Effect of a Surfactant in Microcapsule Synthesis on Self-Healing Behavior of Capsule Embedded Polymeric Films
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of PU Microcapsules
2.3. Preparation of Microcapsule Embedded Self-Healing Membranes
2.4. Instrumentation
3. Results and Discussion
3.1. Microcapsule Properties
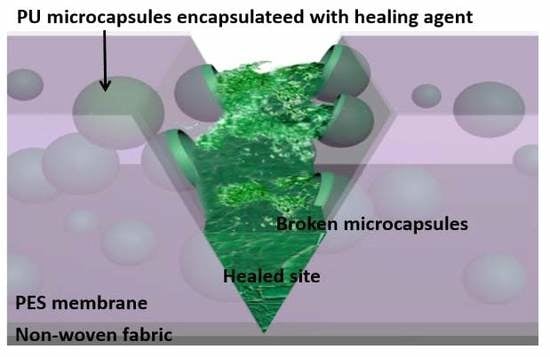
3.2. Self-Healing Behavior
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Getachew, B.A.; Park, S.J.; Kwon, O.S.; Ryu, W.H.; Taylor, A.D.; Bae, J.; Kim, J.H. Toward Microcapsule-Embedded Self-Healing Membranes. Environ. Sci. Technol. Lett. 2016, 3, 216–221. [Google Scholar] [CrossRef]
- Yang, H.I.; Kim, D.M.; Yu, H.C.; Chung, C.M. Microcapsule-Type Organogel-Based Self-Healing System Having Secondary Damage Preventing Capability. ACS Appl. Mater. Interfaces 2016, 8, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, M.A.; Van der Zwaag, S.; Garcia, S.J. Adhesion and Long-Term Barrier Restoration of Intrinsic Self-Healing Hybrid Sol–Gel Coatings. ACS Appl. Mater. Interfaces 2016, 8, 4126–4136. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.M.; Patchan, M.W.; Morris, M.; Maisano, A.J.; Phillips, T.E.; Benkoski, J.J.; Srinivasan, R. Synergy between Galvanic Protection and Self-Healing Paints. Langmuir 2016, 31, 10610–10617. [Google Scholar] [CrossRef] [PubMed]
- Yoonessi, M.; Lerch, B.A.; Peck, J.A.; Rogers, R.B.; Sola-Lopez, F.J.; Meador, M.A. Self-Healing of Core–Shell Magnetic Polystyrene Nanocomposites. ACS Appl. Mater. Interfaces 2016, 7, 16932–16937. [Google Scholar] [CrossRef] [PubMed]
- Na, X.M.; Gao, F.; Zhang, L.Y.; Su, Z.G.; Ma, G.H. Biodegradable Microcapsules Prepared by Self-Healing of Porous Microspheres. ACS Macro Lett. 2016, 1, 697–700. [Google Scholar] [CrossRef]
- Gao, L.; He, J.; Hu, J.; Wang, C. Photoresponsive Self-Healing Polymer Composite with Photoabsorbing Hybrid Microcapsules. ACS Appl. Mater. Interfaces 2015, 7, 25546–25552. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Li, L.; Yang, Y.; An, E. Self-healing properties of protective coatings containing isophorone diisocyanate microcapsules on carbon steel surfaces. Corros. Sci. 2014, 80, 528–533. [Google Scholar] [CrossRef]
- Koh, E.; Kim, N.K.; Shin, J.; Kim, Y.W. Polyurethane microcapsules for self-healing paint coatings. RSC Adv. 2014, 4, 16214–16223. [Google Scholar] [CrossRef]
- Tatiya, P.D.; Hedaoo, R.K.; Mahulikar, P.P.; Gite, V.V. Novel Polyurea Microcapsules Using Dendritic Functional Monomer: Synthesis, Characterization, and Its Use in Self-healing and Anticorrosive Polyurethane Coatings. Ind. Eng. Chem. Res. 2013, 52, 1562–1570. [Google Scholar] [CrossRef]
- Koh, E.; Lee, S.; Shin, J.; Kim, Y.W. Renewable Polyurethane Microcapsules with Isosorbide Derivatives for Self-Healing Anticorrosion Coatings. Ind. Eng. Chem. Res. 2013, 52, 15541–15548. [Google Scholar] [CrossRef]
- Guo, W.; Jia, Y.; Tian, K.; Xu, Z.; Jiao, J.; Li, R.; Wu, Y.; Cao, L.; Wang, H. UV-Triggered Self-Healing of a Single Robust SiO2 Microcapsule Based on Cationic Polymerization for Potential Application in Aerospace Coatings. ACS Appl. Mater. Interfaces 2016, 8, 21046–21054. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Jo, Y.H.; Lim, Y.J.; Cho, S.Y.; Yu, H.C.; Ryu, B.C.; Lee, S.I.; Chung, C.M. Sunlight-Induced Self-Healing of a Microcapsule-Type Protective Coating. ACS Appl. Mater. Interfaces 2013, 5, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; An, J.; Sun, D.; Tang, X.; Xiang, Y.; Yang, J. Robust microcapsules with polyurea/silica hybrid shell for one-part self-healing anticorrosion coatings. J. Mater. Chem. A 2014, 2, 11614–11620. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Yang, X.; Jiang, S.; Lv, W.; Jiang, Z.; Qiao, S.Z. Enhanced Water Retention by Using Polymeric Microcapsules to Confer High Proton Conductivity on Membranes at Low Humidity. Adv. Funct. Mater. 2011, 21, 971–978. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Li, Y.; Liu, Y.; Wang, J.; Zhang, B.; Liu, J. Polyelectrolyte microcapsules as ionic liquid reservoirs within ionomer membrane to confer high anhydrous proton conductivity. J. Power Sources 2015, 279, 667–677. [Google Scholar] [CrossRef]
- Dang, J.; Zhao, L.; Zhang, J.; Liu, J.; Wang, J. Imidazole microcapsules toward enhanced phosphoric acid loading of polymer electrolyte membrane for anhydrous proton conduction. J. Membr. Sci. 2018, 545, 88–98. [Google Scholar] [CrossRef]
- Kardar, P. Preparation of polyurethane microcapsules with different polyols component for encapsulation of isophorone diisocyanate healing agent. Prog. Org. Coat. 2015, 89, 271–276. [Google Scholar] [CrossRef]
- Haghayegh, M.; Mirabedini, S.M.; Yeganeh, H. Microcapsules containing multi-functional reactive isocyanate-terminated polyurethane prepolymer as a healing agent. Part 1: Synthesis and optimization of reaction conditions. J. Mater. Sci. 2016, 51, 3056–3068. [Google Scholar] [CrossRef]
- Credico, B.D.; Levi, M.; Turri, S. An efficient method for the output of new self-repairing materials through a reactive isocyanate encapsulation. Eur. Polym. J. 2013, 49, 2467–2476. [Google Scholar] [CrossRef]
- Blaiszik, B.J.; Caruso, M.M.; Mcllroy, D.A.; Moore, J.S.; White, S.R.; Sottos, N.R. Microcapsules filled with reactive solutions for self-healing materials. Polymer 2009, 50, 990–997. [Google Scholar] [CrossRef]
- Rosenhauer, E.M.; Wagner, M.; Musyanovych, A.; Landfester, K. Controlled Release from Polyurethane Nanocapsules via pH-, UV-Light- or Temperature-Induced Stimuli. Macromolecules 2010, 41, 5083–5093. [Google Scholar] [CrossRef]
- Neuser, S.; Manfredi, E.; Michaud, V. Characterization of solvent-filled polyurethane/urea–formaldehyde core–shell composites. Mater. Chem. Phys. 2014, 143, 1018–1025. [Google Scholar]
- Caruso, M.M.; Blaiszik, B.J.; Jin, H.; Schelkopf, S.R.; Stradley, D.S.; Sottos, N.R.; White, S.R.; Moore, J.S. Robust, Double-Walled Microcapsules for Self-Healing Polymeric Materials. ACS Appl. Mater. Interfaces 2010, 2, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yi, N.; Wu, Y.; Zhang, Y.; Zhang, Q.; Huang, Y.; Ma, Y.; Chen, Y. Multichannel and Repeatable Self-Healing of Mechanical Enhanced Graphene-Thermoplastic Polyurethane Composites. Adv. Mater. 2013, 25, 2224–2228. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of Isocyanates for Self-Healing Polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Huang, M.; Yang, J. Facile microencapsulation of HDI for self-healing anticorrosion coatings. J. Mater. Chem. 2011, 21, 11123–11130. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Influence of the silica content in SPEEK–silica membranes prepared from the sol–gel process of polyethoxysiloxane: Morphology and proton mobility. J. Membr. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Zaribaf, B.H.; Lee, S.J.; Kim, J.H.; Park, P.K.; Kim, J.H. Toward in Situ Healing of Compromised Polymeric Membranes. Environ. Sci. Technol. Lett. 2014, 1, 113–116. [Google Scholar] [CrossRef]
- Saunders, K. Polyurethanes (Chapter Title). In Organic Polymer Chemistry; Saunders, K., Ed.; Springer: Berlin, Germany, 1973; pp. 318–346. [Google Scholar]
- Jeong, E.; Lee, G.; Han, S.W.; Lee, W.J.; Choi, H.S.; Lee, Y.; Kim, J.W. Polyelectrolyte/silica-layered hydrogel microcapsules as vehicles with remarkable shell impermeability. J. Ind. Eng. Chem. 2017, 46, 192–198. [Google Scholar] [CrossRef]
- Prabhakar, A.K.; Lai, H.Y.; Potroz, M.G.; Corliss, M.K.; Park, J.H.; Mundargi, M.C.; Cho, D.; Bang, S.I.; Cho, N.J. Chemical processing strategies to obtain sporopollenin exine capsules from multi-compartmental pine pollen. J. Ind. Eng. Chem. 2017, 53, 375–385. [Google Scholar] [CrossRef]
- Doan, T.; Acosta, E.; Scamehorn, J.F.; Sabatini, D.A. Formulating middle-phase microemulsions using mixed anionic and cationic surfactant systems. J. Surfactants Deterg. 2003, 6, 215–224. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Argillier, J.F. Microencapsulation by complex coacervation: effect of cationic surfactants. J. Surf. Sci. Technol. 2005, 21, 161–168. [Google Scholar]
- Kwon, O.S.; Jang, J.; Bae, J. A Review of Fabrication Methods and Applications of Novel Tailored Microcapsules. Curr. Org. Chem. 2013, 17, 3–13. [Google Scholar]
- Bae, J. Fabrication of Carbon Microcapsules Containing Silicon Nanoparticles for Anode in Lithium Ion Battery. Colloid Polym. Sci. 2011, 289, 1233–1241. [Google Scholar] [CrossRef]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Park, S.J.; Park, C.-S.; Kwon, O.S.; Chung, S.Y.; Shim, J.; Lee, C.-S.; Bae, J. Effect of a Surfactant in Microcapsule Synthesis on Self-Healing Behavior of Capsule Embedded Polymeric Films. Polymers 2018, 10, 675. https://doi.org/10.3390/polym10060675
Lee J, Park SJ, Park C-S, Kwon OS, Chung SY, Shim J, Lee C-S, Bae J. Effect of a Surfactant in Microcapsule Synthesis on Self-Healing Behavior of Capsule Embedded Polymeric Films. Polymers. 2018; 10(6):675. https://doi.org/10.3390/polym10060675
Chicago/Turabian StyleLee, Jiyeon, Seon Joo Park, Chul-Soon Park, Oh Seok Kwon, So Young Chung, Jongwon Shim, Chang-Soo Lee, and Joonwon Bae. 2018. "Effect of a Surfactant in Microcapsule Synthesis on Self-Healing Behavior of Capsule Embedded Polymeric Films" Polymers 10, no. 6: 675. https://doi.org/10.3390/polym10060675
APA StyleLee, J., Park, S. J., Park, C.-S., Kwon, O. S., Chung, S. Y., Shim, J., Lee, C.-S., & Bae, J. (2018). Effect of a Surfactant in Microcapsule Synthesis on Self-Healing Behavior of Capsule Embedded Polymeric Films. Polymers, 10(6), 675. https://doi.org/10.3390/polym10060675