings from zinc oxide nanoparticles embedded in poly(N-isopropylacrylamide) hydrogel surface layers. Adv. Funct. Mater. 2012, 22, 2376–2386. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Han, D.-M.; Zhang, Q.M.; Serpe, M.J. Poly(N-isopropylacrylamide)-co-(acrylic acid) microgel/Ag nanoparticle hybrids for the colorimetric sensing of H2O2. Nanoscale 2015, 7, 2784–2789. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Mann, S.; Goyal, B.; Pal, B.; Goyal, D. CuO nanostructures of variable shapes as an efficient catalyst for [3 + 2] cycloaddition of azides with terminal alkyne. RSC Adv. 2016, 6, 102733–102743. [Google Scholar] [CrossRef]
- Hou, X.; Wang, L. Controllable fabrication and photocatalysis of ZnO/Au nanohybrids via regenerative ion exchange and reduction cycles. RSC Adv. 2014, 4, 56945–56951. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Spinato, C.; Raya, J.; Bianco, A.; Ménard-Moyon, C. Chemical reactivity of graphene oxide towards amines elucidated by solid-state NMR. Nanoscale 2016, 8, 13714–13721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahamed, M.N.; Sankar, S.; Kashif, P.M.; Basha, S.H.; Sastry, T. Evaluation of biomaterial containing regenerated cellulose and chitosan incorporated with silver nanoparticles. Int. J. Biol. Macromol. 2015, 72, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Ahmed, M.; Haq, I.u.; Nisa, S.; Zia, M. PVP and PEG doped CuO nanoparticles are more biologically active: Antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mater. Sci. Eng. C 2017, 79, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, K.; Sivaraj, D. Enhanced antibacterial activity of Cr doped ZnO nanorods synthesized using microwave processing. RSC Adv. 2015, 5, 68461–68469. [Google Scholar] [CrossRef]
- Dragan, E.S.; Cocarta, A.I. Smart macroporous IPN hydrogels responsive to pH, temperature, and ionic strength: Synthesis, characterization, and evaluation of controlled release of drugs. ACS Appl. Mater. Interfaces 2016, 8, 12018–12030. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Namazi, H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2015, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Farhoudian, S.; Barkhordari, S.; Gholamali, I.; Farhadnejad, H.; Motasadizadeh, H. Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. Int. J. Biol. Macromol. 2016, 82, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloids Surf. B 2014, 113, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Shlar, I.; Droby, S.; Rodov, V. Modes of antibacterial action of curcumin under dark and light conditions: A toxicoproteomics approach. J. Proteom. 2017, 160, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.X.; Li, C.M.; Huang, C.Z. Curcumin modified silver nanoparticles for highly efficient inhibition of respiratory syncytial virus infection. Nanoscale 2016, 8, 3040–3048. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Achazi, K.; Schulz, A.; Mülhaupt, R.; Thierbach, S.; Rühl, E.; Adeli, M.; Haag, R. Combination of Surface Charge and Size Controls the Cellular Uptake of Functionalized Graphene Sheets. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Tsekova, P.B.; Spasova, M.G.; Manolova, N.E.; Markova, N.D.; Rashkov, I.B. Electrospun curcumin-loaded cellulose acetate/polyvinylpyrrolidone fibrous materials with complex architecture and antibacterial activity. Mater. Sci. Eng. C 2017, 73, 206–214. [Google Scholar] [CrossRef] [PubMed]







| Formulation Code | Am (mmoL) | AA (mmoL) | PSI (g) | AgNO3 (mmoL) | CuCl2 (mmol) | Zn(NO3)2·6H2O (mmoL) |
|---|---|---|---|---|---|---|
| IPN0 | 0.05 | 0.06 | 0.1 | — | — | — |
| IPN1 | 0.05 | 0.06 | 0.1 | 0.3 | — | — |
| IPN2 | 0.05 | 0.06 | 0.1 | 0.5 | — | — |
| IPN3 | 0.05 | 0.06 | 0.1 | 1 | — | — |
| IPN4 | 0.05 | 0.06 | 0.1 | — | 0.3 | — |
| IPN5 | 0.05 | 0.06 | 0.1 | — | 0.5 | — |
| IPN6 | 0.05 | 0.06 | 0.1 | — | 1 | — |
| IPN7 | 0.05 | 0.06 | 0.1 | — | — | 0.3 |
| IPN8 | 0.05 | 0.06 | 0.1 | — | — | 0.5 |
| IIPN9 | 0.05 | 0.06 | 0.1 | — | — | 1 |
| Formulation Code | % Drug Loading | K, hnr | n | R2 |
|---|---|---|---|---|
| IPN0 | 57.7 | 0.1023 | 0.478 | 0.999 |
| IPN1 | 67.3 | 0.0403 | 0.740 | 0.998 |
| IPN2 | 65.3 | 0.401 | 0.711 | 0997 |
| IPN3 | 70.1 | 0.3721 | 0.955 | 0.997 |
| IPN4 | 67.2 | 0.03372 | 0.938 | 0.997 |
| IPN5 | 64.45 | 0.03483 | 0.933 | 0.997 |
| IPN6 | 60.3 | 0.03167 | 1.051 | 0.997 |
| IPN7 | 65.3 | 0.01702 | 1.257 | 0.998 |
| IPN8 | 62.3 | 0.01661 | 1.303 | 0.997 |
| IPN9 | 60.01 | 0.01521 | 1.330 | 0.998 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sattari, S.; Dadkhah Tehrani, A.; Adeli, M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers 2018, 10, 660. https://doi.org/10.3390/polym10060660
Sattari S, Dadkhah Tehrani A, Adeli M. pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers. 2018; 10(6):660. https://doi.org/10.3390/polym10060660
Chicago/Turabian StyleSattari, Shabnam, Abbas Dadkhah Tehrani, and Mohsen Adeli. 2018. "pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems" Polymers 10, no. 6: 660. https://doi.org/10.3390/polym10060660
APA StyleSattari, S., Dadkhah Tehrani, A., & Adeli, M. (2018). pH-Responsive Hybrid Hydrogels as Antibacterial and Drug Delivery Systems. Polymers, 10(6), 660. https://doi.org/10.3390/polym10060660