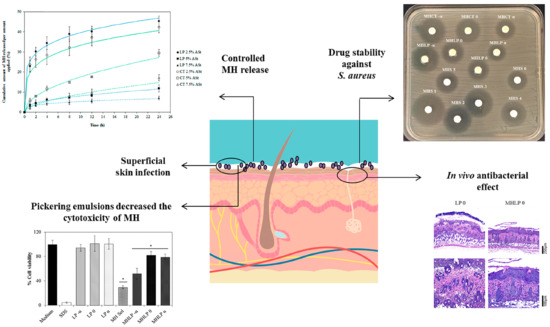
Starch-Based Pickering Emulsions as Platforms for Topical Antibiotic Delivery: In Vitro and In Vivo Studies
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Minocycline Hydrochloride (MH)-Loaded Starch-Stabilized Pickering Emulsions (MHASt-Emulsions)
2.2.2. In Vitro Release Studies
2.2.3. Topical Delivery Studies of MH
In Vitro Skin Permeation and Retention
2.2.4. In Vitro Determination of the Antibacterial Activity of MH in ASt-Emulsions
2.2.5. Skin Adapted Agar Diffusion Test
2.2.6. In Vitro Cytotoxicity Studies
Cell Culture Conditions and Cytotoxicity Assays
2.2.7. Scratch Wound Healing Migration Assay
2.2.8. In Vivo Studies
2.2.8.1. In Vivo Antibacterial Activity Studies: Tape-Stripping and Infection Model
2.2.8.2. Skin Histology
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Formulation Development
3.2. In Vitro Release Studies
3.3. Topical Delivery Studies
3.4. In Vitro Determination of the Antibacterial Activity of MH in ASt-Emulsions
3.5. Skin Adapted Agar Diffusion Test
3.6. In Vitro Cytotoxicity Studies
3.7. In Vitro Scratch Wound-Healing Migration Assay
3.8. In Vivo Studies
3.8.1. In Vivo Antibacterial Activity Studies: Tape-Stripping Infection Model
3.8.2. Skin Histology
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marto, J.; Gouveia, L.; Jorge, I.M.; Duarte, A.; Gonçalves, L.M.; Silva, S.M.C.; Antunes, F.; Pais, A.A.C.C.; Oliveira, E.; Almeida, A.J.; et al. Starch-based Pickering emulsions for topical drug delivery: A QbD approach. Colloids Surf. B Biointerfaces 2015, 135, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Ascenso, A.; Simoes, S.; Almeida, A.J.; Ribeiro, H.M. Pickering emulsions: Challenges and opportunities in topical delivery. Expert Opin. Drug Deliv. 2016, 13, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Hwang, M.-R.; Kim, J.O.; Lee, J.H.; Kim, Y.I.; Kim, J.H.; Chang, S.W.; Jin, S.G.; Kim, J.A.; Lyoo, W.S. Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int. J. Pharm. 2010, 392, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Nava, L.A.; López-Alcalde, J.; Roqué i Figuls, M.; Solà, I.; Bonfill Cosp, X. Antibiotic prophylaxis for preventing burn wound infection. Cochrane Database Syst. Rev. 2013, 6, CD008738. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Ismail, F.A.; Naggar, V.F.; Aboulmagd, E. Comparative Study to Investigate the Effect of Meloxicam or Minocycline HCl In Situ Gel System on Local Treatment of Periodontal Pockets. AAPS PharmSciTech 2014, 15, 1021–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goulden, V.; Glass, D.; Cunliffe, W. Safety of long-term high-dose minocycline in the treatment of acne. Br. J. Dermatol. 1996, 134, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Cherian, P.; Gunson, T.; Borchard, K.; Tai, Y.; Smith, H.; Vinciullo, C. Oral antibiotics versus topical decolonization to prevent surgical site infection after Mohs micrographic surgery—A randomized, controlled trial. Dermatol. Surg. 2013, 39, 1486–1493. [Google Scholar]
- McClain, R.W.; Yentzer, B.A.; Feldman, S.R. Comparison of skin concentrations following topical versus oral corticosteroid treatment: Reconsidering the treatment of common inflammatory dermatoses. J. Drugs Dermatol. JDD 2009, 8, 1076–1079. [Google Scholar]
- Renvert, S.; Lessem, J.; Dahlen, G.; Lindahl, C.; Svensson, M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: A randomized clinical trial. J. Clin. Periodontol. 2006, 33, 362–369. [Google Scholar] [CrossRef]
- Joks, R.O.; Durkin, H.G. Topical Minocycline Ointment for Suppression of Allergic Skin Responses. U.S. Patent Application No. 14/130,995, 6 July 2012. [Google Scholar]
- Group, F. Minocycline Foam Successfully Treats Impetigo with no Side-Effects in Phase II Trial; BMIResearch: London, UK, 2012; pp. 1–2. [Google Scholar]
- Xu, L.; Wang, Y.; Nguyen, V.T.; Chen, J. Effects of Topical Antibiotic Prophylaxis on Wound Healing Following Flapless Implant Surgery: A Pilot Study. J. Periodontol. 2016, 87, 275–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Khan, K. The concept of dissolution efficiency. J. Pharm. Pharmacol. 1975, 27, 48–49. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 428: Skin Absorption: In Vitro Method; OECD Publishing: Paris, France, 2018. [Google Scholar]
- NCCLS. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standards; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2006. [Google Scholar]
- Bicho, J.; Marto, J.; Salgado, A.; Raposo, S.; Simões, S. Lipid Nanocarriers for Keto-conazole Topical Delivery. Gavin J. Dermatol. Res. Ther. 2016, 2016, 7–13. [Google Scholar]
- Kugelberg, E.; Norström, T.; Petersen, T.K.; Duvold, T.; Andersson, D.I.; Hughes, D. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 2005, 49, 3435–3441. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Fausto, N.; Aster, J.C. Robbins and Cotran Pathologic Basis of Disease, Professional Edition: Expert Consult-Online; Elsevier Health Sciences: New York, NY, USA, 2014. [Google Scholar]
- Zbinovsky, V.; Chrekian, G.P. Minocycline. In Analytical Profiles of Drug Substances; Klaus, F., Ed.; Academic Press: New York, NY, USA, 1977; Volume 6, pp. 323–339. [Google Scholar]
- Marku, D.; Wahlgren, M.; Rayner, M.; Sjöö, M.; Timgren, A. Characterization of starch Pickering emulsions for potential applications in topical formulations. Int. J. Pharm. 2012, 428, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Timgren, A.; Sjöö, M.; Dejmek, P.; Rayner, M. Preparation and encapsulation properties of double Pickering emulsions stabilized by quinoa starch granules. Colloids Surf. A Physicochem. Eng. Asp. 2013, 423, 147–153. [Google Scholar] [CrossRef]
- Tadros, T.F. Emulsion Formation and Stability; Wiley-VCH: Weinheim, Germany, 2013; 252p. [Google Scholar]
- Frelichowska, J.; Bolzinger, M.A.; Valour, J.P.; Mouaziz, H.; Pelletier, J.; Chevalier, Y. Pickering w/o emulsions: Drug release and topical delivery. Int. J. Pharm. 2009, 368, 7–15. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, C.; Liu, H.; Zou, S.; Tong, Z. Facile fabrication of biocompatible PLGA drug-carrying microspheres by O/W pickering emulsions. Colloids Surf. B Biointerfaces 2012, 91, 97–105. [Google Scholar] [CrossRef]
- Rolland, A.; Wagner, N.; Chatelus, A.; Shroot, B.; Schaefer, H. Site-specific drug delivery to pilosebaceous structures using polymeric microspheres. Pharm. Res. 1993, 10, 1738–1744. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.; Watts, F.; Papantoniou, C.; Mahieu, C. Cosmetic or pharmaceutical composition containing microspheres of polymers or of fatty substances filled with at least one active product. U.S. Patent 5,292,512, 8 March 1994. [Google Scholar]
- Wissing, S.; Müller, R. Solid lipid nanoparticles as carrier for sunscreens: In vitro release and in vivo skin penetration. J. Control. Release 2002, 81, 225–233. [Google Scholar] [CrossRef]
- Lou, H.; Qiu, N.; Crill, C.; Helms, R.; Almoazen, H. Development of W/O Microemulsion for Transdermal Delivery of Iodide Ions. AAPS Pharm. Sci. Tech. 2013, 14, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Barrett, C. Skin penetration. J. Soc. Cosmet. Chem. 1969, 20, 487–499. [Google Scholar]
- Hafeez, F.; Maibach, H. Occlusion effect on in vivo percutaneous penetration of chemicals in Man and monkey: Partition coefficient effects. Skin Pharmacol. Physiol. 2013, 26, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.; Maibach, H.I. Occlusion vs. skin barrier function. Skin Res. Technol. 2002, 8, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marto, J.; Ascenso, A.; Gonçalves, L.M.; Gouveia, L.F.; Manteigas, P.; Pinto, P.; Oliveira, E.; Almeida, A.J.; Ribeiro, H.M. Melatonin-based pickering emulsion for skin’s photoprotection. Drug Deliv. 2016, 23, 1594–1607. [Google Scholar] [CrossRef]
- Marto, J.; Gouveia, L.; Chiari, B.; Paiva, A.; Isaac, V.; Pinto, P.; Simões, P.; Almeida, A.; Ribeiro, H. The green generation of sunscreens: Using coffee industrial sub-products. Ind. Crops Prod. 2016, 80, 93–100. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.A.; Pelletier, J.; Valour, J.P.; Chevalier, Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef]
- Fargier, A.; Sacha, M.; Keck, C.; Haltner, E. Skin disc antibiogram: A new in-vitro model to test pharmaco-dynamic equivalence of anti-bacterial topic formulations. Wiss. Posterausstellung 2015, 16, 3–5. [Google Scholar]
- OECD. Test No. 439: In Vitro Skin Irritation Reconstructed Human Epidermis Test Method: Reconstructed Human Epidermis Test Method; OECD Publishing: Paris, France, 2010; Volume 4. [Google Scholar]
- Nair, B.; Yamarik, T.A. Final report on the safety assessment of aluminum starch octenylsuccinate. Int. J. Toxicol. 2002, 21 (Suppl. S1), 1–7. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Primorac, M. The influence of cosurfactants and oils on the formation of pharmaceutical microemulsions based on PEG-8 caprylic/capric glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, C.F.; Ugartondo, V.; Mitjans, M.; Rocha-Filho, P.A.; Vinardell, M.P. Low cytotoxicity of creams and lotions formulated with Buriti oil (Mauritia flexuosa) assessed by the neutral red release test. Food Chem. Toxicol. 2008, 46, 2776–2781. [Google Scholar] [CrossRef] [PubMed]
- Santander-Ortega, M.; Stauner, T.; Loretz, B.; Ortega-Vinuesa, J.; Bastos-González, D.; Wenz, G.; Schaefer, U.; Lehr, C. Nanoparticles made from novel starch derivatives for transdermal drug delivery. J. Control. Release 2010, 141, 85–92. [Google Scholar] [CrossRef]
- Leite, L.M.; Carvalho, A.G.G.; Ferreira, P.L.T.; Pessoa, I.X.; Gonçalves, D.O.; de Araújo Lopes, A.; dos Santos Góes, J.G.; de Castro Alves, V.C.; Leal, L.K.A.; Brito, G.A. Anti-inflammatory properties of doxycycline and minocycline in experimental models: An in vivo and in vitro comparative study. Inflammopharmacology 2011, 19, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch-Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Culp, B.; Scheinfeld, N. Rosacea: A review. Pharm. Ther. 2009, 34, 38. [Google Scholar]
- Hartman-Adams, H.; Banvard, C.; Juckett, G. Impetigo: Diagnosis and treatment. Am. Fam. Phys. 2014, 90, 229–235. [Google Scholar]






| Formulations | Quantitative Composition (%, w/w) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ASt-Emulsion with Liquid Paraffin (LP) | ASt-Emulsion with Caprylic/Capric Acid Triglyceride (CT) | MHASt-Emulsion with LP | MHASt-Emulsion with CT | |||||||||
| 2.5% | 5% | 7.5% | 2.5% | 5% | 7.5% | 2.5% | 5% | 7.5% | 2.5% | 5% | 7.5% | |
| Phase A (external) | ||||||||||||
| Liquid paraffin | 72.50 | 70.00 | 67.50 | - | - | - | 72.45 | 69.95 | 67.45 | - | - | - |
| Caprylic/capric acid triglyceride | - | - | - | 72.50 | 70.00 | 67.50 | - | - | - | 72.45 | 69.95 | 67.45 |
| Phase B (solid particles) | ||||||||||||
| Aluminum starch octenylsuccinate | 2.50 | 5.00 | 7.50 | 2.50 | 5.00 | 7.50 | 2.50 | 5.00 | 7.50 | 2.50 | 5.00 | 7.50 |
| Phase C (internal) | ||||||||||||
| Purified water | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 | 25.00 |
| Minocycline hydrochloride | - | - | - | - | - | - | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Model | K | R2adjusted | AIC | T50% (min) | T90% (min) | DE24 h (% ± SD) | |
|---|---|---|---|---|---|---|---|
| 2.5%-MHASt-emulsion with LP | First order | 0.05 | 0.723 | 55.3 | 14.3 | 47.5 | 38.4 ± 5.4 |
| Higuchi | 14.68 | 0.731 | 38.3 | 12.0 | 39.0 | ||
| Korsmeyer–Peppas | 25.63 | 0.973 | 25.6 | 56.3 | 2388.9 | ||
| n—0.18 | |||||||
| Weibull | α—3.14 β—0.20 Ti—0.53 | 0.972 | 26.3 | 83.9 | 12432.0 | ||
| 5%-MHASt-emulsion with LP | First order | 0.01 | 0.838 | 30.4 | 111.1 | 369.1 | 8.1 ± 0.2 |
| Higuchi | 2.53 | 0.960 | 16.6 | 390.1 | 1263.9 | ||
| Korsmeyer–Peppas | 3.53 | 0.989 | 4.2 | 13,683.2 | 185,438.2 | ||
| n—0.39 | |||||||
| Weibull | α—27.17 | 0.985 | 5.1 | 148,476.5 | 1,028,8670.6 | ||
| β—0.35 | |||||||
| Ti—0.40 | |||||||
| 7.5%-MHASt-emulsion with LP | First order | 0.00 | 0.273 | 24.5 | 182.2 | 605.1 | 5.3 ± 0.9 |
| Higuchi | 1.63 | 0.871 | 13.1 | 979.1 | 3172.1 | ||
| Korsmeyer–Peppas | 2.84 | 0.997 | 9.3 | 71,257.0 | 723,399.2 | ||
| n—0.27 | |||||||
| Weibull | α—31.79 | 0.996 | 11.7 | 902,911.8 | 39,677,378.5 | ||
| β—0.24 | |||||||
| Ti—0.40 | |||||||
| 2.5%-MHASt-emulsion with CT | First order | 0.05 | 0.139 | 50.1 | 15.8 | 52.6 | 40.1 ± 8.3 |
| Higuchi | 11.99 | 0.775 | 40.7 | 18.2 | 58.8 | ||
| Korsmeyer–Peppas | 25.10 | 0.988 | 22.2 | 38.8 | 743.7 | ||
| n—0.20 | |||||||
| Weibull | α—3.31 | 0.974 | 28.3 | 283.7 | 2577.2 | ||
| β—0.21 | |||||||
| Ti—0.40 | |||||||
| 5%-MHASt-emulsion with CT | First order | 0.02 | 0.886 | 31.6 | 43.8 | 145.7 | 17.9 ± 0.3 |
| Higuchi | 5.65 | 0.981 | 18.3 | 78.4 | 254.0 | ||
| Korsmeyer–Peppas | 5.60 | 0.988 | 14.4 | 85.8 | 291.9 | ||
| n—0.40 | |||||||
| Weibull | α—14.72 | 0.974 | 22.1 | 153.9 | 1015.0 | ||
| β—0.47 | |||||||
| Ti—0.42 | |||||||
| 7.5%-MHASt-emulsion with CT | First order | 0.01 | 0.8693 | 25.6 | 94.5 | 313.8 | 9.3 ± 1.7 |
| Higuchi | 2.91 | 0.9884 | 8.6 | 317.0 | 1027.2 | ||
| Korsmeyer–Peppas | 2.50 | 0.9967 | 0.5 | 228.4 | 660.9 | ||
| n—0.49 | |||||||
| Weibull | α—33.44 | 0.9846 | 11.8 | 507.4 | 2717.3 | ||
| β—0.51 | |||||||
| Ti—0.40 |
| Formulations | Zone of Inhibition (mm) | Concentration of MH Released (µg/mL) | |
|---|---|---|---|
| MHASt-emulsions with LP | 2.5% ASt | 22.8 ± 0.7 | 1.90 ± 0.12 |
| 5% ASt | 19.3 ± 0.2 | 1.12 ± 0.03 | |
| 7.5% ASt | 14.9 ± 0.3 | 0.45 ± 0.05 | |
| MHASt-emulsions with CT | 2.5% ASt | 22.5 ± 0.4 | 1.88 ± 0.04 |
| 5% ASt | 20.1 ± 0.9 | 1.41 ± 0.15 | |
| 7.5% ASt | 16.5 ± 1.0 | 0.75 ± 0.19 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marto, J.; Duarte, A.; Simões, S.; Gonçalves, L.M.; Gouveia, L.F.; Almeida, A.J.; Ribeiro, H.M. Starch-Based Pickering Emulsions as Platforms for Topical Antibiotic Delivery: In Vitro and In Vivo Studies. Polymers 2019, 11, 108. https://doi.org/10.3390/polym11010108
Marto J, Duarte A, Simões S, Gonçalves LM, Gouveia LF, Almeida AJ, Ribeiro HM. Starch-Based Pickering Emulsions as Platforms for Topical Antibiotic Delivery: In Vitro and In Vivo Studies. Polymers. 2019; 11(1):108. https://doi.org/10.3390/polym11010108
Chicago/Turabian StyleMarto, Joana, Aida Duarte, Sandra Simões, Lídia Maria Gonçalves, Luís Filipe Gouveia, António José Almeida, and Helena Margarida Ribeiro. 2019. "Starch-Based Pickering Emulsions as Platforms for Topical Antibiotic Delivery: In Vitro and In Vivo Studies" Polymers 11, no. 1: 108. https://doi.org/10.3390/polym11010108
APA StyleMarto, J., Duarte, A., Simões, S., Gonçalves, L. M., Gouveia, L. F., Almeida, A. J., & Ribeiro, H. M. (2019). Starch-Based Pickering Emulsions as Platforms for Topical Antibiotic Delivery: In Vitro and In Vivo Studies. Polymers, 11(1), 108. https://doi.org/10.3390/polym11010108