Facile Preparation of Reduction-Responsive Micelles Based on Biodegradable Amphiphilic Polyurethane with Disulfide Bonds in the Backbone
Abstract
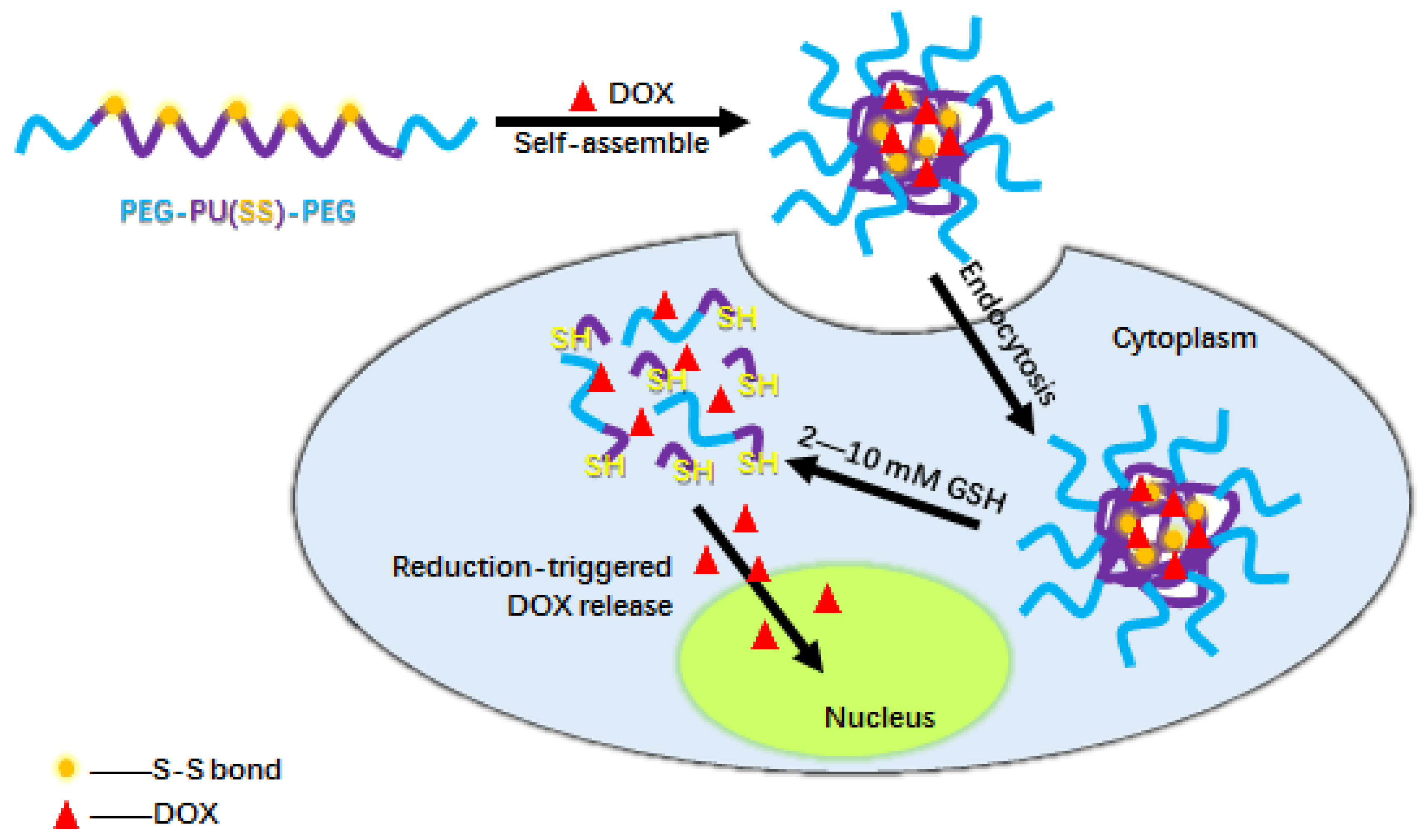
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization
2.3. Synthesis of PEG-PU(SS)-PEG and PEG-PU-PEG
2.4. Preparation and Characterization of PEG-PU(SS)-PEG Micelles
2.5. Degradation of PEG-PU(SS)-PEG Micelles under Reducing Conditions
2.6. Preparation of Encapsulated-DOX PEG-PU(SS)-PEG Micelles and Determination of Drug Loading
2.7. In Vitro Drug Release of Drug-Loaded Micelles
2.8. CCK-8 Assays
- AT: Absorbance of test group
- AC: Absorbance of control group
- AB: Absorbance of blank group
2.9. Cell Internalization
2.10. Flow Cytometry Measurements
3. Results and Discussion
3.1. Synthesis of Reduction-Sensitive Degradable PEG-PU(SS)-PEG Triblock Copolymer
3.2. Formation and Characterization of PEG-PU(SS)-PEG Micelles
3.3. Reduction-Responsive Size Change of PEG-PU(SS)-PEG Micelles
3.4. Loading and In Vitro Release of DOX
3.5. Cellular Uptake
3.6. Cell Viability Analysis of DOX-Loaded PEG-PU(SS)-PEG Micelles
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, J.; Shuai, X.Y.; Wang, R.; He, X.L.; Li, Y.W.; Ding, M.M.; Li, J.H.; Tan, H.; Fu, Q. Clickable and imageable multiblock polymer micelles with magnetically guided and PEG-switched targeting and release property for precise tumor theranosis. Biomaterials 2017, 145, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA-Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Song, N.J.; Zhou, L.J.; Liu, W.K.; He, X.L.; Pan, Z.C.; Ding, M.M.; Wan, X.Y.; Li, J.H.; Tan, H.; Luo, F.; Fu, Q. Effect of trastuzumab on the micellization properties, endocytic pathways and antitumor activities of polyurethane-based drug delivery system. Chin. J. Polym. Sci. 2017, 35, 909–923. [Google Scholar] [CrossRef]
- Gaucher, G.; Marchessault, R.H.; Leroux, J.C. Polyester-based micelles and nanoparticles for the parenteral delivery of taxanes. J. Control. Release 2010, 143, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.X.; Zhang, H.B.; Wei, Y.; Wei, Y.Y.; Xie, Y.P.; Cong, F.S. Reduction- and pH-Sensitive Hyaluronan Nanoparticles for Delivery of Iridium(III) Anticancer Drugs. Biomacromolecules 2017, 18, 2102–2117. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lin, B.B.; Ai, H. Theranostic Nanoparticles for Cancer and Cardiovascular Applications. Pharm. Res. 2014, 31, 1390–1406. [Google Scholar] [CrossRef]
- Min, H.Y.; Lee, H.Y. Oncogene-Driven Metabolic Alterations in Cancer. Biomol. Ther. 2018, 26, 45–56. [Google Scholar] [CrossRef]
- Wang, Z.; Niu, G.; Chen, X.Y. Polymeric Materials for Theranostic Applications. Pharm. Res. 2014, 31, 1358–1376. [Google Scholar] [CrossRef]
- Fu, S.X.; Yang, G.Q.; Wang, J.; Wang, X.; Cheng, X.; Zha, Q.; Tang, R.P. pH-sensitive poly(ortho ester urethanes) copolymers with controlled degradation kinetic: Synthesis, characterization, and in vitro evaluation as drug carriers. Eur. Polym. J. 2017, 95, 275–288. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.J.; Cheng, R.; Meng, F.H.; Zhong, Z.Y. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Huckaby, J.T.; Lai, S.K. PEGylation for enhancing nanoparticle diffusion in mucus. Adv. Drug Deliv. Rev. 2018, 124, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Wang, S.; Zhu, D.D.; Shen, Y.L.; Du, B.X.; Liu, X.J.; Zheng, Y.L. Reversibly cross-linked poly(ethylene glycol)-poly(amino acid)s copolymer micelles: A promising approach to overcome the extracellular stability versus intracellular drug release challenge. RSC Adv. 2015, 5, 20025–20034. [Google Scholar] [CrossRef]
- Huynh, T.T.N.; Padois, K.; Sonvico, F.; Rossi, A.; Zani, F.; Pirot, F.; Doury, J.; Falson, F. Characterization of a polyurethane-based controlled release system for local delivery of chlorhexidine diacetate. Eur. J. Pharm. Biopharm. 2010, 74, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Mukherjee, D.; Mishra, R.; Kundu, P.P. Preparation of polyurethane-alginate/chitosan core shell nanoparticles for the purpose of oral insulin delivery. Eur. Polym. J. 2017, 92, 294–313. [Google Scholar] [CrossRef]
- Omrani, I.; Babanejad, N.; Shendi, H.K.; Nabid, M.R. Fully glutathione degradable waterborne polyurethane nanocarriers: Preparation, redox-sensitivity, and triggered intracellular drug release. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 70, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.M.; Li, J.H.; Tan, H.; Fu, Q. Self-assembly of biodegradable polyurethanes for controlled delivery applications. Soft Matter 2012, 8, 5414–5428. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Hou, T.Y.; Shih, M.F.; Talsma, H.; Hennink, W.E. Polyurethane-based drug delivery systems. Int. J. Pharm. 2013, 450, 145–162. [Google Scholar] [CrossRef]
- Yu, S.J.; Ding, J.X.; He, C.L.; Cao, Y.; Xu, W.G.; Chen, X.S. Disulfide cross-linked polyurethane micelles as a reduction-triggered drug delivery system for cancer therapy. Adv. Healthc. Mater. 2014, 3, 752–760. [Google Scholar] [CrossRef]
- Zhou, L.J.; Liang, D.; He, X.L.; Li, J.H.; Tan, H.; Li, J.S.; Fu, Q.; Gu, Q. The degradation and biocompatibility of pH-sensitive biodegradable polyurethanes for intracellular multifunctional antitumor drug delivery. Biomaterials 2012, 33, 2734–2745. [Google Scholar] [CrossRef]
- Yoo, H.S.; Lee, E.A.; Park, T.G. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkages. J. Control. Release 2002, 82, 17–27. [Google Scholar] [CrossRef]
- Wan, X.J.; Liu, T.; Liu, S.Y. Thermoresponsive Core Cross-Linked Micelles for Selective Ratiometric Fluorescent Detection of Hg2+ Ions. Langmuir 2011, 27, 4082–4090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, X.F.; Chen, L.; Yu, S.J.; Cao, Y.; He, C.L.; Chen, X.S. Intracellular pH-Sensitive PEG-block-Acetalated-Dextrans as Efficient Drug Delivery Platforms. ACS Appl. Mater. Interfaces 2013, 5, 10760–10766. [Google Scholar] [CrossRef]
- Huang, X.N.; Du, F.S.; Cheng, J.; Dong, Y.Q.; Liang, D.H.; Ji, S.P.; Lin, S.S.; Li, Z.C. Acid-Sensitive Polymeric Micelles Based on Thermoresponsive Block Copolymers with Pendent Cyclic Orthoester Groups. Macromolecules 2009, 42, 783–790. [Google Scholar] [CrossRef]
- Guan, Y.Y.; Su, Y.L.; Zhao, L.L.; Meng, F.C.; Wang, Q.X.; Yao, Y.C.; Luo, J.B. Biodegradable polyurethane micelles with pH and reduction responsive properties for intracellular drug delivery. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 75, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- McCarley, R.L.; Systems, R.D.; Cooks, i.R.G.; Yeung, E.S. (Eds.) Annual Review of Analytical Chemistry; Annual Reviews: Palo Alto, CA, USA, 2012; Volume 5, pp. 391–411. [Google Scholar]
- Wang, W.; Sun, H.L.; Meng, F.H.; Ma, S.B.; Liu, H.Y.; Zhong, Z.Y. Precise control of intracellular drug release and anti-tumor activity of biodegradable micellar drugs via reduction-sensitive shell-shedding. Soft Matter 2012, 8, 3949–3956. [Google Scholar] [CrossRef]
- Ding, M.M.; He, X.L.; Wang, Z.G.; Li, J.H.; Tan, H.; Deng, H.; Fu, Q.; Gu, Q. Cellular uptake of polyurethane nanocarriers mediated by gemini quaternary ammonium. Biomaterials 2011, 32, 9515–9524. [Google Scholar] [CrossRef]
- Yoshida, E. Control of Micellar Size and Critical Micelle Concentration for “Nonamphiphilic” Poly(vinyl phenol)-block-Polystyrene Diblock Copolymers. Polym. J. 2003, 35, 965–971. [Google Scholar] [CrossRef]
- Sandoval, R.W.; Williams, D.E.; Kim, J.; Roth, C.B.; Torkelson, J.M. Critical micelle concentrations of block and gradient copolymers in homopolymer: Effects of sequence distribution, composition, and molecular weight. J. Polym. Sci. Part B: Polym. Phys. 2008, 46, 2672–2682. [Google Scholar] [CrossRef]
- Cagel, M.; Grotz, E.; Bernabeu, E.; Moretton, M.A.; Chiappetta, D.A. Doxorubicin: Nanotechnological overviews from bench to bedside. Drug Discov. Today 2017, 22, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics Adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Bhatt, A.N.; Mishra, A.K.; Dwarakanath, B.S.; Jain, S.; Schatz, C.; le Meins, J.F.; Farooque, A.; Chandraiah, G.; Jain, A.K.; et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(gamma-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010, 31, 2882–2892. [Google Scholar] [CrossRef]
- Xiong, J.A.; Meng, F.H.; Wang, C.; Cheng, R.; Liu, Z.A.; Zhong, Z.Y. Folate-conjugated crosslinked biodegradable micelles for receptor-mediated delivery of paclitaxel. J. Mater. Chem. 2011, 21, 5786–5794. [Google Scholar] [CrossRef]
- Xu, P.S.; van Kirk, E.A.; Zhan, Y.H.; Murdoch, W.J.; Radosz, M.; Shen, Y.Q. Targeted charge-reversal nanoparticles for nuclear drug delivery. Angew. Chem.-Int. Ed. 2007, 46, 4999–5002. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.M.; Zeng, X.; He, X.L.; Li, J.H.; Tan, H.; Fu, Q. Cell Internalizable and Intracellularly Degradable Cationic Polyurethane Micelles as a Potential Platform for Efficient Imaging and Drug Delivery. Biomacromolecules 2014, 15, 2896–2906. [Google Scholar] [CrossRef] [PubMed]








| Copolymer | Mna (1H NMR) | Mnb (GPC) | PDI b (GPC) |
|---|---|---|---|
| PEG-PU(SS)-PEG | 15,000 | 28,400 | 1.03 |
| PEG-PU-PEG | 18,800 | 31,400 | 1.02 |
| Block Copolymers | Micelle Size (nm) a | PDI | Zeta Potential (mv) a | CMC (mg/L) b |
|---|---|---|---|---|
| PEG-PU(SS)-PEG | 116.7 ± 1.9 | 0.08 ± 0.03 | −20.8 | 1.64 |
| PEG-PU-PEG | 139.8 ± 1.1 | 0.05 ± 0.01 | −26.6 | 5.62 |
| Sample | Theoretical Drug Loading Content (wt %) | DLC a (wt %) | DLE a (%) | Micelle Size b (nm) | PDI b |
|---|---|---|---|---|---|
| PEG-PU(SS)-PEG | 10 | 6.59 | 65.89 | 116.2 ± 2.8 | 0.20 ± 0.01 |
| 20 | 13.13 | 65.67 | 144.0 ± 0.9 | 0.15 ± 0.02 | |
| 10 | 6.07 | 60.72 | 141.4 ± 1.4 | 0.16 ± 0.02 | |
| PEG-PU-PEG | 20 | 12.54 | 62.70 | 154.9 ± 1.6 | 0.17 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Hu, J.; Bu, L.; Zhang, H.; Du, B.; Zhu, C.; Li, Y. Facile Preparation of Reduction-Responsive Micelles Based on Biodegradable Amphiphilic Polyurethane with Disulfide Bonds in the Backbone. Polymers 2019, 11, 262. https://doi.org/10.3390/polym11020262
Zhang P, Hu J, Bu L, Zhang H, Du B, Zhu C, Li Y. Facile Preparation of Reduction-Responsive Micelles Based on Biodegradable Amphiphilic Polyurethane with Disulfide Bonds in the Backbone. Polymers. 2019; 11(2):262. https://doi.org/10.3390/polym11020262
Chicago/Turabian StyleZhang, Peng, Jiaying Hu, Leran Bu, Hena Zhang, Baixiang Du, Caihong Zhu, and Yuling Li. 2019. "Facile Preparation of Reduction-Responsive Micelles Based on Biodegradable Amphiphilic Polyurethane with Disulfide Bonds in the Backbone" Polymers 11, no. 2: 262. https://doi.org/10.3390/polym11020262
APA StyleZhang, P., Hu, J., Bu, L., Zhang, H., Du, B., Zhu, C., & Li, Y. (2019). Facile Preparation of Reduction-Responsive Micelles Based on Biodegradable Amphiphilic Polyurethane with Disulfide Bonds in the Backbone. Polymers, 11(2), 262. https://doi.org/10.3390/polym11020262




