The Applications of Polymers in Solar Cells: A Review
Abstract
:1. Introduction
2. Polymers in DSSC
2.1. Polymers as the Substrates of DSSC
2.2. Polymers in Mesoporous TiO2 Photoanode of DSSC
2.3. Polymers as the Counter Electrodes of DSSC
2.3.1. Polypyrrole as the Counter Electrodes of DSSC
2.3.2. Polyaniline as the Counter Electrodes of DSSC
2.3.3. Poly(3,4-ethylenedioxythiophene) as the Counter Electrodes of DSSC
2.3.4. The Hybrids Based on Conductive Polymers as the Counter Electrodes of DSSC
2.4. Polymers as the Electrolyte of DSSC
2.4.1. Thermoplastic Polymers as the Electrolyte of DSSC
2.4.2. Thermosetting Polymers as the Electrolyte of DSSC
2.4.3. Composite Polymer as the Electrolyte of DSSC
2.5. Polymers in All-Weather DSSC
3. Polymers in Perovskite Solar Cells
3.1. Polymers in Perovskite Morphology Regulations
3.2. Polymers as Hole Transport Layers (HTLs)
3.2.1. Triarylamine-Based Polymers as HTLs
3.2.2. Conductive Polymers as HTLs
3.2.3. Poly-3-hexylthiophene Based Polymers and Composites as HTLs
3.2.4. Other Polymer as HTLs
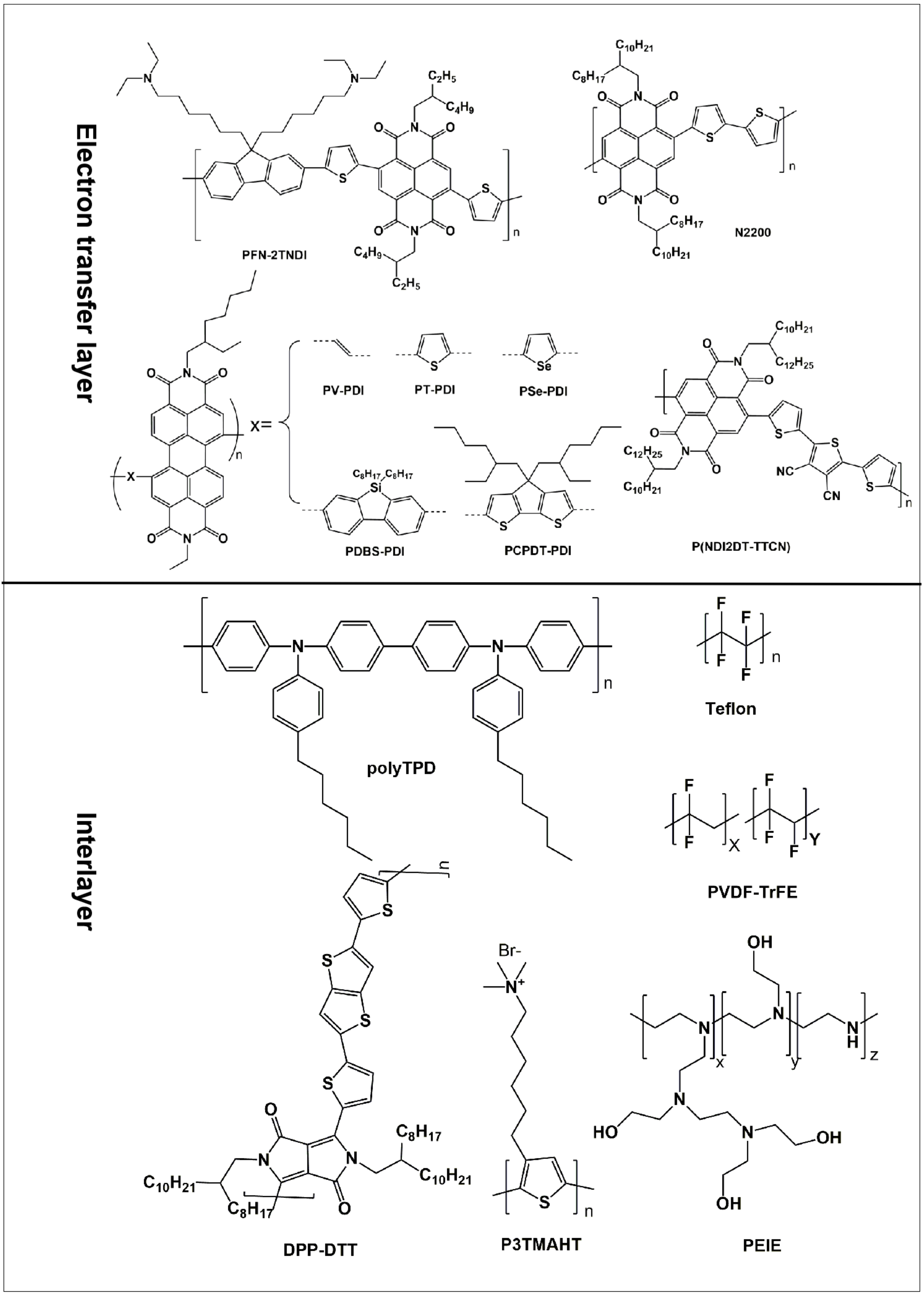
3.3. Polymers as Electron Transport Layers (ETLs)
3.4. Polymer as the Interlayer
4. Polymers in Organic Photovoltaics
4.1. Polymers in Binary OPVs
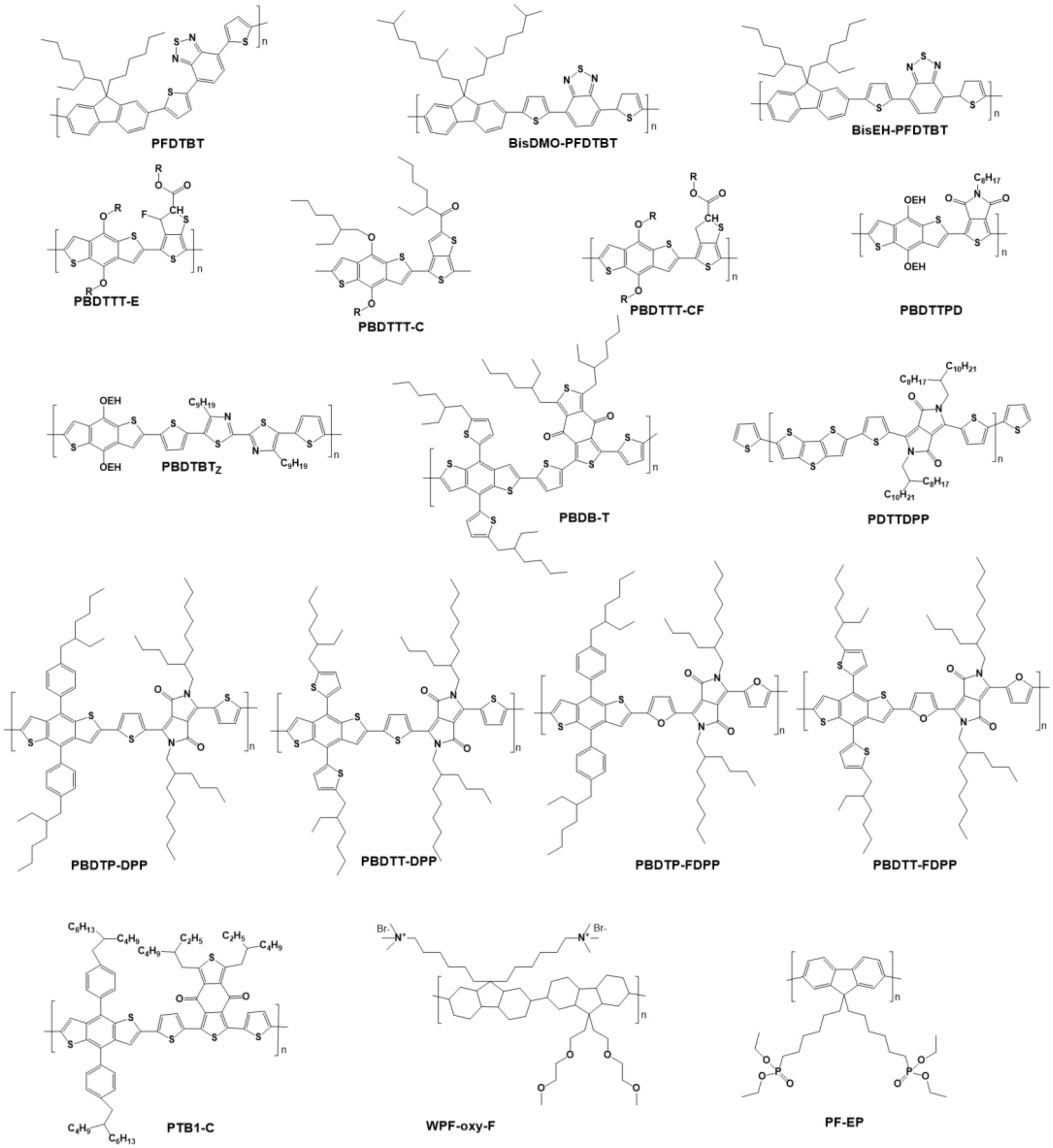
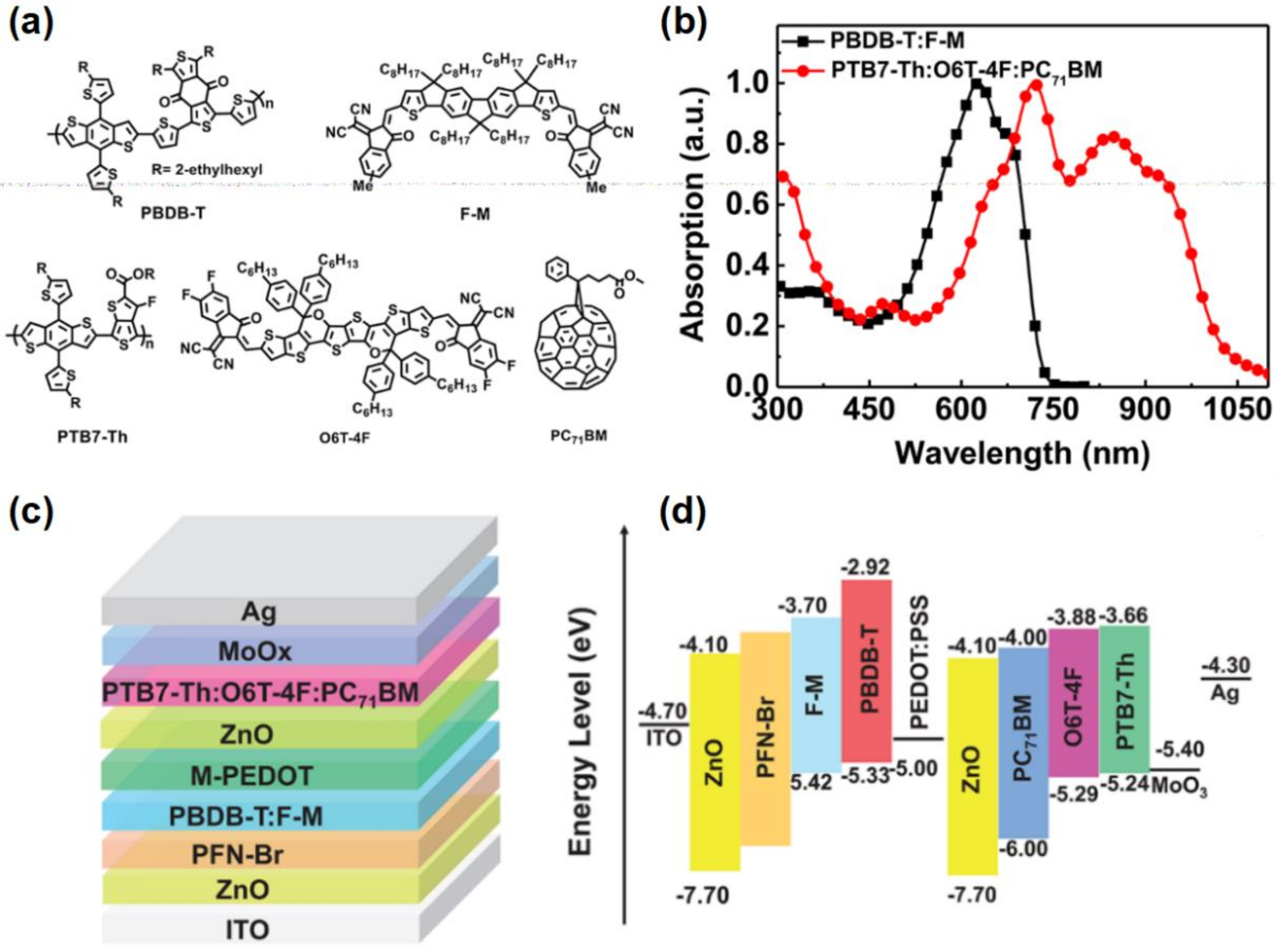
4.1.1. Polymers with Wide Bandgap as the Donor Materials of OPVs
4.1.2. Polymers with Medium Bandgap as the Donor Materials of OPV
4.1.3. Polymers with Narrow Bandgap as the Donor Materials of OPV
4.2. Polymers in Ternary OPVs
4.3. Polymers as Buffer Layers of OPV
4.4. Polymer-Based Micro/Nanostructures in the OPVs
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sovacool, B.K. National context derives concrens. Nat. Energy 2018. [Google Scholar] [CrossRef]
- Quirin, S.; Jeff, T.; Tony, S.; Alexandra, W.; Oliver, M. Energy alternatives: Electricity without carbon. Nature 2008, 454, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A. The path to 25% silicon solar cell efficiency: History of silicon cell evolution. Prog. Photovolt Res. Appl. 2009, 17, 183–189. [Google Scholar] [CrossRef] [Green Version]
- Razykov, T.M.; Ferekides, C.S.; Morel, D.; Stefanakos, E.; Ullal, H.S.; Upadhyaya, H.M. Solar photovoltaic electricity: Current status and future prospects. Sol. Energy 2011, 85, 1580–1608. [Google Scholar] [CrossRef]
- Hubbard, S.M.; Cress, C.D.; Bailey, C.G.; Raffaelle, R.P.; Bailey, S.G.; Wilt, D.M. Effect of strain compensation on quantum dot enhanced GaAs solar cells. Appl. Phys. Lett. 2008, 92, 123512. [Google Scholar] [CrossRef]
- Chirilă, A.; Buecheler, S.; Pianezzi, F.; Bloesch, P.; Gretener, C.; Uh, A.R.; Fella, C.; Kranz, L.; Perrenoud, J.; Seyrling, S.; et al. Highly efficient Cu(In,Ga)Se2 solar cells grown on flexible polymer films. Nat. Mater. 2011, 10, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yang, R.L.; Wang, D.L. CdTe solar cell performance under high-intensity light irradiance. Sol. Energy Mater. Sol. Cells 2014, 123, 249–254. [Google Scholar] [CrossRef]
- Andersen, T.R.; Dam, H.F.; Hosel, M.; Helgesen, M.; Carle, J.E.; Larsen-Olsen, T.T.; Gevorgyan, S.A.; Andreasen, J.W.; Adams, J.; Li, N.; et al. Scalable, ambient atmosphere roll-to-roll manufacture of encapsulated large area, flexible organic tandem solar cell modules. Energy Environ. Sci. 2014, 7, 2925–2933. [Google Scholar] [CrossRef]
- Freitag, M.; Daniel, Q.; Pazoki, M.; Sveinbjornsson, K.; Zhang, J.B.; Sun, L.C.; Hagfeldt, A.; Boschloo, G. High-efficiency dye-sensitized solar cells with molecular copper phenanthroline as solid hole conductor. Energy Environ. Sci. 2015, 8, 2634–2637. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Xie, F.X.; Chen, H.; Yang, X.D.; Su, H.M.; Cai, M.L.; Zhou, Z.M.; Noda, T.; Han, L.Y. Thermally stable MAPbI3 perovskite solar cells with efficiency of 19.19% and area over 1 cm2 achieved by additive engineering. Adv. Mater. 2017, 29, 1701073. [Google Scholar] [CrossRef]
- Ragoussi, M.E.; Torres, T. New generation solar cells: Concepts, trends and perspectives. Chem. Commun. 2015, 51, 3957–3972. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Wu, J.H.; Lin, J.Y.; Yue, G.T.; Lin, J.M.; Huang, M.L.; Lan, Z.; Fan, L.Q. A dual function of high performance counter-electrode for stable quasi-solid-state dye-sensitized solar cells. J. Power Sources 2013, 241, 373–378. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.X.; Chen, C.C.; Wu, Y.Z.; Li, X.; Cai, M.L.; Liu, X.; Yang, X.D.; Han, L.Y. Vertical recrystallization for highly efficient and stable formamidinium-based inverted-structure perovskite solar cells. Energy Environ. Sci. 2017, 10, 1942–1949. [Google Scholar] [CrossRef]
- Research Cell Efficiency Records. Available online: https://www.nrel.gov/pv/assets/images/efficiency-chart-20180716.jpg (accessed on 10 January 2019).
- Cichosz, S.; Masek, A.; Zaborski, M. Polymer-based sensors: A review. Polym. Test. 2018, 67, 342–348. [Google Scholar] [CrossRef]
- Lee, M.M.; Teeuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, H.; Zhao, Y.C.; Zhou, W.K.; Fu, R.; Leprince-Wang, Y.; Yu, D.P.; Zhao, Q. Suppressed hysteresis and improved stability in perovskite solar cells with conductive organic network. Nano Energy 2016, 26, 139–147. [Google Scholar] [CrossRef]
- Stephan, A.M. Review on gel polymer electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 21–42. [Google Scholar] [CrossRef]
- Gao, C.L.; Dong, H.Z.; Bao, X.C.; Zhang, Y.C.; Saparbaev, A.; Yu, L.Y.; Wen, S.G.; Yang, R.Q.; Dong, L.F. Additive engineering to improve efficiency and stability of inverted planar perovskite solar cells. J. Mater. Chem. C 2018, 6, 8234–8241. [Google Scholar] [CrossRef]
- Xue, Q.F.; Hu, Z.C.; Liu, J.; Lin, J.H.; Sun, C.; Chen, Z.M.; Duan, C.H.; Wang, J.; Liao, C.; Lau, W.M.; et al. Highly efficient fullerene/perovskite planar heterojunction solar cells via cathode modification with an amino-functionalized polymer interlayer. J. Mater. Chem. A 2014, 2, 19598–19603. [Google Scholar] [CrossRef]
- Chang, C.M.; Li, W.B.; Guo, X.; Guo, B.; Ye, C.N.; Su, W.Y.; Fan, Q.P.; Zhang, M.J. A narrow-bandgap donor polymer for highly efficient as-cast non-fullerene polymer solar cells with a high open circuit voltage. Organ. Electron. 2018, 58, 82–87. [Google Scholar] [CrossRef]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Yang, Y.; Zhang, Z.; Yan, J.Y.; Lin, Z.H.; Guo, X.Y. Bifacial quasi-solid-state dye-sensitized solar cells with poly (vinyl pyrrolidone)/polyaniline transparent counter electrode. Nano Energy 2016, 26, 123–130. [Google Scholar] [CrossRef]
- Lee, C.P.; Lin, C.A.; Wei, T.C.; Tsai, M.L.; Meng, Y.; Li, C.T.; Huo, K.C.; Wu, C.I.; Lau, S.P.; He, J.H. Economical low-light photovoltaics by using the Pt-free dye-sensitized solar cell with graphene dot/PEDOT:PSS counter electrodes. Nano Energy 2015, 18, 109–117. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Lee, T.H.; Lee, Y.W.; Do, K.; Ko, J.; Im, S.S. Camphorsulfonic acid-doped polyaniline transparent counter electrode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 22743–22748. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Han, L.Y.; Islam, A.; Chen, H.; Malapaka, C.; Chiranjeevi, B.; Zhang, S.F.; Yang, X.D.; Yanagida, M. High-efficiency dye-sensitized solar cell with a novel co-adsorbent. Energy Environ. Sci. 2012, 5, 6057–6060. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Fan, L.Q.; Luo, G.G. Electrolytes in dye-sensitized solar cells. Chem. Rev. 2015, 115, 2136–2173. [Google Scholar] [CrossRef]
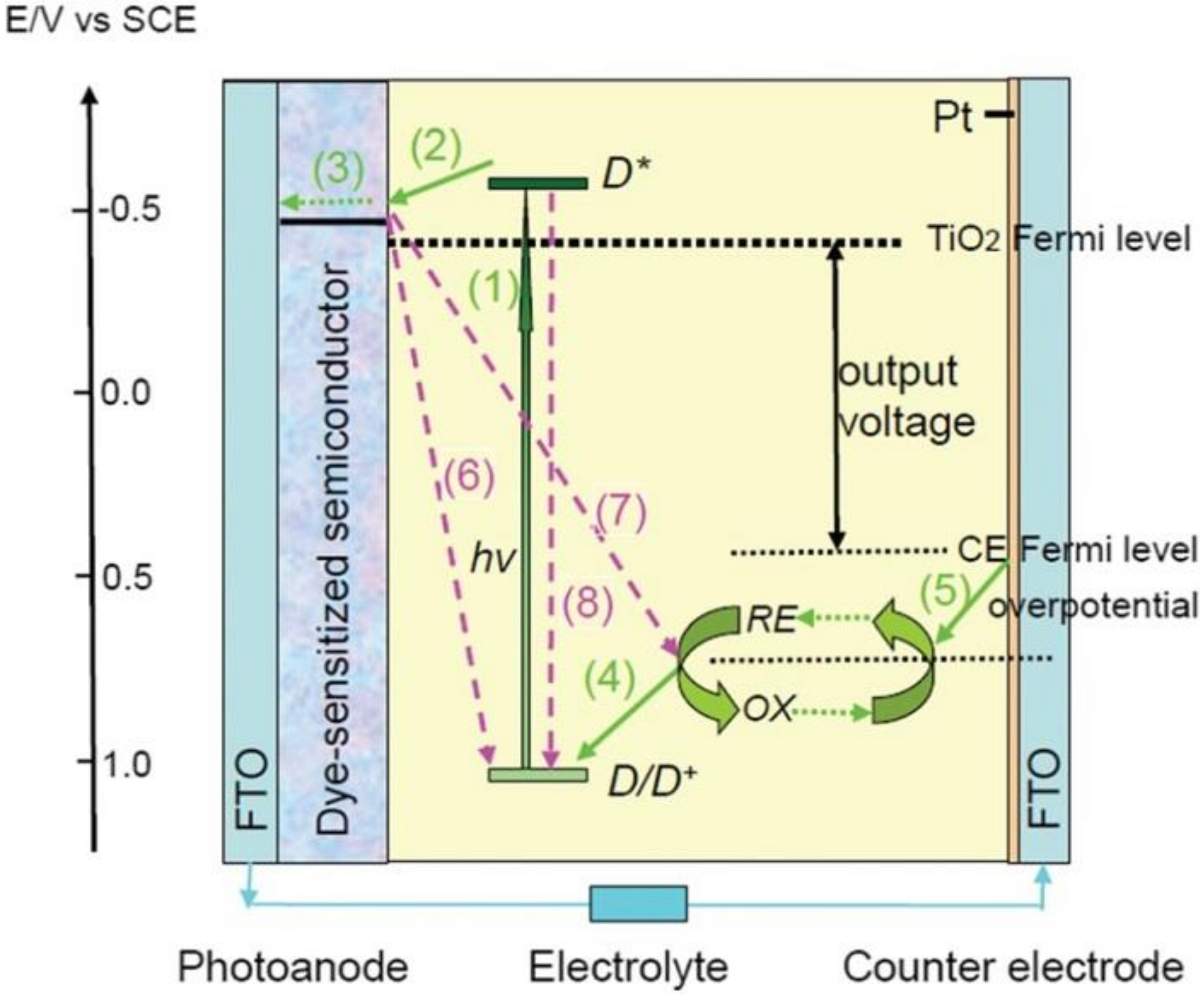
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.C.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Fan, L.Q.; Luo, G.G.; Lin, Y.; Xie, Y.M.; Wei, Y.L. Counter electrodes in dye-sensitized solar cells. Chem. Soc. Rev. 2017, 46, 5975–6023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitag, M.; Boschloo, G. The revival of dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 111–119. [Google Scholar] [CrossRef]
- Zardetto, V.; Brown, T.M.; Reale, A.; Carlo, A.D. Substrates for flexible electronics: A practical investigation on the electrical, film flexibility, optical, temperature, and solvent resistance properties. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 638–648. [Google Scholar] [CrossRef]
- Agarkar, S.A.; Dhas, V.V.; Muduli, S.; Ogale, S.B. Dye sensitized solar cell (DSSC) by a novel fully room temperature process: A solar paint for smart windows and flexible substrates. RSC Adv. 2012, 2, 11645–11649. [Google Scholar] [CrossRef]
- Qin, Q.; Zhang, R. A novel conical structure of polyaniline nanotubes synthesized on ITO-PET conducting substrate by electrochemical method. Electrochim. Acta 2013, 89, 726–731. [Google Scholar] [CrossRef]
- Miettunen, K.; Halme, J.; Lund, P. Metallic and plastic dye solar cells. Wires Energy Environ. 2012, 2, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Zardetto, V.; Giacomo, F.D.; Garcia-Alonso, D.; Keuning, W.; Creatore, M.; Mazzuca, C.; Reale, A.; Carlo, A.D.; Brown, T.M. Fully plastic dye solar cell devices by low-temperature UV-irradiation of both the mesoporous TiO2 photo- and platinized counter-electrodes. Adv. Energy Mater. 2013, 3, 1292–1298. [Google Scholar] [CrossRef]
- Yun, S.N.; Freitas, J.N.; Nogueira, A.F.; Wang, Y.M.; Ahmad, S.; Wang, Z.S. Dye-sensitized solar cells employing polymers. Prog. Polym. Sci. 2016, 59, 1–40. [Google Scholar] [CrossRef]
- Wu, W.Q.; Xu, Y.F.; Rao, H.S.; Feng, H.L.; Su, C.Y.; Kuang, D.B. Constructing 3D branched nanowire coated macroporous metal oxide electrodes with homogeneous or heterogeneous compositions for efficient solar cells. Angew. Chem. Int. Ed. 2014, 53, 4816–4821. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.Q. Dye-sensitized TiO2 nanotube solar cells with markedly enhanced performance via rational surface engineering. Chem. Mater. 2010, 22, 579–584. [Google Scholar] [CrossRef]
- Jang, Y.H.; Xin, X.K.; Byun, M.; Jang, Y.J.; Lin, Z.Q.; Kim, D.H. An unconventional route to high-efficiency dye-sensitized solar cells via embedding graphitic thin films into TiO2 nanoparticle photoanode. Nano Lett. 2012, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.G.; Kang, Y.S.; Sun, Y.K. Anatase TiO2 spheres with high surface area and mesoporous structure via a hydrothermal process for dye-sensitized solar cells. Electrochim. Acta 2010, 55, 4637–4641. [Google Scholar] [CrossRef]
- Low, F.W.; Lai, C.W. Recent developments of graphene-TiO2 composite nanomaterials as efficient photoelectrodes in dye-sensitized solar cells: A review. Renew. Sustain. Energ. Rev. 2018, 82, 103–125. [Google Scholar] [CrossRef]
- Benkstein, K.D.; Kopidakis, N.; Lagemaat, J.; Frank, A.J. Influence of the percolation network geometry on electron transport in dye-sensitized titanium dioxide solar cells. J. Phys. Chem. B 2003, 107, 7759–7767. [Google Scholar] [CrossRef]
- Hou, W.J.; Xiao, Y.M.; Han, G.Y.; Zhou, H.H.; Chang, Y.Z.; Zhang, Y. Preparation of mesoporous titanium dioxide anode by a film- and pore-forming agent for the dye-sensitized solar cell. Mater. Res. Bull. 2016, 76, 140–146. [Google Scholar] [CrossRef]
- Yun, T.K.; Park, S.S.; Kim, D.; Hwang, Y.K.; Huh, S.; Bae, J.Y.; Won, Y.S. Pore-size effect on photovoltaic performance of dye-sensitized solar cells composed of mesoporous anatase-titania. J. Power Sources 2011, 196, 3678–3682. [Google Scholar] [CrossRef]
- Pandikumar, A.; Lim, S.P.; Jayabal, S.; Huang, N.M.; Lim, H.N.; Ramaraj, R. Titania@gold plasmonic nanoarchitectures: An ideal photoanode for dye-sensitized solar cells. Renew. Sustain. Energ. Rev. 2016, 60, 408–420. [Google Scholar] [CrossRef]
- Menon, H.; Gopakumar, G.; Nair, V.S.; Nair, S.V.; Shanmugam, M. 2D-layered MoS2-incorporated TiO2-nanofiber-based dye-sensitized solar cells. Chemistryselect 2018, 3, 5801–5807. [Google Scholar] [CrossRef]
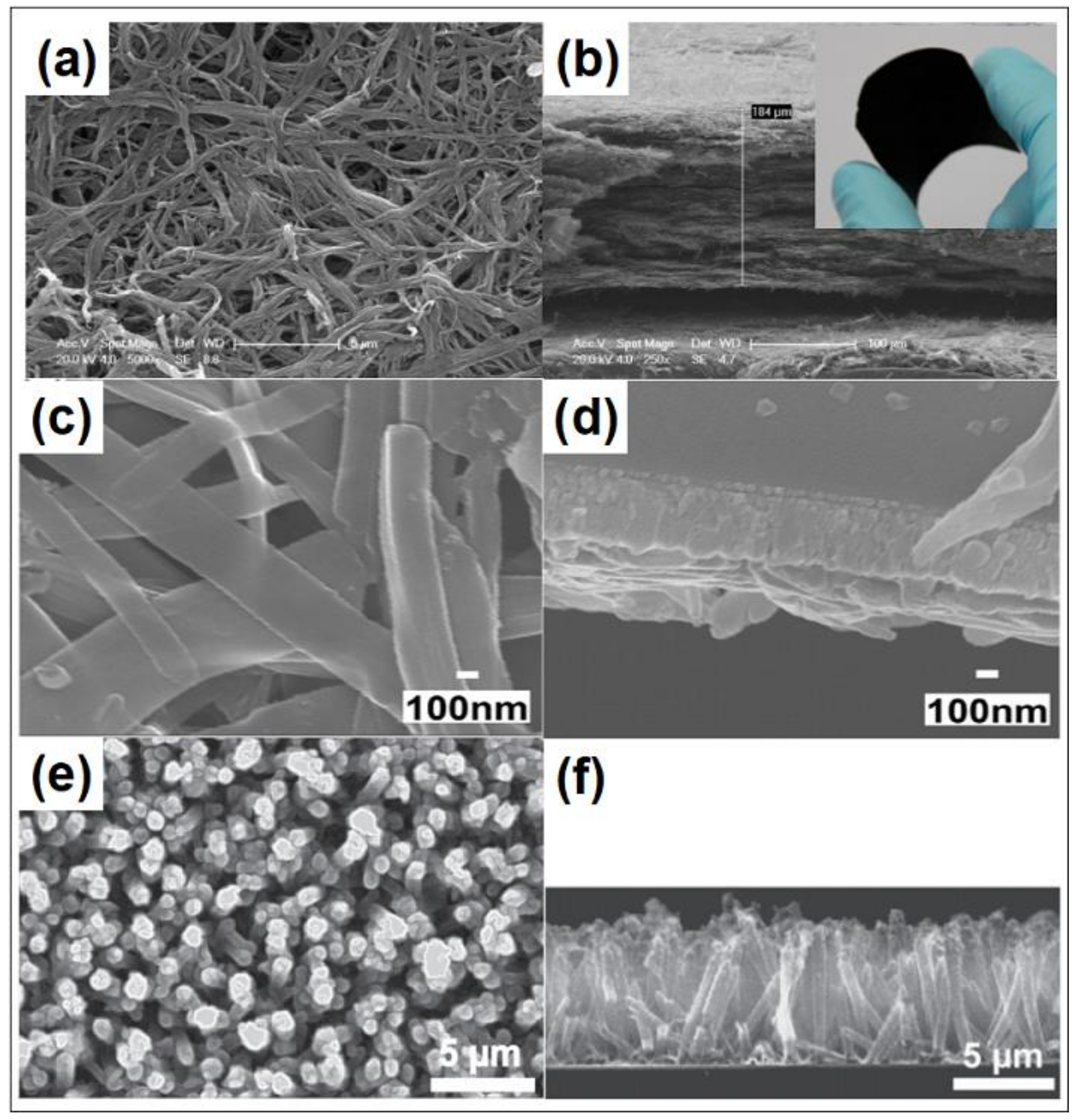
- Kokubo, H.; Ding, B.; Naka, T.; Tsuchihira, H.; Shiratori, S. Multi-core cable-like TiO2 nanofibrous membranes for dye-sensitized solar cells. Nanotechnology 2007, 18, 165604. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.F.; Davoux, D.; Zhu, Z.T.; Galipeau, D.; Fong, H. Composite of TiO2 nanofibers and nanoparticles for dye-sensitized solar cells with significantly improved efficiency. Energy Environ. Sci. 2010, 3, 1507–1510. [Google Scholar] [CrossRef]
- He, G.F.; Wang, X.X.; Xi, M.; Zheng, F.; Zhu, Z.T.; Fong, H. Fabrication and evaluation of dye-sensitized solar cells with photoanodes based on electrospun TiO2 nanotubes. Mater. Lett. 2013, 106, 115–118. [Google Scholar] [CrossRef]
- Briscoe, J.; Dunn, S. The future of using earth-abundant elements in counter electrodes for dye-sensitized solar cells. Adv. Mater. 2016, 28, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.J.; Xiao, Y.M.; Han, G.Y. An interconnected ternary MIn2S4 (M=Fe, Co, Ni) thiospinel nanosheet array: A type of efficient platinum-free counter electrode for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2017, 56, 9146–9150. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Wang, D.Y.; Zhang, W.M.; Li, X.W.; Zhao, X.H.; Zhang, Q.H.; Gu, L.; Yu, Z.; Wu, M.X. Electrospinning synthesis of high performance carbon nanofiber coated flower-like MoS2 nanosheets for dye-sensitized solar cells counter electrode. Electrochim. Acta 2018, 280, 94–100. [Google Scholar] [CrossRef]
- Jing, H.Y.; Shi, Y.T.; Wu, D.Y.; Liang, S.X.; Song, X.D.; An, Y.L.; Hao, C. Well-defined heteroatom-rich porous carbon electrocatalyst derived from biowaste for high-performance counter electrode in dye-sensitized solar cells. Electrochim. Acta 2018, 281, 646–653. [Google Scholar] [CrossRef]
- Zheng, H.Q.; Neo, C.Y.; Mei, X.G.; Qiu, J.; Ouyang, J.Y. Reduced graphene oxide films fabricated by gel coating and their application as platinum-free counter electrodes of highly efficient iodide/triiodide dye-sensitized solar cells. J. Mater. Chem. 2012, 22, 14465–14474. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Han, G.Y.; Li, Y.P.; Li, M.Y.; Lin, J.Y. Three-dimensional hollow platinum-nickel bimetallic nanoframes for use in dye-sensitized solar cells. J. Power Sources 2015, 278, 149–155. [Google Scholar] [CrossRef]
- Tang, Q.W.; Zhang, H.H.; Meng, Y.Y.; He, B.L.; Yu, L.M. Dissolution engineering of platinum alloy counter electrodes in dye-sensitized solar cells. Angew. Chem. Int. Ed. 2015, 54, 11448–11452. [Google Scholar] [CrossRef]
- Yang, W.; Li, Z.H.; Xu, X.W.; Hou, L.Q.; Tang, Y.S.; Deng, B.J.; Yang, F.; Wang, Y.; Li, Y.F. Atomic N-coordinated cobalt sites within nanomesh graphene as highly efficient electrocatalysts for triiodide reduction in dye-sensitized solar cells. Chem. Eng. J. 2018, 349, 782–790. [Google Scholar] [CrossRef]
- Hou, W.J.; Xiao, Y.M.; Han, G.Y. The dye-sensitized solar cells based on the interconnected ternary cobalt diindium sulfide nanosheet array counter electrode. Mater. Res. Bull. 2018, 107, 204–212. [Google Scholar] [CrossRef]
- Tai, Q.D.; Chen, B.L.; Guo, F.; Xu, S.; Hu, H.; Sebo, B.; Zhao, X.Z. In situ prepared transparent polyaniline electrode and its application in bifacial dye-sensitized solar cells. ACS Nano 2011, 5, 3795–3799. [Google Scholar] [CrossRef]
- Tang, Q.W.; Cai, H.Y.; Yuan, S.S.; Wang, X. Counter electrodes from double-layered polyaniline nanostructures for dye-sensitized solar cell applications. J. Mater. Chem. A 2013, 1, 317–323. [Google Scholar] [CrossRef]
- Jeon, S.S.; Kim, C.; Ko, J.; Im, S.S. Spherical polypyrrole nanoparticles as a highly efficient counter electrode for dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 8146–8151. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Wu, J.H.; Zheng, M.; Tang, Q.W.; Liu, Q.; Lin, J.M.; Wang, J.L. High efficient PANI/Pt nanofiber counter electrode used in dye-sensitized solar cell. RSC Adv. 2012, 2, 4062–4064. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J. Fabrication of graphene/free-standing nanofibrillar PEDOT/P(VDF-HFP) hybrid device for wearable and sensitive electronic skin application. Carbon 2015, 87, 275–281. [Google Scholar] [CrossRef]
- Zhou, L.; Yu, M.J.; Chen, X.L.; Nie, S.H.; Lai, W.Y.; Su, W.M.; Cui, Z.; Huang, W. Screen-Printed poly(3,4-ethylenedioxythiophene): poly(styrenesulfonate) grids as ITO-free anodes for flexible organic light-emitting diodes. Adv. Funct. Mater. 2018, 28, 1705955. [Google Scholar] [CrossRef]
- Lee, K.M.; Chiu, W.H.; Wei, H.Y.; Hu, C.W.; Suryanarayanan, V.; Hsieh, W.F.; Ho, K.C. Effects of mesoscopic poly(3,4-ethylenedioxythiophene) films as counter electrodes for dye-sensitized solar cells. Thin Solid Film. 2010, 518, 1716–1721. [Google Scholar] [CrossRef]
- Chen, P.Y.; Li, C.T.; Lee, C.P.; Vittal, R.; Ho, K.C. PEDOT-decorated nitrogen-doped graphene as the transparent composite film for the counter electrode of a dye-sensitized solar cell. Nano Energy 2015, 12, 374–385. [Google Scholar] [CrossRef]
- Lim, S.P.; Pandikumar, A.; Lim, Y.S.; Huang, N.M.; Lim, H.N. In-situ electrochemically deposited polypyrrole nanoparticles incorporated reduced graphene oxide as an efficient counter electrode for platinum-free dye-sensitized solar cells. Sci. Rep. 2014, 4, 5305. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Q.Y.; Wang, H.; Zhou, G.; Wang, Z.S. Reduced graphene oxide-Ta3N5 composite: A potential cathode for efficient Co(bpy)33+/2+ mediated dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 6342–6349. [Google Scholar] [CrossRef]
- Huo, J.H.; Zheng, M.; Tu, Y.G.; Wu, J.H. High-performance and transparent counter electrodes based on polypyrrole and ferrous sulfide nanoparticles for dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 5680–5685. [Google Scholar] [CrossRef]
- Hwang, D.K.; Song, D.; Jeon, S.S.; Han, T.H.; Kang, Y.S.; Im, S.S. Ultrathin polypyrrole nanosheets doped with HCl as counter electrodes in dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 859–865. [Google Scholar] [CrossRef]
- Veerender, P.; Saxena, V.; Jha, P.; Koiry, S.P.; Gusain, A.; Samanta, S.; Chauhan, A.K.; Aswal, D.K.; Gupta, S.K. Free-standing polypyrrole films as substrate-free and pt-free counterelectrodes for quasi-solid dye-sensitized solar cells. Org. Electron. 2012, 13, 3032–3039. [Google Scholar] [CrossRef]
- Peng, T.; Sun, W.W.; Huang, C.L.; Yu, W.J.; Sebo, B.; Dai, Z.G.; Guo, S.S.; Zhao, X.Z. Self-assembled free-standing polypyrrole nanotube membrane as an efficient FTO- and pt-free counter electrode for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Li, Q.H.; Fan, L.Q.; Lan, Z.; Li, P.J.; Lin, J.M.; Hao, S.C. High-performance polypyrrole nanoparticles counter electrode for dye-sensitized solar cells. J. Power Sources 2008, 181, 172–176. [Google Scholar] [CrossRef]
- Bu, C.H.; Tai, Q.D.; Liu, Y.M.; Guo, S.S.; Zhao, X.Z. A transparent and stable polypyrrole counter electrode for dye-sensitized solar cell. J. Power Sources 2013, 221, 78–83. [Google Scholar] [CrossRef]
- Wang, G.Q.; Dong, W.N.; Yan, C.; Hou, S.; Zhang, W. Facile synthesis of hierarchical nanostructured polypyrrole and its application in the counter electrode of dye-sensitized solar cells. Mater. Lett. 2018, 214, 158–161. [Google Scholar] [CrossRef]
- Xu, Q.; Li, M.X.; Yan, P.; Wei, C.Z.; Fang, L.L.; Wei, W.; Bao, H.F.; Xu, J.; Xu, W.L. Polypyrrole-coated cotton fabrics prepared by electrochemical polymerization as textile counter electrode for dye-sensitized solar cells. Org. Electron. 2016, 29, 107–113. [Google Scholar] [CrossRef]
- Saranya, K.; Rameez, M.; Subramania, A. Developments in conducting polymer based counter electrodes for dye-sensitized solar cells-an overview. Eur. Polym. J. 2015, 66, 207–227. [Google Scholar] [CrossRef]
- Li, Q.H.; Wu, J.H.; Tang, Q.W.; Lan, Z.; Li, P.J.; Lin, J.M.; Fan, L.Q. Application of microporous polyaniline counter electrode for dye-sensitized solar cells. Electrochem. Commun. 2008, 10, 1299–1302. [Google Scholar] [CrossRef]
- Hou, W.J.; Xiao, Y.Y.; Han, G.Y.; Fu, D.Y.; Wu, R.F. Serrated, flexible and ultrathin polyaniline nanoribbons: An efficient counter electrode for the dye-sensitized solar cell. J. Power Sources 2016, 322, 155–162. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Q.Y.; Gong, F.; Li, Y.; Zhou, G.; Wang, Z.S. In situ growth of oriented polyaniline nanowires array for efficient cathode of Co(III)/Co(II) mediated dye-sensitized solar cell. J. Mater. Chem. A 2013, 1, 97–104. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Lin, J.Y.; Wang, W.Y.; Tai, S.Y.; Yue, G.T.; Wu, J.H. Enhanced performance of low-cost dye-sensitized solar cells with pulse-electropolymerized polyaniline counter electrodes. Electrochim. Acta 2013, 90, 468–474. [Google Scholar] [CrossRef]
- Burschka, J.; Brault, V.; Ahmad, S.; Breau, L.; Nazeeruddin, M.K.; Marsan, B.; Zakeeruddin, S.M.; Grätzel, M. Influence of the counter electrode on the photovoltaic performance of dye-sensitized solar cells using a disulfide/thiolate redox electrolyte. Energy Environ. Sci. 2012, 5, 6089–6097. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.P.; Ye, B.X.; Hu, X.D.; Ma, X.Y.; Zhang, X.P.; Deng, Y.Q. Facile electropolymerized-PANI as counter electrode for low cost dye-sensitized solar cell. Electrochem. Commun. 2009, 11, 1768–1771. [Google Scholar] [CrossRef]
- Yun, S.N.; Hagfeldt, A.; Ma, T.L. Pt-free counter electrode for dye-sensitized solar cells with high efficiency. Adv. Mater. 2014, 26, 6210–6237. [Google Scholar] [CrossRef]
- Wei, W.; Wang, H.; Hu, Y.H. A review on PEDOT-based counter electrodes for dye-sensitized solar cells. Int. J. Energy Res. 2014, 38, 1099–1111. [Google Scholar] [CrossRef]
- Worfolk, B.J.; Andrews, S.C.; Park, S.; Reinspach, J.; Liu, N.; Toney, M.F.; Mannsfeld, S.C.B.; Bao, Z.N. Ultrahigh electrical conductivity in solution-sheared polymeric transparent films. Proc. Natl. Acad. Sci. USA 2015, 112, 14138–14143. [Google Scholar] [CrossRef]
- Lee, K.M.; Chen, P.Y.; Hsu, C.Y.; Huang, J.H.; Ho, W.H.; Chen, H.C.; Ho, K.C. A high-performance counter electrode based on poly(3,4-alkylenedioxythiophene) for dye-sensitized solar cells. J. Power Sources 2009, 188, 313–318. [Google Scholar] [CrossRef]
- Li, H.G.; Xiao, Y.M.; Han, G.Y.; Hou, W.J. Honeycomb-like poly(3,4-ethylenedioxythiophene) as an effective and transparent counter electrode in bifacial dye-sensitized solar cells. J. Power Sources 2017, 342, 709–716. [Google Scholar] [CrossRef]
- Trevisan, R.; Döbbelin, M.; Boix, P.P.; Barea, E.M.; Tena-Zaera, R.; Mora-Seró, I.; Bisquert, J. PEDOT nanotube arrays as high performing counter electrodes for dye sensitized solar cells. Study of the interactions among electrolytes and counter electrodes. Adv. Energy Mater. 2011, 1, 781–784. [Google Scholar] [CrossRef]
- Xia, J.B.; Masaki, N.; Jiang, K.J.; Yanagida, S. The influence of doping ions on poly(3,4-ethylenedioxythiophene) as a counter electrode of a dye-sensitized solar cell. J. Mater. Chem. 2007, 17, 2845–2850. [Google Scholar] [CrossRef]
- Li, R.; Tang, Q.W.; Yu, L.M.; Yan, X.F.; Zhang, Z.M.; Yang, P.Z. Counter electrodes from conducting polymer intercalated graphene for dye-sensitized solar cells. J. Power Sources 2016, 309, 231–237. [Google Scholar] [CrossRef]
- He, B.L.; Tang, Q.W.; Wang, M.; Ma, C.Q.; Yuan, S.S. Complexation of polyaniline and graphene for efficient counter electrodes in dye-sensitized solar cells: Enhanced charge transfer ability. J. Power Sources 2014, 256, 8–13. [Google Scholar] [CrossRef]
- Zhang, H.H.; He, B.L.; Tang, Q.W.; Yu, L.M. Bifacial dye-sensitized solar cells from covalent-bonded polyanilineemultiwalled carbon nanotube complex counter electrodes. J. Power Sources 2015, 275, 489–497. [Google Scholar] [CrossRef]
- He, B.L.; Tang, Q.W.; Luo, J.H.; Li, Q.H.; Chen, X.X.; Cai, H.Y. Rapid charge-transfer in polypyrrole-single wall carbon nanotube complex counter electrodes: Improved photovoltaic performances of dye-sensitized solar cells. J. Power Sources 2014, 256, 170–177. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Lin, J.Y.; Wu, J.H.; Tai, S.Y.; Yue, G.T.; Lin, T.W. Dye-sensitized solar cells with high-performance polyaniline/multi-wall carbon nanotube counter electrodes electropolymerized by a pulse potentiostatic technique. J. Power Sources 2013, 233, 320–325. [Google Scholar] [CrossRef]
- Li, H.G.; Xiao, Y.M.; Han, G.Y.; Zhang, Y. A transparent honeycomb-like poly(3,4-ethylenedioxythiophene)/multi-wall carbon nanotube counter electrode for bifacial dyesensitized solar cells. Org. Electron. 2017, 50, 161–169. [Google Scholar] [CrossRef]
- Hou, W.J.; Xiao, Y.M.; Han, G.Y.; Zhou, H.H. Electro-polymerization of polypyrrole/multi-wall carbon nanotube counter electrodes for use in platinum-free dye-sensitized solar cells. Electrochim. Acta 2016, 190, 720–728. [Google Scholar] [CrossRef]
- Xu, H.X.; Zhang, X.Y.; Zhang, C.J.; Liu, Z.H.; Zhou, X.H.; Pang, S.P.; Chen, X.; Dong, S.M.; Zhang, Z.Y.; Zhang, L.X.; et al. Nanostructured titanium nitride/PEDOT:PSS composite films as counter electrodes of dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2012, 4, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Di, Y.; Xiao, Z.H.; Liu, Z.T.; Chen, B.; Feng, J.W. Hybrid films of PEDOT containing transition metal phosphates as high effective Pt-free counter electrodes for dye sensitized solar cells. Org. Electron. 2018, 57, 171–177. [Google Scholar] [CrossRef]
- Ramasamy, M.S.; Nikolakapoulou, A.; Raptis, D.; Dracopoulos, V.; Paterakis, G.; Lianos, P. Reduced graphene oxide/Polypyrrole/PEDOT composite films as efficient Pt-free counter electrode for dye-sensitized solar cells. Electrochim. Acta 2015, 173, 276–281. [Google Scholar] [CrossRef]
- Yue, G.T.; Wu, J.H.; Xiao, Y.M.; Lin, J.M.; Huang, M.L.; Lan, Z. Application of poly(3,4-ethylenedioxythiophene):polystyrenesulfonate/polypyrrole counter electrode for dye-sensitized solar cells. J. Phys. Chem. C 2012, 116, 18057–18063. [Google Scholar] [CrossRef]
- Goncalves, L.M.; Bermudez, V.D.Z.; Ribeiro, H.A.; Mendes, A.M. Dye-sensitized solar cells: A safe bet for the future. Energy Environ. Sci. 2008, 1, 655–667. [Google Scholar] [CrossRef]
- Grätzel, M. Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef]
- Li, B.; Guo, E.Y.; Wang, C.X.; Yin, L.W. Novel Au inlaid Zn2SnO4/SnO2 hollow rounded cubes for dye-sensitized solar cells with enhanced photoelectric conversion performance. J. Mater. Chem. A 2016, 4, 466–477. [Google Scholar] [CrossRef]
- Yu, Z.; Vlachopoulos, N.; Gorlov, M.; Kloo, L. Liquid electrolytes for dye-sensitized solar cells. Dalton Trans. 2011, 40, 10289–10303. [Google Scholar] [CrossRef]
- Hamann, T.W.; Ondersma, J.W. Dye-sensitized solar cell redox shuttles. Energy Environ. Sci. 2011, 4, 370–381. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Wang, P.; Wenger, B.; Humphry-Baker, R.; Moser, J.E.; Teuscher, J.; Kantlehner, W.; Mezger, J.; Stoyanov, E.V.; Zakeeruddin, S.M.; Grätzel, M. Charge separation and efficient light energy conversion in sensitized mesoscopic solar cells based on binary ionic liquids. J. Am. Chem. Soc. 2005, 127, 6850–6856. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Lan, Z.; Hao, S.C.; Li, P.J.; Lin, J.M.; Huang, M.L.; Fang, L.Q.; Huang, Y.F. Progress on the electrolytes for dye-sensitized solar cells. Pure Appl. Chem. 2008, 80, 2241–2258. [Google Scholar] [CrossRef]
- Chalkias, D.A.; Giannopoulos, D.I.; Kollia, E.; Petala, A.; Kostopoulos, V.; Papanicolaou, G.C. Preparation of polyvinylpyrrolidone-based polymer electrolytes and their application by in-situ gelation in dye-sensitized solar cells. Electrochim. Acta 2018, 271, 632–640. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Nazeeruddin, M.K.; Sekiguchi, T.; Grätzel, M. A stable quasi-solid-state dye-sensitized solar cell with an amphiphilic ruthenium sensitizer and polymer gel electrolyte. Nat. Mater. 2003, 2, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, A.F.; Longo, C.; Paoli, M.A.D. Polymers in dye sensitized solar cells: Overview and perspectives. Coord. Chem. Rev. 2004, 248, 1455–1468. [Google Scholar] [CrossRef]
- Nogueira, A.F.; Paoli, M.A.D. Electron transfer dynamics in dye sensitized nanocrystalline solar cells using a polymer electrolyte. J. Phys. Chem. B 2001, 105, 7517–7524. [Google Scholar] [CrossRef]
- Cao, F.; Oskam, G.; Searson, P.C. A solid state, dye sensitized photoelectrochemical cell. J. Phys. Chem. 1995, 99, 17071–17073. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Wang, D.B.; Hao, S.C.; Lin, J.M.; Huang, Y.F.; Yin, S.; Sato, T. Gel polymer electrolyte based on poly(acrylonitrile-co-styrene) and a novel organic iodide salt for quasi-solid state dye-sensitized solar cell. Electrochim. Acta 2006, 51, 4243–4249. [Google Scholar] [CrossRef]
- Wu, J.H.; Hao, S.C.; Lan, Z.; Lin, J.M.; Huang, M.L.; Huang, Y.F.; Fang, L.Q.; Yin, S.; Sato, T. A thermoplastic gel electrolyte for stable quasi-solid-state dye-sensitized solar cells. Adv. Funct. Mater. 2007, 17, 2645–2652. [Google Scholar] [CrossRef]
- Shi, Y.T.; Zhan, C.; Wang, L.D.; Ma, B.B.; Gao, R.; Zhu, Y.F.; Qiu, Y. The electrically conductive function of high-molecular weight poly(ethylene oxide) in polymer gel electrolytes used for dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2009, 11, 4230–4235. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Jun, Y.; Park, J.H. Controlled dissolution of polystyrene nanobeads: Transition from liquid electrolyte to gel electrolyte. Nano Lett. 2012, 12, 2233–2237. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Wu, J.H.; Wang, D.B.; Hao, S.C.; Lin, J.M.; Huang, Y.F. Quasi-solid-state dye-sensitized solar cells based on a sol-gel organic-inorganic composite electrolyte containing an organic iodide salt. Sol. Energy 2007, 81, 117–122. [Google Scholar] [CrossRef]
- Su, J.Y.; Tsai, C.H.; Wang, S.A.; Huang, T.W.; Wu, C.C.; Wong, K.T. Functionalizing organic dye with cross-linked electrolyte-blocking shell as a new strategy for improving DSSC efficiency. RSC Adv. 2012, 2, 3722–3728. [Google Scholar] [CrossRef]
- Lan, Z.; Wu, J.H.; Hao, S.C.; Lin, J.M.; Huang, M.L.; Huang, Y.F. Template-free synthesis of closed-microporous hybrid and its application in quasi-solid-state dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 524–528. [Google Scholar] [CrossRef]
- Parvez, M.K.; In, I.; Park, J.M.; Lee, S.H.; Kim, S.R. Long-term stable dye-sensitized solar cells based on UV photo-crosslinkable poly(ethylene glycol) and poly(ethylene glycol) diacrylate based electrolytes. Sol. Energy Mater. Sol. Cells 2011, 95, 318–322. [Google Scholar] [CrossRef]
- Wang, L.; Fang, S.B.; Lin, Y.; Zhou, X.W.; Li, M.Y. A 7.72% efficient dye sensitized solar cell based on novel necklace-like polymer gel electrolyte containing latent chemically cross-linked gel electrolyte precursors. Chem. Commun. 2005, 45, 5687–5689. [Google Scholar] [CrossRef]
- Wu, J.H.; Lan, Z.; Lin, J.M.; Huang, M.L.; Hao, S.C.; Sato, T.; Yin, S. A novel thermosetting gel electrolyte for stable quasi-solid-state dye-sensitized solar cells. Adv. Mater. 2007, 19, 4006–4011. [Google Scholar] [CrossRef]
- Stergiopoulos, T.; Arabatzis, I.M.; Katsaros, G.; Falaras, P. Binary polyethylene oxide/titania solid-state redox electrolyte for highly efficient nanocrystalline TiO2 photoelectrochemical cells. Nano Lett. 2002, 2, 1259–1261. [Google Scholar] [CrossRef]
- Katsaros, G.; Stergiopoulos, T.; Arabatzis, I.M.; Papadokostaki, K.G.; Falaras, P. A solvent-free composite polymer/inorganic oxide electrolyte for high efficiency solid-state dye-sensitized solar cells. J. Photochem. Photobiol. A Chem. 2002, 149, 191–198. [Google Scholar] [CrossRef]
- Yoon, I.N.; Song, H.K.; Won, J.; Kang, Y.S. Shape dependence of SiO2 nanomaterials in a quasi-solid electrolyte for application in dye-sensitized solar cells. J. Phys. Chem. C 2014, 118, 3918–3924. [Google Scholar] [CrossRef]
- Huo, Z.P.; Dai, S.Y.; Wang, K.J.; Kong, F.T.; Zhang, C.N.; Pan, X.; Fang, X.Q. Nanocomposite gel electrolyte with large enhanced charge transport properties of an I3−/I− redox couple for quasi-solid-state dye-sensitized solar cells. Sol. Energy Mater. Sol. Cells 2007, 91, 1959–1965. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Q.W.; He, B.L.; Yang, P.Z. Graphene enabled all-weather solar cells for electricity harvest from sun and rain. J. Mater. Chem. A 2016, 4, 13235–13241. [Google Scholar] [CrossRef]
- Tang, Q.W.; Zhang, H.N.; He, B.L.; Yang, P.Z. An all-weather solar cell that can harvest energy from sunlight and rain. Nano Energy 2016, 30, 818–824. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Zhang, Y.; Sun, W.Y.; Wang, M.; He, B.L.; Chen, H.Y.; Tang, Q.W. Biomass converted carbon quantum dots for all-weather solar cells. Electrochim. Acta 2017, 257, 259–266. [Google Scholar] [CrossRef]
- Zhu, W.L.; Wang, M.; Wang, Z.L.; Sun, W.Y.; He, B.L.; Tang, Q.W. Photoelectric engineering of all-weather bifacial solar cells in the dark. Electrochim. Acta 2017, 254, 299–307. [Google Scholar] [CrossRef]
- Duan, J.L.; Duan, Y.Y.; Zhao, Y.Y.; Wang, Y.L.; Tang, Q.W.; He, B.L. Bifunctional polyaniline electrode tailored hybridized solar cells for energy harvesting from sun and rain. J. Energy Chem. 2018, 27, 742–747. [Google Scholar] [CrossRef]
- Ye, F.; Chen, H.; Xie, F.X.; Tang, W.T.; Yin, M.S.; He, J.J.; Bi, E.B.; Wang, Y.B.; Yang, X.D.; Han, L.Y. Soft-cover deposition of scaling-up uniform perovskite thin films for high cost-performance solar cells. Energy Environ. Sci. 2016, 9, 2295–2301. [Google Scholar] [CrossRef]
- Yang, W.S.; Park, B.W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide management in formamidinium-lead-halide- based perovskite layers for efficient solar cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Zhang, T.Y.; Li, G.; Xu, F.; Wang, Y.P.; Guo, N.J.; Qian, X.F.; Zhao, Y.X. In situ gas/solid reaction for the formation of luminescent quantum confined CH3NH3PbBr3 perovskite planar film. Chem. Commun. 2016, 52, 11080–11083. [Google Scholar] [CrossRef]
- Huang, J.B.; Tan, S.Q.; Lund, P.D.; Zhou, H.P. Impact of H2O on organic-inorganic hybrid perovskite solar cells. Energy Environ. Sci. 2017, 10, 2284–2311. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photon. 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-transport materials for perovskite solar cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef] [PubMed]
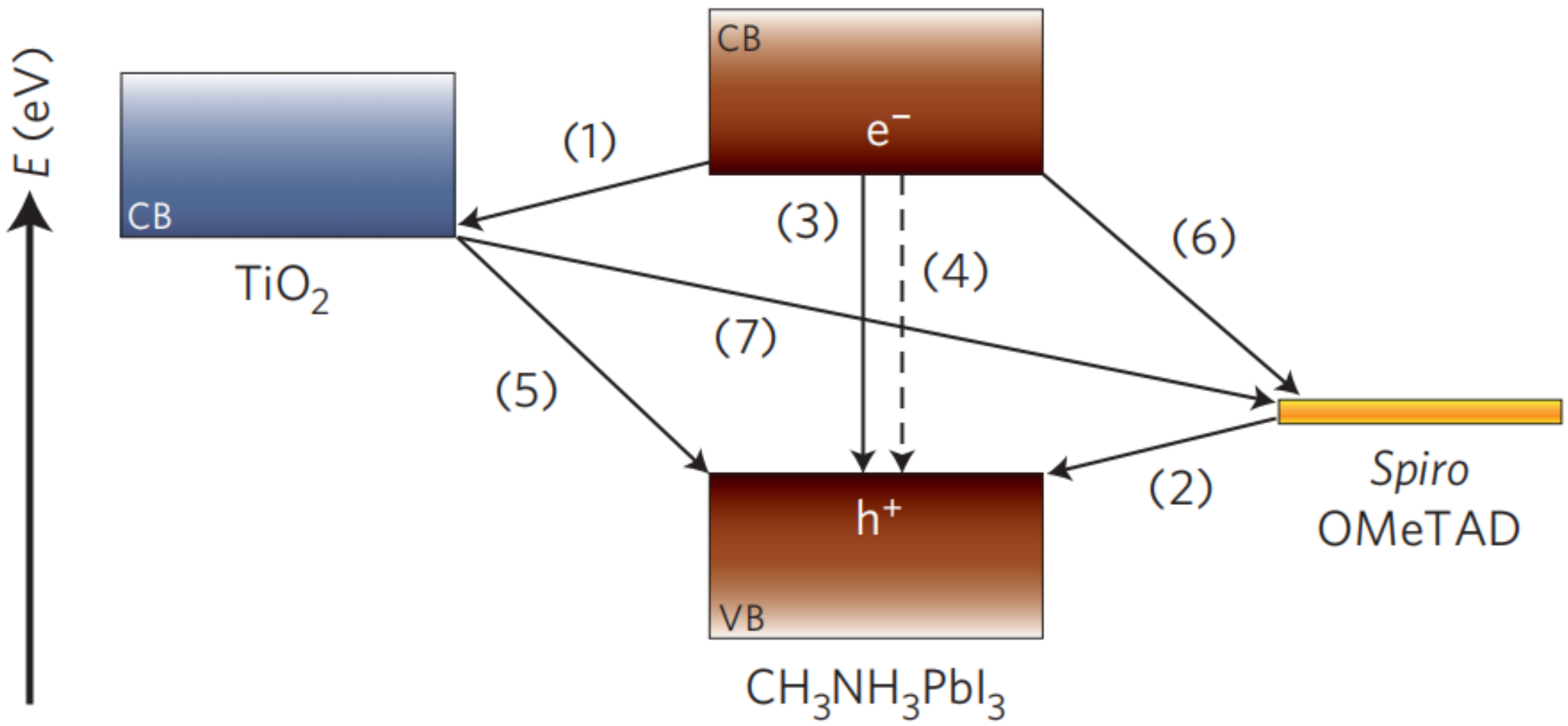
- Marchioro, A.; Teuscher, J.; Friedrich, D.; Kunst, M.; Krol, R.V.D.; Moehl, T.; Grätzel, M.; Moser, J.E. Unravelling the mechanism of photoinduced charge transfer processes in lead iodide perovskite solar cells. Nat. Photonics 2014, 8, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Sum, T.C.; Mathews, N. Advancements in perovskite solar cells: Photophysics behind the photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef]
- Shi, S.W.; Li, Y.F.; Li, X.Y.; Wang, H.Q. Advancements in all-solid-state hybrid solar cells based on organometal halide perovskites. Mater. Horiz. 2015, 2, 378–405. [Google Scholar] [CrossRef]
- Shen, Q.; Ogomi, Y.; Chang, J.; Toyoda, T.; Fujiwara, K.; Yoshino, K.; Sato, K.; Yamazaki, K.; Akimoto, M.; Kuga, Y.; et al. Optical absorption, charge separation and recombination dynamics in Sn/Pb cocktail perovskite solar cells and their relationships to photovoltaic performances. J. Mater. Chem. A 2015, 3, 9308–9316. [Google Scholar] [CrossRef]
- Eperon, G.E.; Burlakov, V.M.; Docampo, P.; Goriely, A.; Snaith, H.J. Morphological control for high performance, solution-processed planar heterojunction perovskite solar cells. Adv. Funct. Mater. 2014, 24, 151–157. [Google Scholar] [CrossRef]
- Salim, T.; Sun, S.Y.; Abe, Y.; Krishna, A.; Grimsdale, A.C.; Lam, Y.M. Perovskite-based solar cells: Impact of morphology and device architecture on device performance. J. Mater. Chem. A 2015, 3, 8943–8969. [Google Scholar] [CrossRef]
- Nie, W.Y.; Tsai, H.; Asadpour, R.; Blancon, J.C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.D.; Huang, F.Z.; Huang, W.C.; Dkhissi, Y.; Zhu, Y.; Etheridge, J.; Gray-Weale, A.; Bach, U.; Cheng, Y.B.; Spiccia, L. A fast deposition-crystallization procedure for highly efficient lead iodide perovskite thin-film solar cells. Angew. Chem. Int. Ed. 2014, 53, 9898–9903. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.W.; Liao, C.Y.; Chueh, C.C.; Zuo, F.; Williams, S.T.; Xin, X.K.; Lin, J.J.; Jen, A.K.Y. Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells. Adv. Mater. 2014, 26, 3748–3754. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.X.; Buin, A.; Ip, A.H.; Li, W.; Voznyy, O.; Comin, R.; Yuan, M.J.; Jeon, S.; Ning, Z.J.; McDowell, J.J.; et al. Perovskite-fullerene hybrid materials suppress hysteresis in planar diodes. Nat. Cmmun. 2015, 6, 7081. [Google Scholar] [CrossRef] [PubMed]
- Saliba, M.; Matsui, T.; Domanski, K.; Seo, J.Y.; Ummadisingu, A.; Zakeeruddin, S.M.; Correa-Baena, J.P.; Tress, W.R.; Abate, A.; Hagfeldt, A.; et al. Incorporation of rubidium cations into perovskite solar cells improves photovoltaic performance. Science 2016, 354, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Jahandar, M.; Heo, J.H.; Song, C.E.; Kong, K.J.; Shin, W.S.; Lee, J.C.; Im, S.H.; Moon, S.J. Highly efficient metal halide substituted CH3NH3I(PbI2)1-X(CuBr2)X planar perovskite solar cells. Nano Energy 2016, 27, 330–339. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Zhu, K. Efficient planar perovskite solar cells based on 1.8 eV band gap CH3NH3PbI2Br nanosheets via thermal decomposition. J. Am. Chem. Soc. 2014, 136, 12241–12244. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.C.; Luo, D.Y.; Wu, J.; Hu, Q.; Zhang, W.; Chen, K.; Liu, T.H.; Liu, Y.; Zhang, Y.F.; Liu, F.; et al. High-performance inverted planar heterojunction perovskite solar cells based on lead acetate precursor with efficiency exceeding 18%. Adv. Funct. Mater. 2016, 26, 3508–3514. [Google Scholar] [CrossRef]
- Li, S.S.; Chang, C.H.; Wang, Y.C.; Lin, C.W.; Wang, D.Y.; Lin, J.C.; Chen, C.C.; Sheu, H.S.; Chia, H.C.; Wu, W.R.; et al. Intermixing-seeded growth for high-performance planar heterojunction perovskite solar cells assisted by precursor-capped nanoparticles. Energy Environ. Sci. 2016, 9, 1282–1289. [Google Scholar] [CrossRef]
- Tripathi, N.; Shirai, Y.; Yanagida, M.; Karen, A.; Miyano, K. Novel surface passivation technique for low-temperature solution-processed perovskite PV cells. ACS Appl. Mater. Interfaces 2016, 8, 4644–4650. [Google Scholar] [CrossRef]
- Li, T.T.; Pan, Y.F.; Wang, Z.; Xia, Y.D.; Chen, Y.H.; Huang, W. Additive engineering for highly efficient organic-inorganic halide perovskite solar cells: Recent advances and perspectives. J. Mater. Chem. A 2017, 5, 12602–12652. [Google Scholar] [CrossRef]
- Zuo, C.T.; Ding, L.M. An 80.11% FF record achieved for perovskite solar cells by using the NH4Cl additive. Nanoscale 2014, 6, 9935–9938. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.G.; Wang, D.; Dong, Q.F.; Wang, Q.; Wei, W.; Dai, J.; Zeng, X.C.; Huang, J.S. Unraveling the hidden function of a stabilizer in a precursor in improving hybrid perovskite film morphology for high efficiency solar cells. Energy Environ. Sci. 2016, 9, 867–872. [Google Scholar] [CrossRef]
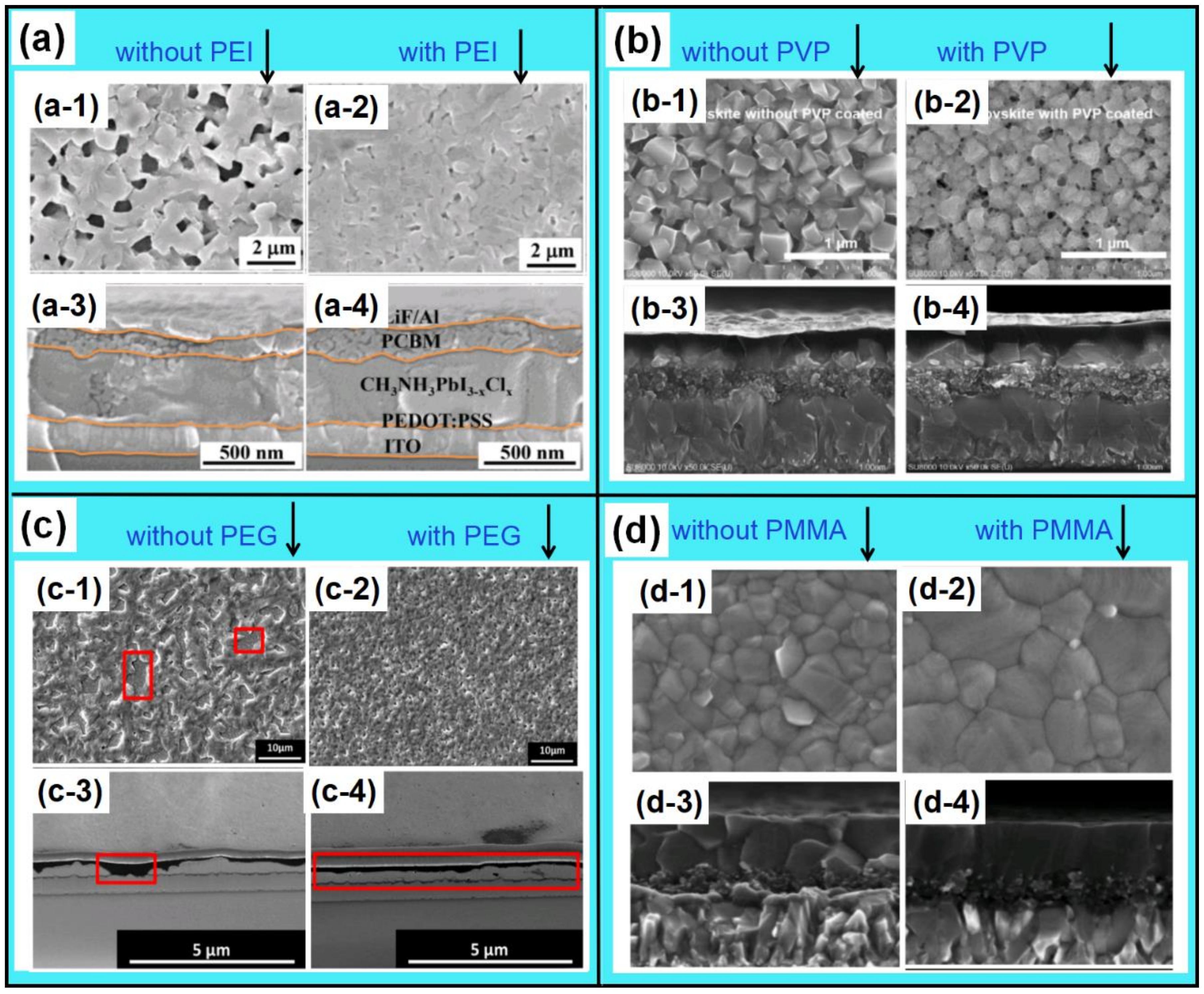
- Zuo, L.J.; Guo, H.X.; DeQuilettes, D.W.; Jariwala, S.; Marco, N.D.; Dong, S.Q.; DeBlock, R.; Ginger, D.S.; Dunn, B.; Wang, M.K.; et al. Polymer-modified halide perovskite films for efficient and stable planar heterojunction solar cells. Sci. Adv. 2017, 3, e1700106. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.Q.; Wang, Z.W.; Zhang, K.C.; Yu, H.; Huang, P.; Liu, X.D.; Zhou, Y.; Chen, N.; Song, B. Easily accessible polymer additives for tuning the crystal-growth of perovskite thin-films for highly efficient solar cells. Nanoscale 2016, 8, 5552–5558. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, B.; Kulkarni, A.; Jena, A.K.; Ikegami, M.; Udagawa, Y.; Kunugita, H.; Ema, K.; Miyasaka, T. Poly(4-Vinylpyridine)-based interfacial passivation to enhance voltage and moisture stability of lead halide perovskite solar cells. ChemSusChem 2017, 10, 2473–2479. [Google Scholar] [CrossRef] [PubMed]
- Masi, S.; Rizzo, A.; Aiello, F.; Balzano, F.; Uccello-Barretta, G.; Listorti, A.; Giglia, G.; Colella, S. Multiscale morphology design of hybrid halide perovskites through a polymeric template. Nanoscale 2015, 7, 18956–18963. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.F.; Hu, Z.C.; Sun, C.; Chen, Z.M.; Huang, F.; Yip, H.L.; Cao, Y. Metallohalide perovskite-polymer composite film for hybrid planar heterojunction solar cells. RSC Adv. 2015, 5, 775–783. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Luo, J.M.; Nie, R.M.; Deng, X.Y. Planar perovskite solar cells using CH3NH3PbI3 films: A simple process suitable for large-scale production. Energy Technol. 2016, 4, 473–478. [Google Scholar] [CrossRef]
- Chang, C.Y.; Chu, C.Y.; Huang, Y.C.; Huang, C.W.; Chang, S.Y.; Chen, C.A.; Chao, C.Y.; Su, W.F. Tuning perovskite morphology by polymer additive for high efficiency solar cell. ACS Appl. Mater. Interfaces 2015, 7, 4955–4961. [Google Scholar] [CrossRef]
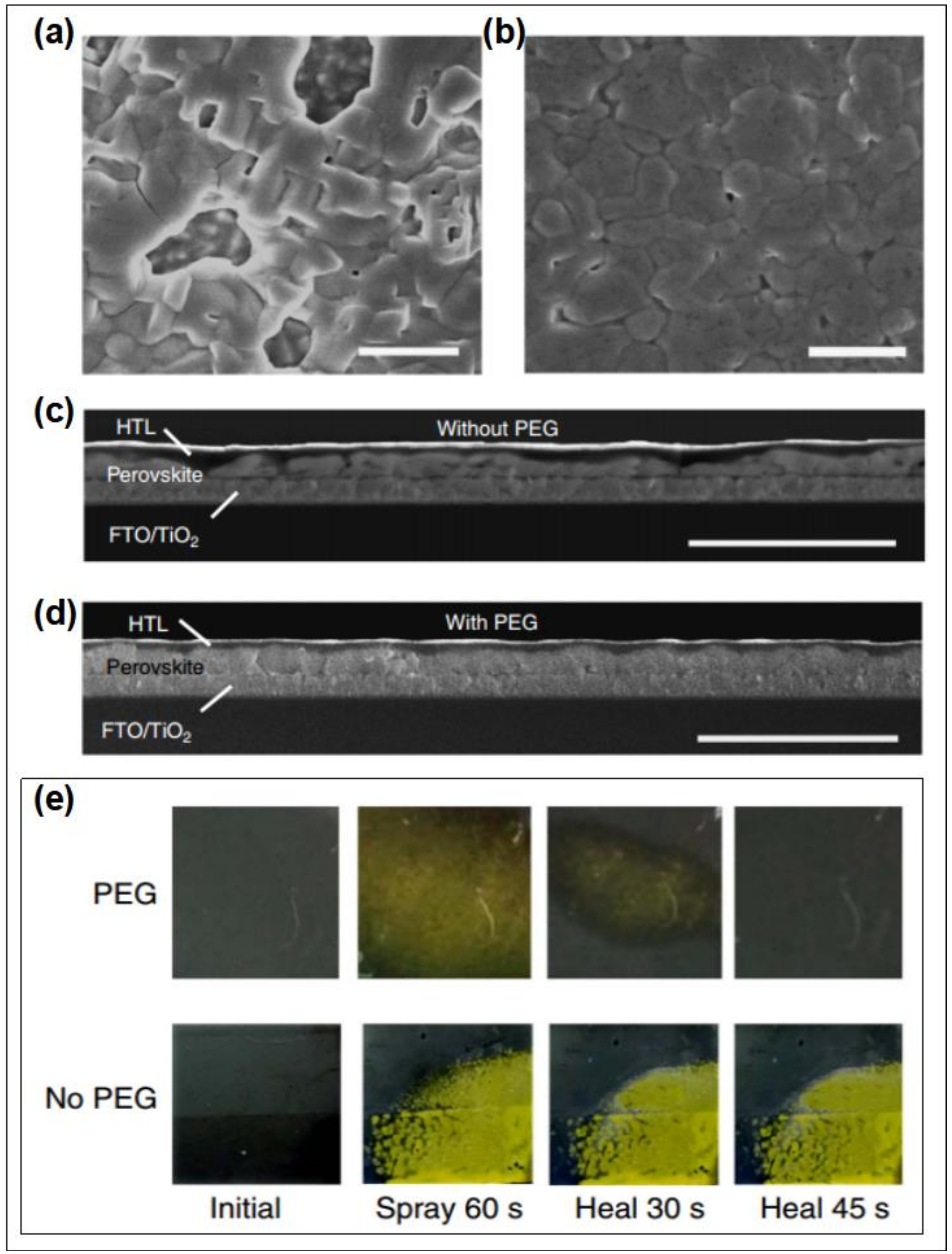
- Zhao, Y.C.; Wei, J.; Li, H.; Yan, Y.; Zhou, W.K.; Yu, D.P.; Zhao, Q. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016, 7, 10228. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.L.; Shoyama, K.; Sato, W.; Nakamura, E. Polymer stabilization of lead(II) perovskite cubic nanocrystals for semitransparent solar cells. Adv. Energy Mater. 2016, 6, 1502317. [Google Scholar] [CrossRef]
- Bi, D.Q.; Yi, C.Y.; Luo, J.S.; Décoppet, J.D.; Zhang, F.; Zakeeruddin, S.M.; Li, X.; Hagfeldt, A.; Grätzel, M. Polymer-templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 2016, 1, 16142. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.J.; Shimazaki, A.; Yang, F.J.; Kanahashi, K.; Matsuki, K.; Miyauchi, Y.; Takenobu, T.; Wakamiya, A.; Murata, Y.; Matsuda, K. Highly efficient and stable perovskite solar cells by interfacial engineering using solution-processed polymer layer. J. Phys. Chem. C 2017, 121, 1562–1568. [Google Scholar] [CrossRef]
- Ji, X.X.; Peng, X.F.; Wang, Q.; Ren, J.; Xiong, Z.H.; Yang, X.H. On the performance of polymer:organometal halide perovskite composite light emitting devices: The effects of polymer additives. Org. Electron. 2018, 52, 350–355. [Google Scholar] [CrossRef]
- Piatkowski, P.; Cohen, B.; Ramos, F.J.; Nunzio, M.D.; Nazeeruddin, M.K.; Grätzel, M.; Ahmad, S.; Douhal, A. Direct monitoring of ultrafast electron and hole dynamics in perovskite solar cells. Phys. Chem. Chem. Phys. 2015, 17, 14674–14684. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Sun, L.C. Recent progress on hole-transporting materials for emerging organometal halide perovskite solar cells. Adv. Energy Mater. 2015, 5, 1500213. [Google Scholar] [CrossRef]
- Leijtens, T.; Lim, J.; Teuscher, J.; Park, T.; Snaith, H.J. Charge density dependent mobility of organic hole-transporters and mesoporous TiO2 determined by transient mobility spectroscopy: Implications to dye-sensitized and organic solar cells. Adv. Mater. 2013, 25, 3227–3233. [Google Scholar] [CrossRef]
- Cappel, U.B.; Daeneke, T.; Bach, U. Oxygen-induced doping of Spiro-MeOTAD in solid-state dye-sensitized solar cells and its impact on device performance. Nano Lett. 2012, 12, 4925–4931. [Google Scholar] [CrossRef]
- Nguyen, W.H.; Bailie, C.D.; Unger, E.L.; McGehee, M.D. Enhancing the hole-conductivity of Spiro-OMeTAD without oxygen or lithium salts by using Spiro(TFSI)2 in perovskite and dye-sensitized solar cells. J. Am. Chem. Soc. 2014, 136, 10996–11001. [Google Scholar] [CrossRef]
- Lee, I.; Yun, J.H.; Son, H.J.; Kim, T.S. Accelerated degradation due to weakened adhesion from Li-TFSI additives in perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 7029–7035. [Google Scholar] [CrossRef]
- Abate, A.; Leijtens, T.; Pathak, S.; Teuscher, J.; Avolio, R.; Errico, M.E.; Kirkpatrik, J.; Ball, J.M.; Docampo, P.; McPhersonc, I.; et al. Lithium salts as “redox active” p-type dopants for organic semiconductors and their impact in solid-state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2013, 15, 2572–2579. [Google Scholar] [CrossRef] [PubMed]
- Bakr, Z.H.; Wali, Q.; Fakharuddin, A.; Schmidt-Mendec, L.; Brown, T.M.; Jose, R. Advances in hole transport materials engineering for stable and efficient perovskite solar cells. Nano Energy 2017, 34, 271–305. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Wu, Y.Z.; Yue, Y.F.; Liu, J.; Zhang, W.J.; Yang, X.D.; Chen, H.; Bi, E.B.; Ashraful, I.; Grätzel, M.; et al. Efficient and stable large-area perovskite solar cells with inorganic charge extraction layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christians, J.A.; Fung, R.C.M.; Kamat, P.V. An inorganic hole conductor for organo-lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 2014, 136, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Tanaka, S.; Ito, S.; Tetreault, N.; Manabe, K.; Nishino, H.; Nazeeruddin, M.K.; Grätzel, M. Inorganic hole conductor-based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 2014, 5, 3834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.C.; Wang, H.X.; Cai, B.; Yu, Z.; Sun, L.C. Progress in hole-transporting materials for perovskite solar cells. J. Energy Chem. 2018, 27, 650–672. [Google Scholar] [CrossRef]
- Heo, J.H.; Im, S.H.; Noh, J.H.; Mandal, T.N.; Lim, C.S.; Chang, J.A.; Lee, Y.H.; Kim, H.J.; Sarkar, A.; Nazeeruddin, M.K.; et al. Efficient inorganic-organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 2013, 7, 486–491. [Google Scholar] [CrossRef]
- Yang, W.S.; Noh, J.H.; Jeon, N.J.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 2015, 348, 1234–1237. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Tetreault, N.; Dar, M.I.; Gao, P.; McCall, K.L.; Rutter, S.R.; Ogier, S.D.; Forrest, N.D.; Bissett, J.S.; Simms, M.J.; et al. A novel oligomer as a hole transporting material for efficient perovskite solar cells. Adv. Energy Mater. 2014, 5, 1400980. [Google Scholar] [CrossRef]
- Sin, D.H.; Ko, H.; Jo, S.B.; Kim, M.; Bae, G.Y.; Cho, K. Decoupling charge transfer and transport at polymeric hole transport layer in perovskite solar cells. ACS Appl. Mater. Interfaces 2016, 8, 6546–6553. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.B.; Ye, S.Y.; Li, Y.L.; Sun, W.H.; Rao, H.X.; Liu, Z.W.; Bian, Z.Q.; Huang, C.H. Hole-transporting materials in inverted planar perovskite solar cells. Adv. Energy Mater. 2016, 6, 1600474. [Google Scholar] [CrossRef]
- Chen, K.; Wu, P.; Yang, W.Q.; Su, R.; Luo, D.Y.; Yang, X.Y.; Tu, Y.G.; Zhu, R.; Gong, Q.H. Low-dimensional perovskite interlayer for highly efficient lead-free formamidinium tin iodide perovskite solar cells. Nano Energy 2018, 49, 411–418. [Google Scholar] [CrossRef]
- Bai, L.B.; Wang, Z.; Han, Y.M.; Zuo, Z.Y.; Liu, B.; Yu, M.N.; Zhang, H.J.; Lin, J.Y.; Xia, Y.D.; Yin, C.R.; et al. Diarylfluorene-based nano-molecules as dopant-free hole-transporting materials without post-treatment process for flexible p-i-n type perovskite solar cells. Nano Energy 2018, 46, 241–248. [Google Scholar] [CrossRef]
- Zhang, F.; Song, J.; Zhang, L.X.; Niu, F.F.; Hao, Y.Y.; Zeng, P.J.; Niu, H.B.; Huang, J.S.; Lian, J.R. Film-through large perovskite grains formation via a combination of sequential thermal and solvent treatment. J. Mater. Chem. A 2016, 4, 8554–8561. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.H.; Nazeeruddin, M.K.; Grätzel, M.; Wu, C.G. The synergistic effect of H2O and DMF towards stable and 20% efficiency inverted perovskite solar cells. Energy Environ. Sci. 2017, 10, 808–817. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Xue, Q.F.; Chen, G.T.; Liu, M.Y.; Xiao, J.Y.; Chen, Z.M.; Hu, Z.C.; Jiang, X.F.; Zhang, B.; Huang, F.; Yang, W.; et al. Improving film formation and photovoltage of highly efficient inverted-type perovskite solar cells through the incorporation of new polymeric hole selective layers. Adv. Energy Mater. 2016, 6, 1502021. [Google Scholar] [CrossRef]
- Zhao, D.W.; Sexton, M.; Park, H.Y.; Baure, G.; Nino, J.C.; So, F. High-efficiency solution-processed planar perovskite solar cells with a polymer hole transport layer. Adv. Energy Mater. 2014, 5, 1401855. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, K.F.; Chiu, K.Y.; Tsai, C.L.; Cheng, H.M.; Yeh, S.C.; Wu, W.T.; Chen, W.N.; Chen, C.T.; Chen, S.H.; et al. Improving the efficiency of CH3NH3PbI3 based photovoltaics by tuning the work function of the PEDOT:PSS hole transport layer. Sol. Energy 2015, 122, 892–899. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, W.Y.; Cheng, T.; Zhang, B.; Yao, J.X.; Alsaedi, A.; Hayat, T.; Ding, Y.; Tan, Z.A.; Dai, S.Y. Optical-electrical-chemical engineering of PEDOT:PSS by incorporation of hydrophobic nafion for efficient and stable perovskite solar cells. ACS Appl. Mater. Interfaces 2018, 10, 3902–3911. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.M.; Han, G.Y.; Chang, Y.Z.; Zhou, H.H.; Li, M.Y.; Li, Y.P. An all-solid-state perovskite-sensitized solar cell based on the dual function polyaniline as the sensitizer and p-type hole-transporting material. J. Power Sources 2014, 267, 1–8. [Google Scholar] [CrossRef]
- Xiao, Y.M.; Han, G.Y.; Wu, J.H.; Lin, J.Y. Efficient bifacial perovskite solar cell based on a highly transparent poly(3,4-ethylenedioxythiophene) as the p-type hole-transporting material. J. Power Sources 2016, 306, 171–177. [Google Scholar] [CrossRef]
- Bi, D.Q.; Yang, L.; Boschloo, G.; Hagfeldt, A.; Johansson, E.M.J. Effect of different hole transport materials on recombination in CH3NH3PbI3 perovskite-sensitized mesoscopic solar cells. J. Phys. Chem. Lett. 2013, 4, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Edri, E.; Kirmayer, S.; Cahen, D.; Hodes, G. High open-circuit voltage solar cells based on organic-inorganic lead bromide perovskite. J. Phys. Chem. Lett. 2013, 4, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.Q.; Bao, X.C.; Yu, J.H.; Zhu, D.Q.; Qiu, M.; Yang, R.Q.; Dong, L.F. Compact layer free perovskite solar cells with a high-mobility hole-transporting layer. ACS Appl. Mater. Interfaces 2016, 8, 2652–2657. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.H.; Im, S.H. CH3NH3PbI3/poly-3-hexylthiophen perovskite mesoscopic solar cells: Performance enhancement by Li-assisted hole conduction. Phys. Status Solidi RRL 2014, 8, 816–821. [Google Scholar] [CrossRef]
- Cai, M.L.; Tiong, V.T.; Hreid, T.; Bell, J.; Wang, H.X. An efficient hole transport material composite based on poly(3-hexylthiophene) and bamboostructured carbon nanotubes for high performance perovskite solar cells. J. Mater. Chem. A 2015, 3, 2784–2793. [Google Scholar] [CrossRef]
- Xiao, J.Y.; Shi, J.J.; Liu, H.B.; Xu, Y.Z.; Lv, S.T.; Luo, Y.H.; Li, D.M.; Meng, Q.B.; Li, Y.L. Efficient CH3NH3PbI3 perovskite solar cells based on graphdiyne (GD)-modified P3HT hole-transporting material. Adv. Energy Mater. 2015, 5, 1401943. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon nanotube/polymer composites as a highly stable hole collection layer in perovskite solar cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lim, J.; Yun, H.J.; Kim, Y.H.; Park, T. A diketopyrrolopyrrole-containing hole transporting conjugated polymer for use in efficient stable organic-inorganic hybrid solar cells based on a perovskite. Energy Environ. Sci. 2014, 7, 1454–1460. [Google Scholar] [CrossRef]
- Kim, G.W.; Kang, G.; Kim, J.; Lee, G.Y.; Kim, H.I.; Pyeon, L.; Leeb, J.; Park, T. Dopant-free polymeric hole transport materials for highly efficient and stable perovskite solar cells. Energy Environ. Sci. 2016, 9, 2326–2333. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, D.S.; Ann, M.H.; Im, S.H.; Kim, J.H.; Kwon, O.P. Non-fullerene organic electron-transporting materials for perovskite solar cells. ChemSusChem 2018, 11, 3882–3892. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.H.; Yip, H.L.; Zhang, H.; Jiang, X.F.; Xue, Q.F.; Hu, Z.C.; Hu, Z.H.; Shen, Y.; Wang, M.K.; et al. Amino-functionalized conjugated polymer as an efficient electron transport layer for high-performance planar-heterojunction perovskite solar cells. Adv. Energy Mater. 2016, 6, 1501534. [Google Scholar] [CrossRef]
- Wang, W.W.; Yuan, J.Y.; Shi, G.Z.; Zhu, X.X.; Shi, S.H.; Liu, Z.K.; Han, L.; Wang, H.Q.; Ma, W.L. Inverted planar heterojunction perovskite solar cells employing polymer as the electron conductor. ACS Appl. Mater. Interfaces 2015, 7, 3994–3999. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Kim, M.J.; Choi, K.; Lim, C.; Kim, Y.H.; Kwon, S.K.; Park, T. Improving the performance and stability of inverted planar flexible perovskite solar cells employing a novel NDI-based polymer as the electron transport layer. Adv. Energy Mater. 2018, 1702872. [Google Scholar] [CrossRef]
- Guo, Q.; Xu, Y.X.; Xiao, B.; Zhang, B.; Zhou, E.J.; Wang, F.Z.; Bai, Y.M.; Hayat, T.; Alsaedi, A.; Tan, Z.A. Effect of energy alignment, electron mobility, and film morphology of perylene diimide based polymers as electron transport layer on the performance of perovskite solar cells. ACS Appl. Mater. Interfaces 2017, 9, 10983–10991. [Google Scholar] [CrossRef]
- Malinkiewicz, O.; Yella, A.; Lee, Y.H.; Espallargas, G.M.; Graetzel, M.; Nazeeruddin, M.K.; Bolink, H.J. Perovskite solar cells employing organic charge-transport layers. Nat. Photonics 2014, 8, 128–132. [Google Scholar] [CrossRef]
- Lin, Q.Q.; Armin, A.; Nagiri, R.C.R.; Burn, P.L.; Meredith, P. Electro-optics of perovskite solar cells. Nat. Photonics 2015, 9, 106–112. [Google Scholar] [CrossRef]
- Wen, X.R.; Wu, J.M.; Ye, M.D.; Gao, D.; Lin, C.J. Interface engineering via an insulating polymer for highly efficient and environmentally stable perovskite solar cells. Chem. Commun. 2016, 52, 11355–11358. [Google Scholar] [CrossRef]
- Snaitha, H.J.; Grätzel, M. Enhanced charge mobility in a molecular hole transporter via addition of redox inactive ionic dopant: Implication to dye-sensitized solar cells. Appl. Phys. Lett. 2006, 89, 262114. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, Q.F.; Li, T.; Gruverman, G.; Huang, J.S. Thin insulating tunneling contacts for effcient and water-resistant perovskite solar cells. Adv. Mater. 2016, 28, 6734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Azimi, H.; Hou, Y.; Ameri, T.; Przybilla, T.; Spiecker, E.; Kraft, M.; Scherf, U.; Brabec, C.J. Improved high-efficiency perovskite planar heterojunction solar cells via incorporation of a polyelectrolyte interlayer. Chem. Mater. 2014, 26, 5190–5193. [Google Scholar] [CrossRef]
- Li, G.; Chang, W.H.; Yang, Y. Low-bandgap conjugated polymers enabling solution-processable tandem solar cells. Nat. Rev. Mater. 2017, 2, 17043. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Yu, G.; Heeger, A.J. Charge separation and photovoltaic conversion in polymer composites with internal donor-acceptor heterojunctions. J. Appl. Phys. 1995, 78, 4510–4515. [Google Scholar] [CrossRef]
- Koster, L.J.A.; Smits, E.C.P.; Mihailetchi, V.D.; Blom, P.W.M. Device model for the operation of polymer/fullerene bulk heterojunction solar cells. Phys. Rev. B 2005, 72, 085205. [Google Scholar] [CrossRef]
- Lu, L.Y.; Yu, L.P. Understanding low bandgap polymer PTB7 and optimizing polymer solar cells based on it. Adv. Mater. 2014, 26, 4413–4430. [Google Scholar] [CrossRef]
- He, Z.C.; Zhong, C.M.; Huang, X.; Wong, W.Y.; Wu, H.B.; Chen, L.W.; Su, S.J.; Cao, Y. Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk-heterojunction solar cells-towards 10% energy-conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Li, Y.F. Molecular design of photovoltaic materials for polymer solar cells: Toward suitable electronic energy levels and broad absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Frechet, J.M.J. Molecular design and ordering effects in π-functional materials for transistor and solar cell applications. J. Am. Chem. Soc. 2011, 133, 20009–20029. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.F.; Ye, L.; Zhang, H.; Li, S.S.; Zhang, S.Q.; Hou, J.H. Molecular design of benzodithiophene-based organic photovoltaic materials. Chem. Rev. 2016, 116, 7397–7457. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, L.P. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells-enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Yang, H.; Shin, T.J.; Yang, L.; Cho, K.; Ryu, C.Y.; Bao, Z.N. Effect of mesoscale crystalline structure on the field-effect mobility of regioregular poly(3-hexyl thiophene) in thin-film transistors. Adv. Funct. Mater. 2010, 15, 671–676. [Google Scholar] [CrossRef]
- Ma, W.L.; Yang, C.Y.; Gong, X.; Lee, K.; Heeger, A.J. Thermally stable, efficient polymer solar cells with nanoscale control of the interpenetrating network morphology. Adv. Funct. Mater. 2005, 15, 1617–1622. [Google Scholar] [CrossRef]
- Zhao, G.J.; He, Y.J.; Li, Y.F. 6.5% Efficiency of polymer solar cells based on poly(3-hexylthiophene) and indene-C60 bisadduct by device optimization. Adv. Mater. 2010, 22, 4355–4358. [Google Scholar] [CrossRef]
- Liao, S.H.; Li, Y.L.; Jen, T.H.; Cheng, Y.S.; Chen, S.A. Multiple functionalities of polyfluorene grafted with metal ion-intercalated crown ether as an electron transport layer for bulk-heterojunction polymer solar cells: Optical interference, hole blocking, interfacial dipole, and electron conduction. J. Am. Chem. Soc. 2012, 134, 14271–14274. [Google Scholar] [CrossRef]
- Inganäs, O.; Svensson, M.; Zhang, F.; Gadisa, A.; Persson, N.K.; Wang, X.; Andersson, M.R. Low bandgap alternating polyfluorene copolymers in plastic photodiodes and solar cells. Appl. Phys. A 2004, 79, 31–35. [Google Scholar] [CrossRef]
- Campbell, A.J.; Bradley, D.D.C.; Antoniadis, H. Dispersive electron transport in an electroluminescent polyfluorene copolymer measured by the current integration time-of-flight method. Appl. Phys. Lett. 2001, 79, 2133–2135. [Google Scholar] [CrossRef]
- Svensson, M.; Zhang, F.L.; Veenstra, S.C.; Verhees, W.J.H.; Hummelen, J.C.; Kroon, J.M.; Inganäs, O.; Andersson, M.R. High-performance polymer solar cells of an alternating polyfluorene copolymer and a fullerene derivative. Adv. Mater. 2003, 15, 988–991. [Google Scholar] [CrossRef]
- Grell, M.; Bradley, D.D.C.; Inbasekaran, M.; Woo, E.P. A glass-forming conjugated main-chain liquid crystal polymer for polarized electroluminescence applications. Adv. Mater. 1997, 9, 798–802. [Google Scholar] [CrossRef]
- Russell, D.M.; Arias, A.C.; Friend, R.H.; Silva, C.; Ego, C.; Grimsdale, A.C.; Mullen, K. Efficient light harvesting in a photovoltaic diode composed of a semiconductor conjugated copolymer blend. Appl. Phys. Lett. 2002, 80, 2204–2206. [Google Scholar] [CrossRef]
- Gadisa, A.; Mammo, W.; Andersson, L.M.; Admassie, S.; Zhang, F.L.; Andersson, M.R.; Inganäs, O. A new donor-acceptor-donor polyfluorene copolymer with balanced electron and hole mobility. Adv. Funct. Mater. 2007, 17, 3836–3842. [Google Scholar] [CrossRef]
- Chen, M.H.; Hou, J.; Hong, Z.R.; Yang, G.W.; Sista, S.; Chen, L.M.; Yang, Y. Efficient polymer solar cells with thin active layers based on alternating polyfluorene copolymer/fullerene bulk heterojunctions. Adv. Mater. 2009, 21, 4238–4242. [Google Scholar] [CrossRef]
- Umeyama, T.; Imahori, H. Design and control of organic semiconductors and their nanostructures for polymer-fullerene-based photovoltaic devices. J. Mater. Chem. A 2014, 2, 11545–11560. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Wu, Y.; Feng, D.Q.; Tsai, S.T.; Son, H.J.; Li, G.; Yu, L.P. Development of new semiconducting polymers for high performance solar cells. J. Am. Chem. Soc. 2009, 131, 56–57. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Xu, Z.; Xia, J.B.; Tsai, S.T.; Wu, Y.; Li, G.; Ray, C.; Yu, L.P. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef]
- Chen, H.Y.; Hou, J.H.; Zhang, S.Q.; Liang, Y.Y.; Yang, G.W.; Yang, Y.; Yu, L.P.; Wu, Y.; Li, G. Polymer solar cells with enhanced open-circuit voltage and efficiency. Nat. Photonics 2009, 3, 649–653. [Google Scholar] [CrossRef]
- He, Z.C.; Zhong, C.M.; Su, S.J.; Xu, M.; Wu, H.B.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Xu, Y.X.; Chueh, C.C.; Yip, H.L.; Ding, F.Z.; Li, Y.X.; Li, C.Z.; Li, X.S.; Chen, W.C.; Jen, A.K.Y. Improved charge transport and absorption coefficient in indacenodithieno[3,2-b]thiophene-based ladder-type polymer leading to highly efficient polymer solar cells. Adv. Mater. 2012, 24, 6356–6361. [Google Scholar] [CrossRef]
- Qian, D.P.; Ye, L.; Zhang, M.J.; Liang, Y.R.; Li, L.J.; Huang, Y.; Guo, X.; Zhang, S.Q.; Tan, Z.A.; Hou, J.H. Design, application, and morphology study of a new photovoltaic polymer with strong aggregation in solution state. Macromolecules 2012, 45, 9611–9617. [Google Scholar] [CrossRef]
- Huo, L.J.; Liu, T.; Sun, X.B.; Cai, Y.H.; Heeger, A.J.; Sun, Y.M. Single-junction organic solar cells based on a novel wide-bandgap polymer with efficiency of 9.7%. Adv. Mater. 2015, 27, 2938. [Google Scholar] [CrossRef] [PubMed]
- Winder, C.; Sariciftci, N.S. Low bandgap polymers for photon harvesting in bulk heterojunction solar cells. J. Mater. Chem. 2004, 14, 1077–1086. [Google Scholar] [CrossRef]
- Hou, J.H.; Park, M.H.; Zhang, S.Q.; Yao, Y.; Chen, L.M.; Li, J.H.; Yang, Y. Bandgap and molecular energy level control of conjugated polymer photovoltaic materials based on benzo[1,2-b:4,5-b’]dithiophene. Macromolecules 2008, 41, 6012–6018. [Google Scholar] [CrossRef]
- Hou, J.H.; Chen, H.Y.; Zhang, S.Q.; Chen, R.I.; Yang, Y.; Wu, Y.; Li, G. Synthesis of a low band gap polymer and its application in highly efficient polymer solar cells. J. Am. Chem. Soc. 2009, 131, 15586–15587. [Google Scholar] [CrossRef]
- Zhang, Y.; Hau, S.K.; Yip, H.L.; Sun, Y.; Acton, O.; Jen, A.K.Y. Efficient polymer solar cells based on the copolymers of benzodithiophene and thienopyrroledione. Chem. Mater. 2010, 22, 2696–2698. [Google Scholar] [CrossRef]
- Zhang, M.J.; Fan, H.J.; Guo, X.; He, Y.J.; Zhang, Z.G.; Min, J.; Zhang, J.; Zhao, G.J.; Zhan, X.W.; Li, Y.F. Synthesis and photovoltaic properties of a copolymer of Benzo[1,2-b:4,5-b’]dithiophene and bithiazole. Macromolecules 2010, 43, 8714–8717. [Google Scholar] [CrossRef]
- Tang, C.G.; Ang, M.C.Y.; Choo, K.K.; Keerthi, V.; Tan, J.K.; Syafiqah, M.N.; Kugler, T.; Burroughes, J.H.; Png, R.Q.; Chua, L.L.; et al. Doped polymer semiconductors with ultrahigh and ultralow work functions for ohmic contacts. Nature 2016, 539, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.C.; Qian, D.P.; Zhang, S.Q.; Li, S.S.; Inganäs, O.; Gao, F.; Hou, J.H. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.X.; Zhang, Y.M.; Wan, X.J.; Li, C.X.; Zhang, X.; Wang, Y.B.; Ke, X.; Xiao, Z.; Ding, L.M.; Xia, R.X.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.T.; Gao, J.; Richard, E.; You, J.B.; Chen, C.C.; Cha, K.C.; He, Y.J.; Li, G.; Yang, Y. Systematic investigation of benzodithiophene- and diketopyrrolopyrrole-based low-bandgap polymers designed for single junction and tandem polymer solar cells. J. Am. Chem. Soc. 2012, 134, 10071–10079. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Liu, F.; Russell, T.P.; Jo, W.H. A high mobility conjugated polymer based on dithienothiophene and diketopyrrolopyrrole for organic photovoltaics. Energy Environ. Sci. 2012, 5, 6857–6861. [Google Scholar] [CrossRef]
- Wang, C.F.; Xu, X.F.; Zhang, W.; Dkhil, S.B.; Meng, X.Y.; Liu, X.J.; Margeat, O.; Yartsev, A.; Ma, W.; Ackermann, J.; et al. Ternary organic solar cells with enhanced open circuit voltage. Nano Energy 2017, 37, 24–31. [Google Scholar] [CrossRef]
- Fu, H.T.; Li, C.; Bi, P.Q.; Hao, X.T.; Liu, F.; Li, Y.; Wang, Z.H.; Sun, Y.M. Effcient ternary organic solar cells enabled by the integration of nonfullerene and fullerene acceptors with a broad composition tolerance. Adv. Funct. Mater. 2018, 1807006. [Google Scholar] [CrossRef]
- Xie, Y.P.; Yang, F.; Li, Y.X.; Uddin, M.A.; Bi, P.Q.; Fan, B.B.; Cai, Y.H.; Hao, X.T.; Woo, H.Y.; Li, W.W.; et al. Morphology control enables effcient ternary organic solar cells. Adv. Mater. 2018, 30, 1803045. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.H.; Noh, J.; Han, S.H. Performance and stability of electroluminescent device with self-assembled layers of poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) and polyelectrolytes. Thin Solid Film 2006, 510, 305–310. [Google Scholar] [CrossRef]
- He, Z.C.; Zhang, C.; Xu, X.F.; Zhang, L.J.; Huang, L.; Chen, J.W.; Wu, H.B.; Cao, Y. Largely enhanced efficiency with a PFN/Al bilayer cathode in high efficiency bulk heterojunction photovoltaic cells with a low bandgap polycarbazole donor. Adv. Mater. 2011, 23, 3086–3089. [Google Scholar] [CrossRef]
- Na, S.I.; Oh, S.H.; Kim, S.S.; Kim, D.Y. Efficient organic solar cells with polyfluorene derivatives as a cathode interfacial layer. Org. Electron. 2009, 10, 496–500. [Google Scholar] [CrossRef]
- Zhao, Y.; Xie, Z.Y.; Qin, C.J.; Qu, Y.; Geng, Y.H.; Wang, L.X. Enhanced charge collection in polymer photovoltaic cells by using an ethanol-soluble conjugated polyfluorene as cathode buffer layer. Sol. Energy Mater. Sol. Cells 2009, 93, 604–608. [Google Scholar] [CrossRef]
- Kirchartz, T.; Taretto, K.; Rau, U. Efficiency limits of organic bulk heterojunction solar cells. J. Phys. Chem. C 2009, 113, 17958–17966. [Google Scholar] [CrossRef]
- Mikhnenko, O.V.; Azimi, H.; Scharber, M.; Morana, M.; Blom, P.W.M.; Loi, M.A. Exciton diffusion length in narrow bandgap polymers. Energy Environ. Sci. 2012, 5, 6960–6965. [Google Scholar] [CrossRef]
- Nalwa, K.S.; Park, J.M.; Ho, K.M.; Chaudhary, S. On realizing higher efficiency polymer solar cells using a textured substrate platform. Adv. Mater. 2011, 23, 112–116. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Andersson, V.; Zhang, F.L.; Inganäs, O. Folded reflective tandem polymer solar cell doubles efficiency. Appl. Phys. Lett. 2007, 91, 123514. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Elshobaki, M.; Ye, Z.; Park, J.M.; Noack, M.A.; Ho, K.M.; Chaudhary, S. Microlens array induced light absorption enhancement in polymer solar cells. Phys. Chem. Chem. Phys. 2013, 15, 4297–4302. [Google Scholar] [CrossRef]
- Tvingstedt, K.; Zilio, S.D.; Inganäs, O.; Tormen, M. Trapping light with micro lenses in thin film organic photovoltaic cells. Opt. Express 2008, 16, 21608–21615. [Google Scholar] [CrossRef]
- Peer, A.; Biswas, R. Nanophotonic organic solar cell architecture for advanced light trapping with dual photonic crystals. ACS Photonics 2014, 1, 840–847. [Google Scholar] [CrossRef]
- Peer, A.; Biswas, R.; Park, J.M.; Shinar, R.; Shinar, J. Light management in perovskite solar cells and organic LEDs with microlens arrays. Opt. Express 2017, 25, 10704–10709. [Google Scholar] [CrossRef]
- He, X.M.; Gao, F.; Tu, G.L.; Hasko, D.; Huttner, S.; Steiner, U.; Greenham, N.C.; Friend, R.H.; Huck, W.T.S. Formation of nanopatterned polymer blends in photovoltaic devices. Nano Lett. 2010, 10, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, W.; Sims, L.; Abdellah, A.; Exner, A.; Meier, R.; Musselman, K.P.; MacManus-Driscoll, J.L.; Müller-Buschbaum, P.; Scarpa, G.; Lugli, P.; et al. Nanostructured interfaces in polymer solar cells. Appl. Phys. Lett. 2010, 96, 263109–263111. [Google Scholar] [CrossRef]






| Preparation Methods of PPy Counter Electrode | Electrolyte | JSC (mA cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|---|
| FeCl3 chemical polymerization | I3−/I− | 13.80 | 0.771 | 26.3 | 2.86 | [64] |
| Potentiostatical electrodeposition | I3−/I− | 13.10 | 0.660 | 53.9 | 4.65 | [64] |
| FeCl3 oxidation/Drop-casting | I3−/I− | 15.50 | 0.791 | 67.0 | 8.20 | [65] |
| FeCl3 oxidation | I3−/I− | 13.96 | 0.712 | 64.9 | 6.45 | [73] |
| FeCl3 oxidation/with HCl vapor post-doping | I3−/I− | 11.90 | 0.725 | 63.2 | 5.70 | [74] |
| FeCl3 oxidation/without HCl vapor post-doping | I3−/I− | 15.20 | 0.721 | 61.8 | 6.80 | [74] |
| Liquid-liquid biphasic interfacial polymerization | I3−/I− | 11.31 | 0.49 | 63.0 | 3.50 | [75] |
| FeCl3 oxidation/Heating pulp-like suspensions | I3−/I− | 13.10 | 0.716 | 56.0 | 5.27 | [76] |
| I2 oxidation | I3−/I− | 15.01 | 0.74 | 69.0 | 7.66 | [77] |
| APS oxidation | I3−/I− | 12.19 | 0.725 | 52.0 | 5.74 | [78] |
| V2O5 nanofibers/FeCl3 oxidation | I3−/I− | 15.37 | 0.69 | 64.0 | 6.78 | [79] |
| Preparation Methods of PANI Counter Electrode | Electrolyte | JSC (mA cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|---|
| APS oxidation | I3−/I− | 15.24 | 0.71 | 60.4 | 6.54 | [63] |
| KPS oxidation | I3−/I− | 13.50 | 0.76 | 38.3 | 3.92 | [64] |
| cyclic voltammetry deposition | I3−/I− | 13.40 | 0.728 | 67.6 | 6.58 | [64] |
| APS oxidation | I3−/I− | 14.60 | 0.714 | 69.0 | 7.15 | [82] |
| V2O5 nanofibers oxidation/template etching | I3−/I− | 17.92 | 0.72 | 56.0 | 7.23 | [83] |
| In situ oxidation with fixed content of APS | Co(bpy)33+/2+ | 15.09 | 0.78 | 70.0 | 8.24 | [84] |
| Drop-casting | Co(bpy)33+/2+ | 12.76 | 0.72 | 65.0 | 5.97 | [84] |
| I-t electropolymerization | I3−/I− | 10.88 | 0.69 | 58.0 | 4.35 | [85] |
| Pulse electropolymerization | I3−/I− | 12.18 | 0.71 | 60.0 | 5.19 | [85] |
| Cyclic voltammetry deposition with SO42− doping | I3−/I− | 10.70 | 0.81 | 64.0 | 5.60 | [87] |
| Preparation Methods of PEDOT Counter Electrodes | Electrolyte | JSC (mA cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|---|
| Fe(OTs)3 oxidative polymerization | I3−/I− | 17.00 | 0.72 | 66.0 | 8.08 | [69] |
| Constant current deposition | I3−/I− | 13.96 | 0.7116 | 70.0 | 6.96 | [70] |
| Potentiostatical electrodeposition | Disulfide/thiolate | 15.90 | 0.687 | 72.0 | 7.90 | [86] |
| Electrochemical polymerization | I3−/I− | 8.84 | 0.705 | 63.0 | 3.93 | [91] |
| cyclic voltammetry/ template etching | I3−/I− | 16.75 | 0.75 | 64.0 | 8.05 (front) | [92] |
| cyclic voltammetry/ template etching | I3−/I− | 7.84 | 0.719 | 67.0 | 3.78 (rear) | [92] |
| cyclic voltammetry electrodeposition | I3−/I− | 17.72 | 0.768 | 67.0 | 9.12 (front) | [92] |
| cyclic voltammetry electrodeposition | I3−/I− | 11.23 | 0.731 | 70.0 | 5.75 (rear) | [92] |
| ZnO nanowire template/electropolerization | I3−/I− | 16.24 | 0.72 | 70.0 | 8.30 | [93] |
| Electropolymerization with ClO4 doping | I3−/I− | 9.60 | 0.68 | 66.0 | 4.20 | [94] |
| Electropolymerization with TsO doping | I3−/I− | 9.10 | 0.68 | 67.0 | 4.20 | [94] |
| Electropolymerization with TsO doping | I3−/I− | 9.20 | 0.665 | 66.0 | 4.0 | [94] |
| Counter Electrodes | Preparation Methods | Electrolyte | JSC (mA·cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|---|---|
| PEDOT/N-doped GO | Constant current deposition | I3−/I− | 15.60 | 0.7392 | 72.0 | 8.30 | [70] |
| rGO@PPy | Electrochemical polymerization | I3−/I− | 7.49 | 0.70 | 42.0 | 2.21 | [71] |
| PPy/FeS | FeCl3 oxidation/ Na2S reduction | I3−/I− | 15.87 | 0.711 | 66.3 | 7.48 | [73] |
| PANI-graphene | Cyclic voltametric | I3−/I− | 16.55 | 0.699 | 67.0 | 7.70 | [96] |
| PANI-MWCNT | Cyclic voltametric | I3−/I− | 22.25 | 0.691 | 60.1 | 9.24 (both) | [97] |
| PANI-MWCNT | Cyclic voltametric | I3−/I− | 17.95 | 0.675 | 65.3 | 7.91 (front) | [97] |
| PANI-MWCNT | Cyclic voltametric | I3−/I− | 4.30 | 0.622 | 64.3 | 1.72 (rear) | [97] |
| PPy-SWCNT | FeCl3 oxidation | I3−/I− | 15.68 | 0.742 | 71.0 | 8.30 | [98] |
| PANI-MWCNT | Pulse potentiostatic | I3−/I− | 13.53 | 0.721 | 64.0 | 6.24 | [99] |
| PEDOT/MWCNT | Cyclic voltametric/ PMMA template | I3−/I− | 17.09 | 0.792 | 67.0 | 9.07 (front) | [100] |
| PEDOT/MWCNT | Cyclic voltametric/ PMMA template | I3−/I− | 10.76 | 0.757 | 69.0 | 5.62 (rear) | [100] |
| TiN(P)-PEDOT:PSS | Doctor blade | I3−/I− | 14.45 | 0.727 | 67.18 | 7.06 | [102] |
| TiN(R)-PEDOT:PSS | Doctor blade | I3−/I− | 14.53 | 0.727 | 65.26 | 6.89 | [102] |
| TiN(S)-PEDOT:PSS | Doctor blade | I3−/I− | 14.35 | 0.724 | 59.48 | 6.18 | [102] |
| PEDOT-Ni3(PO4)2 | Spin-coating | I3−/I− | 12.21 | 0.746 | 70.3 | 6.412 | [103] |
| PEDOT-Co3(PO4)2 | Spin-coating | I3−/I− | 12.05 | 0.724 | 69.6 | 6.109 | [103] |
| PEDOT-Ag3(PO4)2 | Spin-coating | I3−/I− | 11.08 | 0.762 | 44.1 | 3.731 | [103] |
| rGO/PPy/PEDOT | APS oxidation/ potentiostatic deposition | I3−/I− | 17.0 | 0.76 | 55.0 | 7.1 | [104] |
| PEDOT:PSS/PPy | Electrochemical polymerization | I3−/I− | 14.27 | 0.75 | 71.0 | 7.60 | [105] |
| Electrolyte System | JSC (mA cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|
| Epichlomer-16, 0.3 g elastomer, 25 mL acetone, 0.03 g NaI | 4.20 | 0.82 | 47 | 1.6 | [118] |
| 1.4 g polyacrylonitrile, 10 g ethylene carbonate, 5 mL propylene carbonate, 5 mL acetonitrile, 1.5 g NaI, 0.1 g I2 | 3.40 | 0.58 | 67.0 | 4.40 | [119] |
| 17.5 wt % Poly(acrylonitrile-co-styrene), 0.5 M N-methyl pyridine iodide, 0.05 M iodine, ethylene carbonate:propylene carbonate | 7.82 | 0.708 | 56.0 | 3.10 | [120] |
| 40 wt % PEG, 60 wt % PC, 0.65M KI, 0.065 M I2, | 14.89 | 0.73 | 66.45 | 7.22 | [121] |
| 10 wt % PEO, 0.1 M LiI, 0.1 M I2, 0.6 M DMPII, 0.45 M NMBI in MePN | 9.13 | 0.76 | 68.1 | 4.72 | [122] |
| 2 wt % PS, 0.6 M butylmethylimidazolium iodide, 0.03 M I2, 0.1 M guanidinium thiocyanate, 0.5 M tBP in acetonitrile/valeronitrile (85:15, V/V) | 15.30 | 0.77 | 64.0 | 7.54 | [123] |
| N-methyl pyridine iodide, iodine, γ-BL, Triton X-100, 0.5 mL glacial acetic acid, 2 mL titanium isopropoxide | 5.63 | 0.635 | 51.4 | 3.06 | [124] |
| PAA-PEG, 0.5 M NaI, 0.05 M I2, 0.4 M pyridine, 30 vol % NMP, 70 vol % GBL | 15.28 | 0.661 | 62.4 | 6.30 | [126] |
| PAA-PEG, 1.0 M NaI, 0.15 M I2, 0.4 M pyridine, 30 vol % NMP, 70 vol % GBL | 11.41 | 0.724 | 63.5 | 5.25 | [126] |
| PEG:LiI/I2 + 15 wt % PEGDA | --- | --- | --- | 4.18 | [127] |
| PPDD, 0.5 M iodide, 0–0.5 M inorganic salt, 0.5 M NMBI | 17.10 | 0.70 | 64.0 | 7.72 | [128] |
| PAA-PEG 20,000, 0.5 M NaI, 0.05M I2, 30 vol % NMP, 70 vol % GBL, 0.4 M PY, DMF | 12.55 | 0.735 | 66.1 | 6.10 | [129] |
| PEO-TiO2(I−/I3−) | 7.20 | 0.664 | 58.0 | 4.20 | [130] |
| PEO/TiO2/I−/I3− | 2.05 | 0.67 | 39.0 | 0.96 | [131] |
| 9 wt % SiO2 nanorod/PMII/I2/PEGDME | 12.0 | 0.621 | 62.8 | 4.68 | [132] |
| 10 wt % P (VDF-HFP), 0.6 M DMPII, 0.1 M LiI, 0.1 M I2, 0.45 M NMBI | 14.746 | 0.621 | 62.5 | 5.72 | [133] |
| Device Structure of PSCs with Polymer HTLs | JSC (mA·cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|
| FTO/mp-TiO2/CH3NH3PbI3/PTAA/Au | 16.40 | 0.90 | 61.4 | 9.0 | [189] |
| FTO/bl-TiO2/mp-TiO2/FAPbI3-based perovskite/PTAA/Au | 24.70 | 1.06 | 77.5 | 20.2 | [190] |
| FTO/TiO2/CH3NH3PbI3/S197/Au | 17.60 | 0.967 | 70.0 | 12.0 | [191] |
| ITO/PEDOT:PSS/FASnI3/C60/BCP/Cu | 24.87 | 0.45 | 0.63 | 7.05 | [194] |
| ITO/PEDOT:PSS/CH3NH3PbI3/PC61BM/Au/LiF | 19.35 | 0.80 | 73.0 | 11.90 | [195] |
| ITO/PEDOT:PSS/CH3NH3PbI3/PCBM/Bphen/Al | 22.53 | 0.983 | 77.3 | 17.16 | [196] |
| FTO/(PEDOT:PSS):Nafion/CH3NH3PbI3/PCBM/Al | 22.17 | 1.02 | 73.47 | 16.68 | [202] |
| FTO/TiO2/CH3NH3PbI3/PANI | 14.48 | 0.78 | 65.0 | 7.34 | [203] |
| FTO/TiO2/CH3NH3PbI3/PPEDOT | 20.08 | 0.89 | 69.0 | 12.33 | [204] |
| FTO/Mp-TiO2/CH3NH3PbI3/P3HT/Au | 12.60 | 0.73 | 73.2 | 6.70 | [189] |
| FTO/TiO2/CH3NH3PbBr3/P3HT/Au | 1.13 | 0.84 | 54.0 | 0.52 | [206] |
| ITO/perovskite/P3HT/MoO3/Ag | 17.90 | 0.87 | 66.0 | 10.80 | [207] |
| ITO/perovskite/P/MoO3/Ag | 13.80 | 0.81 | 52.0 | 5.62 | [207] |
| FTO/bl-TiO2/CH3NH3PbI3/P3HT+Li-TFSI+tBP/Au | 20.10 | 0.92 | 74.0 | 13.70 | [208] |
| FTO/Mp-TiO2/CH3NH3PbI3/P3HT+BCN/Au | 17.75 | 0.83 | 49.0 | 7.60 | [209] |
| FTO/Mp-TiO2/CH3NH3PbI3/P3HT-graphdiyne/Au | 19.63 | 0.939 | 71.5 | 13.17 | [210] |
| FTO/Mp-TiO2/CH3NH3PbI3/P3HT/SWCNT-PMMA/Au | 22.71 | 1.02 | 66.0 | 15.30 | [211] |
| FTO/Mp-TiO2/CH3NH3PbI3/PCPDTBT/Au | 10.30 | 0.77 | 66.7 | 5.30 | [189] |
| FTO/Mp-TiO2/CH3NH3PbI3/PCDTBT/Au | 10.50 | 0.92 | 43.7 | 4.20 | [189] |
| FTO/bl-TiO2/mp-TiO2/CH3NH3PbI3/PDPPDBTE/Au | 14.40 | 0.8553 | 74.9 | 9.20 | [212] |
| FTO/bl-SnO2/CH3NH3PbI3/RCP/Au | 21.9 | 1.08 | 75.0 | 17.30 | [213] |
| Device Structure of OPVs with Polymer Donors | JSC (mA·cm−2) | VOC (V) | FF (%) | η (%) | Ref. |
|---|---|---|---|---|---|
| ITO/PEDOT:PSS/P3HT:ICBA/Ca/Al | 10.61 | 0.84 | 72.7 | 6.48 | [240] |
| ITO/PEDOT:PSS/P3HT:ICBA/PFCn6:K+/Ca/Al | 11.65 | 0.89 | 72.6 | 7.50 | [241] |
| ITO/PEDOT:PSS/DiO/Al | 3.55 | 1.01 | 58.0 | 2.10 | [242] |
| ITO/PEDOT:PSS/APFO-15:PCBM/LiF/Al | 6.00 | 1.00 | 63.0 | 3.70 | [247] |
| ITO/PEDOT:PSS/BisEH-PFDTBT:PC71BM/Ca/Al | 8.40 | 0.95 | 44.0 | 3.50 | [248] |
| ITO/PEDOT:PSS/BisDMO-PFDTBT:PC71BM/Ca/Al | 9.10 | 0.97 | 51.0 | 4.50 | [248] |
| ITO/PEDOT:PSS/PTB7:PC71BM/Ca/Al | 14.50 | 0.74 | 68.97 | 7.40 | [251] |
| ITO/PEDOT:PSS/PBDTTT-E:PC70BM/Ca/Al | 13.20 | 0.62 | 63.0 | 5.15 | [252] |
| ITO/PEDOT:PSS/PBDTTT-C:PC70BM/Ca/Al | 14.70 | 0.70 | 64.1 | 6.58 | [252] |
| ITO/PEDOT:PSS/PBDTTT-CF:PC70BM/Ca/Al | 15.20 | 0.76 | 66.9 | 7.73 | [252] |
| ITO/PEDOT:PSS/PTB7:PC71BM/PFN interlayer/Al | 15.40 | 0.759 | 70.6 | 8.24 | [253] |
| ITO/PFN interlayer/PTB7:PC71BM/MoO3/Al | 17.20 | 0.740 | 72.0 | 9.15 | [253] |
| ITO/PEDOT:PSS/PIDT-DFBT:PC71BM/Ca/Al | 11.20 | 0.97 | 55.0 | 5.97 | [254] |
| ITO/PEDOT:PSS/PIDTT-DFBT:PC71BM/Ca/Al | 12.21 | 0.95 | 61.0 | 7.03 | [254] |
| ITO/PEDOT:PSS/PBDTBDD:PC61BM/Ca/Al | 10.68 | 0.86 | 72.27 | 6.67 | [255] |
| ITO/PEDOT:PSS/PDBT-T1:PC70BM/ZrAcac/Al | 14.11 | 0.92 | 75.0 | 9.74 | [256] |
| ITO/PEDOT:PSS/PBDTTPD:PC71BM/LiF/Al | 9.10 | 0.87 | 53.8 | 4.20 | [260] |
| ITO/PEDOT:PSS/PBDTBTz:PC70BM/Al | 7.84 | 0.86 | 57.0 | 3.82 | [261] |
| ITO/ZnO/PBDB-T:ITIC/MoO3/Al | 16.81 | 0.899 | 74.2 | 11.21 | [263] |
| ITO/ZnO/PFN-Br/PBDB-T:F-M/PEDOT:PSS/ZnO/ PTB7-Th:O6T-4F:PC71BM/MoO3/Ag | 14.35 | 1.642 | 73.7 | 17.36 | [264] |
| ITO/PEDOT:PSS/PBDTT-DPP:PC71BM/Ca/Al | 14.00 | 0.73 | 65.0 | 6.60 | [265] |
| ITO/PEDOT:PSS/PBDTP-DPP:PC71BM/Ca/Al | 13.60 | 0.76 | 60.0 | 6.20 | [265] |
| ITO/PEDOT:PSS/PBDTT-FDPP:PC71BM/Ca/Al | 13.80 | 0.77 | 55.0 | 5.80 | [265] |
| ITO/PEDOT:PSS/PBDTP-FDPP:PC71BM/Ca/Al | 7.42 | 0.77 | 59.0 | 3.30 | [265] |
| ITO/PEDOT:PSS/PDTTDPP:PC71BM/LiF/Al | 13.70 | 0.66 | 66.1 | 6.05 | [265] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The Applications of Polymers in Solar Cells: A Review. Polymers 2019, 11, 143. https://doi.org/10.3390/polym11010143
Hou W, Xiao Y, Han G, Lin J-Y. The Applications of Polymers in Solar Cells: A Review. Polymers. 2019; 11(1):143. https://doi.org/10.3390/polym11010143
Chicago/Turabian StyleHou, Wenjing, Yaoming Xiao, Gaoyi Han, and Jeng-Yu Lin. 2019. "The Applications of Polymers in Solar Cells: A Review" Polymers 11, no. 1: 143. https://doi.org/10.3390/polym11010143
APA StyleHou, W., Xiao, Y., Han, G., & Lin, J.-Y. (2019). The Applications of Polymers in Solar Cells: A Review. Polymers, 11(1), 143. https://doi.org/10.3390/polym11010143






