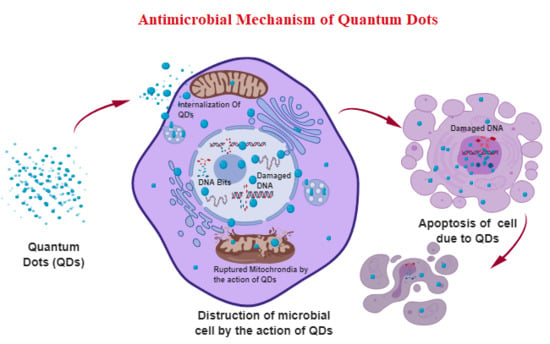
Antimicrobial Activity and Mechanism of Functionalized Quantum Dots
Abstract
1. Introduction
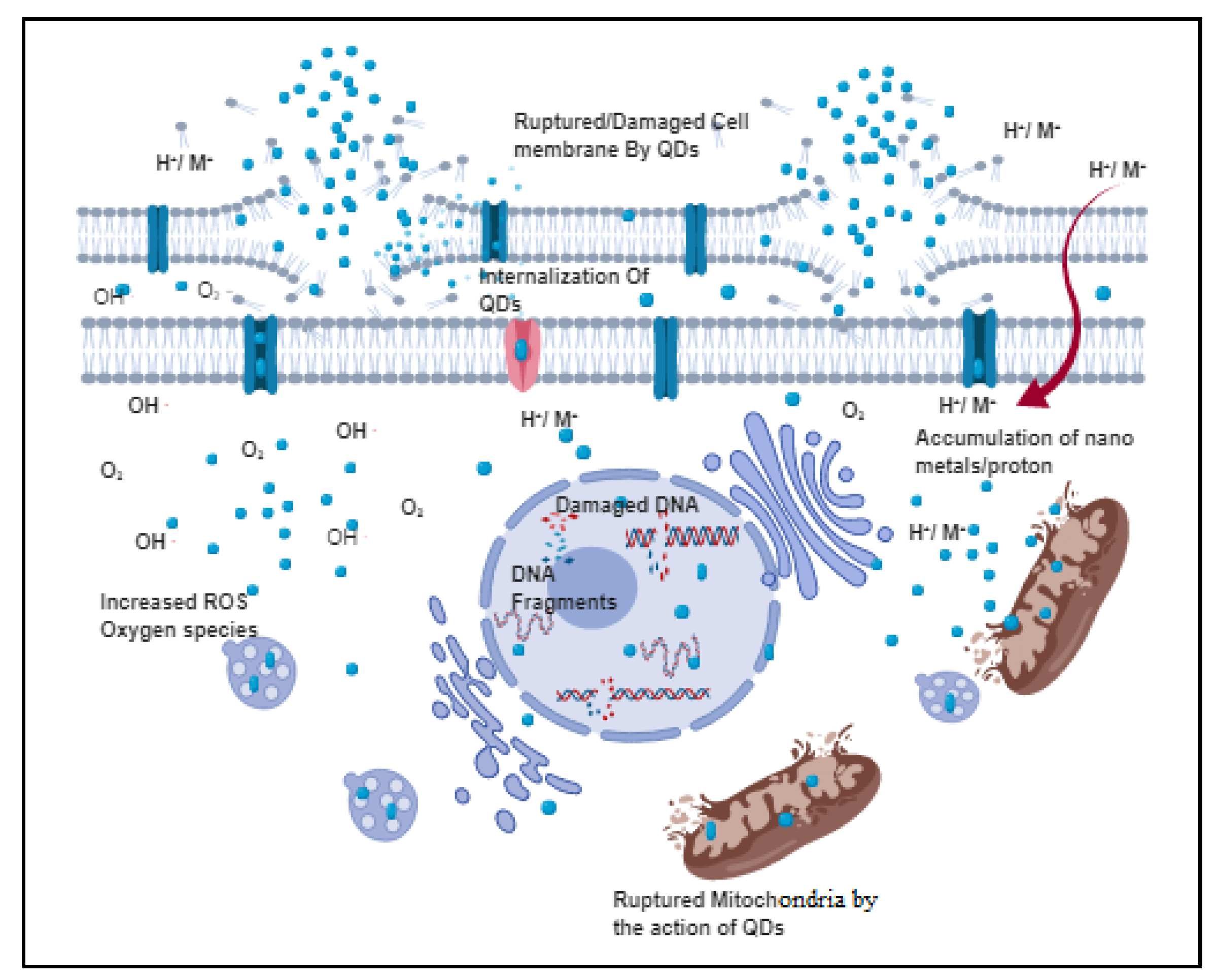
2. The General Antibacterial Mechanism of QDs
2.1. Destruction of Cell Wall/Cell Membrane
2.2. Production of ROS
2.3. QD Interaction with Nucleus Components
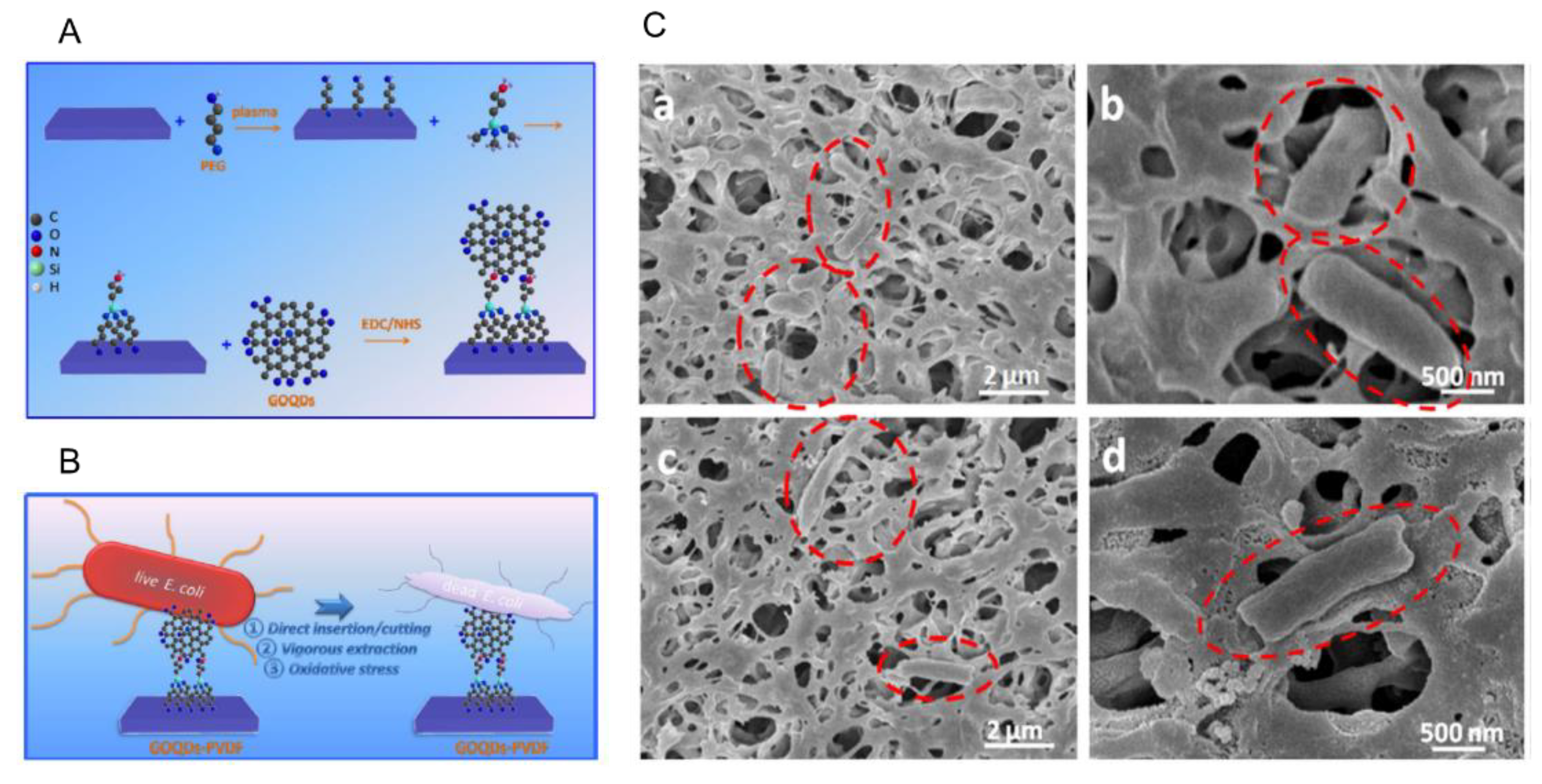
3. Antibacterial Activity of Graphene QDs
3.1. Antibacterial Mechanism of the Functionalized GQDs
3.2. Toxicity of GQDs on Mammalian Cells
4. Functionalized CdTe QDs against Multi-Drug-Resistant Bacteria and Fungi
4.1. Mechanism of the QDs against Multi-Drug-Resistant Bacterial Strains
4.2. Antifungal Mechanism of CdTe QDs
4.3. Toxicity of CdTe QDs on Human Cells
5. Antibacterial Activity of CdSe QDs
6. Antimicrobial Activity of ZnO QDs
6.1. Antibacterial Mechanism of ZnO QDs
6.2. Antifungal Mechanism of the ZnO QDs
7. Others
8. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Sugden, R.; Kelly, R.; Davies, S. Combatting antimicrobial resistance globally. Nature Microbiol. 2016, 1, 16187. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, P.; Feng, Q.; Meng, T.; Wei, J.; Xu, C. Green preparation of fluorescent carbon quantum dots from cyanobacteria for biological imaging. Polymers 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.A.; Gilani, S.J.; Muheem, A.; Jafar, M.; Aslam, M.; Ansari, M.T. Quantum dots: next generation of smart nano-systems. Pharm. Nanotechnol. 2019, 7, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.R.; Moffitt, M.G. Amphiphilic quantum dots with asymmetric, mixed polymer brush layers: from single core-shell nanoparticles to salt-induced vesicle formation. Polymers 2018, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Owusu, E.G.A.; MacRobert, A.J.; Naasani, I.; Parkin, I.P.; Allan, E.; Yaghini, E. Photoactivable polymers embedded with cadmium-free quantum dots and crystal violet: efficient bactericidal activity against clinical strains of antibiotic-resistant bacteria. ACS Appl. Mater. Interfaces 2019, 11, 12367–12378. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.; Liu, A.H. Luminescent molecularly imprinted polymers based on covalent organic frameworks and quantum dots with strong optical response to quinoxaline-2-carboxylicacid. Polymers 2019, 11, 708. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gao, N.; Dong, K.; Ren, J.; Qu, X. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano 2014, 8, 6202–6210. [Google Scholar] [CrossRef]
- Habiba, K.; Bracho-Rincon, D.P.; Gonzalez-Feliciano, J.A.; Villalobos-Santos, J.C.; Makarov, V.I.; Ortiz, D. Synergistic antibacterial activity of PEGylated silver–graphene quantum dots nanocomposites. App. Mater. Today 2015, 1, 80–87. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.M.; Bao, H.; Qiao, Y.; Toh, Y.; Yang, X. Mechanism of antimicrobial activity of CdTe quantum dots. Langmuir 2008, 24, 5445–5452. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, Q.; Zhang, M.; Li, P.; Ding, Y. Cooperative antimicrobial activity of CdTe quantum dots with Rocephin and fluorescence monitoring for Escherichia coli. J. Colloid Interface Sci. 2011, 362, 100–106. [Google Scholar] [CrossRef]
- Geraldo, D.A.; Arancibia-Miranda, N.; Villagra, N.A.; Mora, G.C.; Arratia-Perez, R. Synthesis of CdTe QDs/single-walled aluminosilicate nanotubes hybrid compound and their antimicrobial activity on bacteria. J. Nanoparticle Res. 2012, 14, 1286–1294. [Google Scholar] [CrossRef]
- Priester, J.H.; Stoimenov, P.K.; Mielke, R.E.; Webb, S.M.; Ehrhardt, C.; Zhang, J.P. Effects of soluble cadmium salts versus CdSe quantum dots on the growth of planktonic pseudomonas aeruginosa. Environ. Sci. Technol. 2009, 43, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Dhar, R.; Singh, S.; Kumar, A. Effect of capping agents on optical and antibacterial properties of cadmium selenide quantum dots. Bull. Mater. Sci. 2015, 38, 1247–1252. [Google Scholar]
- Ma, X.; Xiang, Q.; Liao, Y.; Wen, T.; Zhang, H. Visible-light-driven CdSe quantum dots/graphene/TiO2 nanosheets composite with excellent photocatalytic activity for E. coli disinfection and organic pollutant degradation. App. Surface Sci. 2018, 457, 846–855. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf. B Biointerfaces 2012, 100, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Chakraborti, S.; Chakrabarti, P.; Haranath, D.; Shanker, V.; Ansari, Z.A. Role of surface absorbed anionic species in antibacterial activity of ZnO quantum dots against Escherichia coli. J. Nanosci. Nanotechnol. 2009, 9, 6427–6433. [Google Scholar] [CrossRef]
- Jin, T.; Sun, D.; Su, J.Y.; Zhang, H.; Sue, H.J. Antimicrobial efficacy of ainc oxide quantum dots against Listeria monocytogenes, Salmonella enteritidis, and Escherichia coli O157: H7. J. Food Sci. 2009, 74, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mir, I.A.; Radhakrishanan, V.S.; Rawat, K.; Prasad, T.; Bohidar, H.B. Bandgap tunable AgInS based quantum dots for high contrast cell imaging with enhanced photodynamic and antifungal applications. Sci. Rep. 2018, 8, 9322–9333. [Google Scholar] [CrossRef]
- Pati, R.; Sahu, R.; Panda, J.; Sonawane, A. Encapsulation of zinc-rifampicin complex into transferrin-conjugated silver quantum-dots improves its antimycobacterial activity and stability and facilitates drug delivery into macrophages. Sci. Rep. 2016, 6, 24184–24198. [Google Scholar] [CrossRef]
- Patra, P.; Roy, S.; Sarkar, S.; Mitra, S.; Pradhan, S.; Debnath, N.; Goswami, A. Damage of lipopolysaccharides in outer cell membrane and production of ROS-mediated stress within bacteria makes nano zinc oxide a bactericidal agent. Applied Nanosci. 2014, 5, 857–866. [Google Scholar] [CrossRef]
- Thangamuthu, M.; Hsieh, K.Y.; Kumar, P.V.; Chen, G.Y. Graphene- and graphene oxide-based nanocomposite platforms for electrochemical biosensing applications. Int. J. Mol. Sci. 2019, 20, 2975. [Google Scholar] [CrossRef]
- Fan, H.Y.; Yu, X.H.; Wang, K.; Yin, Y.J.; Tang, Y.J.; Tang, Y.L. Graphene quantum dots (GQDs)-based nanomaterials for improving photodynamic therapy in cancer treatment. Eur. J. Med. Chem. 2019, 182, 111620–111646. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Huang, J.; Chen, G.; Zhu, Y.; Yang, L. Antibacterial property of graphene quantum dots (both source material and bacterial shape matter). ACS Appl. Mater. Interfaces 2016, 8, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Kholikov, K.; Ilhom, S.; Sajjad, M.; Smith, M.E.; Monroe, J.D.; San, O. Improved singlet oxygen generation and antimicrobial activity of sulphur-doped graphene quantum dots coupled with methylene blue for photodynamic therapy applications. Photodiagnosis Photodyn. Ther. 2018, 24, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Khandelwal, P.; Das, R.; Salunke, G.; Alam, A.; Ghorai, S. Oxidant-mediated one-step complete conversion of multi-walled carbon nanotubes to graphene quantum dots and their bioactivity against mammalian and bacterial cells. J. Mater. Chem. B 2017, 5, 785–796. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.X.; Zhang, Y. Graphene oxide quantum dots covalently functionalized PVDF membrane with significantly-enhanced bactericidal and antibiofouling performances. Sci. Rep. 2016, 6, 20142–20152. [Google Scholar] [CrossRef]
- Liu, J.; Rojas-Andrade, M.D.; Chata, G.; Peng, Y.; Roseman, G.; Lu, J.E. Photo-enhanced antibacterial activity of ZnO/graphene quantum dot nanocomposites. Nanoscale 2018, 10, 158–166. [Google Scholar] [CrossRef]
- Kuo, W.S.; Shao, Y.T.; Huang, K.S.; Chou, T.M.; Yang, C.H. Antimicrobial amino-functionalized nitrogen-doped graphene quantum dots for eliminating multidrug-resistant species in dual-modality photodynamic therapy and bioimaging under two-photon excitation. ACS Appl. Mater. Interfaces 2018, 10, 14438–14446. [Google Scholar] [CrossRef]
- Deng, S.; Fu, A.; Junaid, M.; Wang, Y.; Yin, Q.; Fu, C.; Liu, L.; Su, D.S.; Bian, W.P.; Pei, D.S. Nitrogen-doped graphene quantum dots (N-GQDs) perturb redox-sensitive system via the selective inhibition of antioxidant enzyme activities in zebrafish. Biomaterials 2019, 206, 61–72. [Google Scholar] [CrossRef]
- Deng, S.; Jia, P.P.; Zhang, J.H.; Junaid, M.; Niu, A.; Ma, Y.B. Transcriptomic response and perturbation of toxicity pathways in zebrafish larvae after exposure to graphene quantum dots (GQDs). J. Hazard. Mater. 2018, 357, 146–158. [Google Scholar] [CrossRef]
- Shang, W.; Zhang, X.; Zhang, M.; Fan, Z.; Sun, Y.; Han, M.; Fan, L. The uptake mechanism and biocompatibility of graphene quantum dots with human neural stem cells. Nanoscale 2014, 6, 5799–5806. [Google Scholar] [CrossRef]
- Thakur, M.; Mewada, A.; Pandey, S.; Bhori, M.; Singh, K.; Sharon, M.; Sharon, M. Milk-derived multi-fluorescent graphene quantum dot-based cancer theranostic system. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.T.; Li, X.; Wang, Q.S.; Zhang, Z.J.; Liu, P.; Zhang, C.C. Toxicity evaluation of CdTe quantum dots with different sizes on Escherichia coli. Toxicol. In Vitro 2012, 26, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Moradi Alvand, Z.; Rajabi, H.R.; Mirzaei, A.; Masoumiasl, A.; Sadatfaraji, H. Rapid and green synthesis of cadmium telluride quantum dots with low toxicity based on a plant-mediated approach after microwave and ultrasonic assisted extraction: Synthesis, characterization, biological potentials and comparison study. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Khare, S.K.; Kundu, J. The adverse effect of CdTe quantum dots on the cell membrane of Bacillus subtilis: Insight from microscopy. Nano-Structure Nano-Objects 2017, 12, 19–26. [Google Scholar] [CrossRef]
- Ananth, D.A.; Rameshkumar, A.; Jeyadevi, R.; Jagadeeswari, S.; Nagarajan, N.; Renganathan, R.; Sivasudha, T. Antibacterial potential of rutin conjugated with thioglycolic acid capped cadmium telluride quantum dots (TGA–CdTe QDs). Spectrochim. Acta, Part A 2015, 138, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, X.W.; Li, W.Y.; Zhang, Y.K. Thermo-sensitive imprinted polymer coating CdTe quantum dots for target protein specific recognition. Chem. Commun. 2012, 48, 1757–1759. [Google Scholar] [CrossRef]
- Li, X.; Lu, Z.; Li, Q. Multilayered films incorporating CdTe quantum dots with tunable optical properties for antibacterial application. Thin Solid Films 2013, 548, 336–342. [Google Scholar] [CrossRef]
- Courtney, C.M.; Goodman, S.M.; McDaniel, J.A.; Madinger, N.E.; Chatterjee, A.; Nagpal, P. Photoexcited quantum dots for killing multidrug-resistant bacteria. Nat. Mater. 2016, 15, 529–534. [Google Scholar] [CrossRef]
- Lai, L.; Li, S.J.; Feng, J.; Mei, P.; Ren, Z.H.; Chang, Y.L.; Liu, Y. Effects of surface charges on the bactericide activity of CdTe/ZnS quantum dots: a cell membrane disruption perspective. Langmuir 2017, 33, 2378–2386. [Google Scholar] [CrossRef]
- Han, X.; Lai, L.; Tian, F.; Jiang, F.L.; Xiao, Q.; Li, Y.; Liu, Y. Toxicity of CdTe quantum dots on yeast saccharomyces cerevisiae. Small 2012, 8, 2680–2689. [Google Scholar] [CrossRef]
- Mei, J.; Yang, L.Y.; Lai, L.; Xu, Z.Q.; Wang, C.; Zhao, J.; Liu, Y. The interactions between CdSe quantum dots and yeast saccharomyces cerevisiae: adhesion of quantum dots to the cell surface and the protective effect of ZnS shell. Chemosphere 2014, 112, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, L.; Yu, B.; Chen, Y.; Shen, Y.; Cong, H. ZnO quantum dots modified by pH-activated charge-reversal polymer for tumor targeted drug delivery. Polymers 2018, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine 2011, 7, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Meshram, J.V.; Koli, V.; Kumbhar, S.G.; Borade, L.; Phadatare, M.R.; Pawar, S. Structural, spectroscopic and anti-microbial inspection of peg capped ZnO nanoparticles for biomedical applications. Mater. Res. Exp. 2018, 5, 045016–045029. [Google Scholar] [CrossRef]
- Repp, S.; Erdem, E. Controlling the exciton energy of zinc oxide (ZnO) quantum dots by changing the confinement conditions. Spectrochim. Acta, Part A. 2016, 152, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Soheyli, E.; Azad, D.; Sahraei, R.; Hatamnia, A.A.; Rostamzad, A.; Alinazari, M. Synthesis and optimization of emission characteristics of water-dispersible ag-in-s quantum dots and their bactericidal activity. Colloids Surf. B Biointerfaces 2019, 182, 110389–110425. [Google Scholar] [CrossRef] [PubMed]
- Midya, L.; Patra, A.S.; Banerjee, C.; Panda, A.B.; Pal, S. Novel nanocomposite derived from ZnO/ CdS QDs embedded crosslinked chitosan: An efficient photocatalyst and effective antibacterial agent. J. Hazard. Mater. 2019, 369, 398–407. [Google Scholar] [CrossRef]
- Abu Rabe, D.I.; Al Awak, M.M.; Yang, F.; Okonjo, P.A.; Dong, X.; Teisl, L.R.; Wang, P.; Tang, Y.; Pan, N.; Sun, Y.P.; et al. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int. J. Nanomedicine 2019, 14, 2655–2665. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng., C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Park, T.J.; Rohit, J.V.; Koduru, J.R. Antimicrobial activity of silver nanoparticles. Nanoparticles in Pharmacotherapy. 2019, 1, 461–484. [Google Scholar]
- Ivask, A.; Titma, T.; Visnapuu, M.; Vija, H.; Kakinen, A.; Sihtmae, M.; Pokhrel, S.; Madler, L.; Heinlaan, M.; Kisand, V.; et al. Toxicity of 11 metal oxide nanoparticles to three mammalian cell types in vitro. Curr. Top. Med. Chem. 2015, 15, 1914–1929. [Google Scholar] [CrossRef] [PubMed]

| Quantum Dots (QDs) | Size (nm) | Microorganism (Bacteria and Fungi) | Main Mechanism of Inhibitory Action | References |
|---|---|---|---|---|
| Graphene QDs | 3–8 nm | Escherichia coli, Staphylococcus aureus | Production of free radicals to damage the cell wall | [7] |
| Polyethyleneglycol (PEGylated) silver graphene QDs | 5–8 nm | Pseudomonas aeruginosa, Staphylococcus aureus | The PEG-Ag graphene QDs bind with the thiol of enzymes and proteins of the cell wall/membrane, leading to leakage of cell metabolites | [8] |
| CdTe QDs | 5–10 nm | Escherichia coli | The QDs insert into the cell membrane to cause membrane stress; furthermore, the heavy metal ions are released into the cells to decline the gene expression of superoxide dismutase (SOD) | [9] |
| CdTe–Rocephin QD complex | 3 nm | Escherichia coli | Rochepins damage the cell wall and make pits in the membrane; then, CdTe QDs enter the cell cytoplasm, and attach to the nucleic material, preventing the gene expression of anti-oxidase | [10] |
| 3-mercaptopropionic acid (MPA) -capped CdTe QDs | 1–10 nm | Salmonella typhimurium, Acinetobacter baumanni, Pseudomonas aeruginosa | The QDs attach to the phospholipid layer of bacteria; also, Cd2+ disrupts the cellular pathway and retards cell respiration | [11] |
| CdSe QDs | 7 nm | Pseudomonas aeruginosa | Internalization of Cd2+ cause cell toxicity and genomic toxicity | [12] |
| thioglycolic acid (TGA) and mercapto-acetohydrazide (TGH) lysine-capped CdSe QDs | 8 nm | Staphylococcus aureus | Increased toxicity of Cd2+ causes cell death | [13] |
| CdSe QDs/TiO2/nano graphene sheets | 10 nm | Escherichia coli | The delocalized photogenerated π electrons create oxidative stress | [14] |
| CdS/Ag2S QDs | 2–19 nm | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | The QDs penetrate through the cell wall and attach to DNA; then, the DNA molecules get condensed and damage DNA structure | [15] |
| ZnO QDs | 3–7 nm | Escherichia coli | The photoexcitation of ZnO QDs produces an electron–hole pair; then, the electron trapped by the oxygen induces excessive reactive oxygen species (ROS) | [16] |
| Polyvinyl pyrrolidone (PVP)-capped ZnO QDs | 2–10 nm | Listeria monocytogenes, Salmonella enteritis, Escherichia coli | The QDs penetrate through the cell membrane and cause cell organelle damage | [17] |
| Ag/In/S QDs | 9.5–10 nm | Candida albicans | Promotion of ROS production | [18] |
| Zn/rifampicin/Tf QDs | 10 nm | Mycobacterium smegmatis, Mycobacterium bovis BCG | Cell toxicity resulting in apoptosis | [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendiran, K.; Zhao, Z.; Pei, D.-S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670. https://doi.org/10.3390/polym11101670
Rajendiran K, Zhao Z, Pei D-S, Fu A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers. 2019; 11(10):1670. https://doi.org/10.3390/polym11101670
Chicago/Turabian StyleRajendiran, Keerthiga, Zizhen Zhao, De-Sheng Pei, and Ailing Fu. 2019. "Antimicrobial Activity and Mechanism of Functionalized Quantum Dots" Polymers 11, no. 10: 1670. https://doi.org/10.3390/polym11101670
APA StyleRajendiran, K., Zhao, Z., Pei, D.-S., & Fu, A. (2019). Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers, 11(10), 1670. https://doi.org/10.3390/polym11101670