Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview
Abstract
1. Introduction
2. Preparation of Guanidine and Biguanidine-Based Antimicrobial Polymers
3. Surface Modification Using Guanidine and Biguanidine Polymers
4. Antimicrobial Beads or Latex and Star Polymer Containing Guanidine Chains
5. Complexation of PHGH with Other Polymers
6. Rendering Biodegradable Polymers and Cellulose-Based Foam Materials Antimicrobial
7. Gemini Antimicrobial Surfactants and Polymers
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Munoz-Bonilla, A.; Ernández-García, M. Polymeric materials with antimicrobial activity. Prog. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Choi, H.; Chakraborty, S.; Liu, R.; Gellman, S.H.; Weisshaar, J.C. Single-cell, time-resolved antimicrobial effects of a highly cationic, random nylon-3 copolymer on live escherichia coli. ACS Chem. Biol. 2016, 11, 113–120. [Google Scholar] [CrossRef]
- Russell, A.D. The mechanism of action of some antibacterial agents. Prog. Med. Chem. 1969, 6, 135–199. [Google Scholar]
- Nurdin, N.; Helary, G.; Sauvet, G. Biocidal polymers active by contact. Ii. Biological evaluation of polyurethane coatings with pendant quaternary ammonium salts. J. Appl. Polym. Sci. 1993, 50, 663–670. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Wei, D.F.; Guan, Y.; Zheng, A.N.; Zhong, J.J. Damage of escherichia coli membrane by bactericidal agent polyhexamethylene guanidine hydrochloride: Micrographic evidences. J. Appl. Microbiol. 2010, 108, 898–907. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, A.; Zhong, J. Interactions of biocidal guanidine hydrochloride polymer analogs with model membranes: A comparative biophysical study. Acta Biochem. Biophys. Sin. 2011, 43, 729–737. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Klibanov, A.M. Permanently microbicidal materials coatings. J. Mater. Chem. 2007, 17, 2479–2482. [Google Scholar] [CrossRef]
- Zana, R.; Talmon, Y. Dependence of aggregate morphology on structure of dimeric surfactants. Nature 1993, 362, 228–230. [Google Scholar] [CrossRef]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. X. Antibacterial activity of filters incorporating phosphonium biocides. J. Appl. Polym. Sci. 1994, 54, 1305–1310. [Google Scholar] [CrossRef]
- Lin, J.; Qiu, S.Y.; Lewis, K.; Klibanov, A.M. Mechanism of bactericidal and fungicidal activities of textiles covalently modified with alkylated polyethylenimine. Biotechnol. Bioeng. 2003, 83, 168–172. [Google Scholar] [CrossRef]
- Roy, D.; Knapp, J.S.; Guthrie, J.T.; Perrier, S. Antibacterial cellulose fiber via raft surface graft polymerization. Biomacromolecules 2008, 9, 91–99. [Google Scholar] [CrossRef]
- Kugler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiol. SGM 2005, 151, 1341–1348. [Google Scholar] [CrossRef]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; Chen, Y. Synthesis and antimicrobial activity of polymeric guanidine and biguanidine salts. Polymer 1999, 40, 6189–6198. [Google Scholar] [CrossRef]
- Grace, J.L.; Schneider-Futschik, E.K.; Elliott, A.G.; Amado, M.; Truong, N.P.; Cooper, M.A.; Li, J.; Davis, T.P.; Quinn, J.F.; Velkov, T.; et al. Exploiting macromolecular design to optimize the antibacterial activity of alkylated cationic oligomers. Biomacromolecules 2018, 19, 4629–4640. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, R.; Zhang, Y.; Guan, Y.; Zheng, A. Acrylonitrile copolymers containing guanidine oligomer: Synthesis and use for the preparation of nonleaching antimicrobial acrylic fibers. J. Appl. Polym. Sci. 2013, 130, 419–425. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Cao, W.; Wei, D.; Zheng, A.; Guan, Y. Permanent antimicrobial cotton fabrics obtained by surface treatment with modified guanidine. Carbohydr. Polym. 2018, 180, 192–199. [Google Scholar] [CrossRef]
- Wei, D.; Ding, Y.; Wang, T.; Yang, J.; Guan, Y.; Zheng, A. Preparation of nonleaching antimicrobial polypropylene wax and its application in polypropylene. J. Appl. Polym. Sci. 2017, 134, 44190. [Google Scholar] [CrossRef]
- Guan, Y.; Xiao, H.; Sullivan, H.; Zheng, A. Antimicrobial-modified sulfite pulps prepared by in situ copolymerization. Carbohydr. Polym. 2007, 69, 688–696. [Google Scholar] [CrossRef]
- Khan, A.; Li, Y.; Xiao, H.; Zou, X. Rendering packaging paper antimicrobial with functional-modified starch: Pilot paper machine trial at fpinnovations. J-For J. Sci. Techn. Forest Prod. Processes 2016, 5, 11–16. [Google Scholar]
- Wei, D.; Zhou, R.; Guan, Y.; Zheng, A.; Zhang, Y. Investigation on the reaction between polyhexamethylene guanidine hydrochloride oligomer and glycidyl methacrylate. J. Appl. Polym. Sci. 2013, 127, 666–674. [Google Scholar] [CrossRef]
- Guan, Y.; Qian, L.; Xiao, H.; Zheng, A. Preparation of novel antimicrobial-modified starch and its adsorption on cellulose fibers: Part I. Optimization of synthetic conditions and antimicrobial activities. Cellulose 2008, 15, 609–618. [Google Scholar] [CrossRef]
- Guan, Y.; Qian, L.; Xiao, H.; Zheng, A.; He, B. Synthesis of a novel antimicrobial-modified starch and its adsorption on cellulose fibers: Part ii—Adsorption behaviors of cationic starch on cellulose fibers. Cellulose 2008, 15, 619–629. [Google Scholar] [CrossRef]
- Ni, S.; Zhang, H.; Dai, H.; Xiao, H. Starch-based flexible coating for food packaging paper with exceptional hydrophobicity and antimicrobial activity. Polymers 2018, 10, 1260. [Google Scholar] [CrossRef]
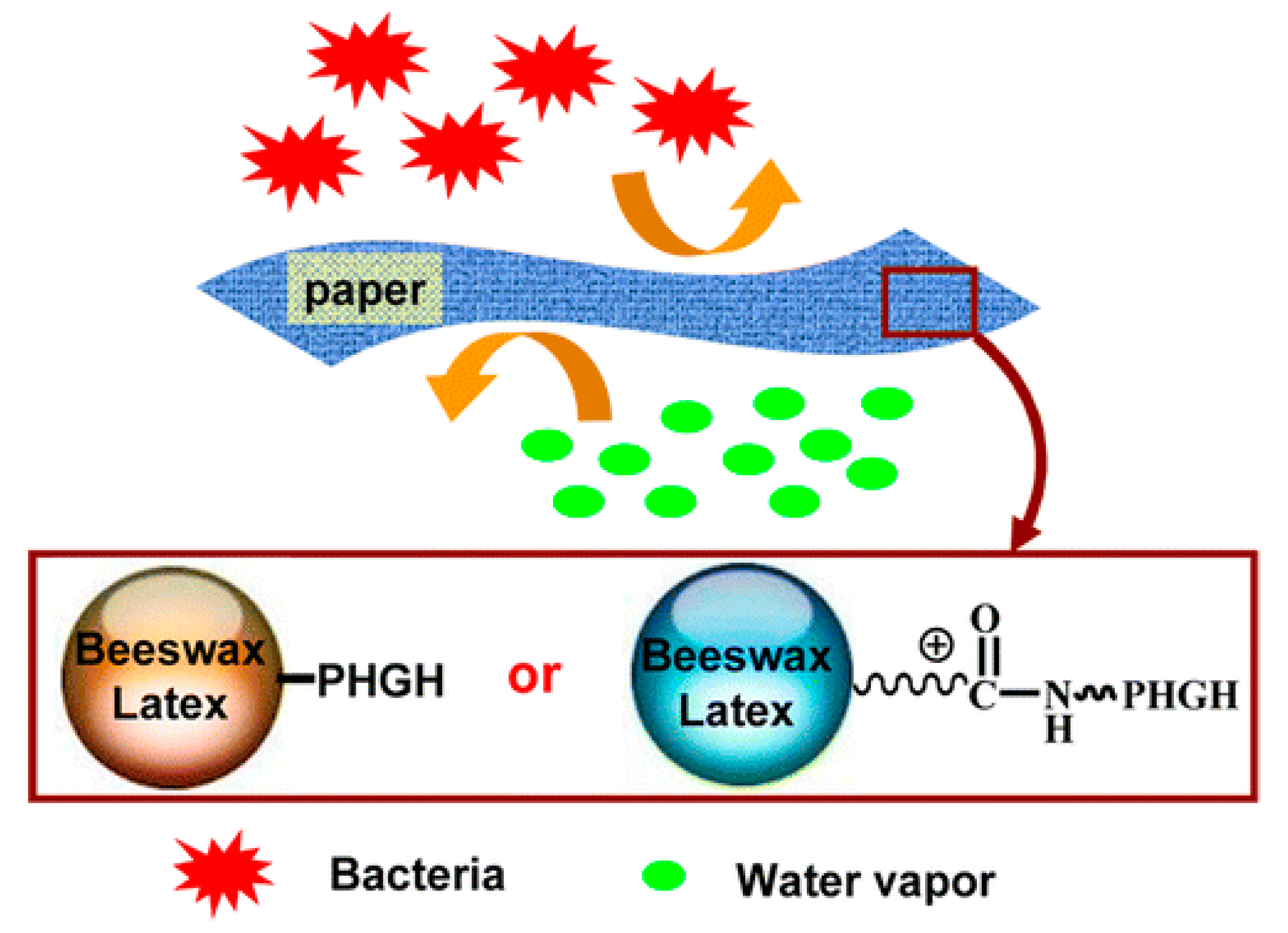
- Zhang, D.; Xiao, H. Dual-functional beeswaxes on enhancing antimicrobial activity and water vapor barrier property of paper. ACS Appl. Mater. Interfaces 2013, 5, 3464–3468. [Google Scholar] [CrossRef]
- Zhang, D.; Yu, G.; Long, Z.; Xiao, H.; Qian, L. Bio-wax latex-modified paper as antimicrobial and water-vapor-resistant packaging material. J. Wood Chem. Technol. 2016, 36, 182–191. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, A.; Zhou, X.; Guan, Y.; Xiao, H. Antimicrobial polyethylene wax emulsion and its application on active paper-based packaging material. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, H.; Cai, P.; Colpitts, M. Cellulose fibers modified with nano-sized antimicrobial polymer latex for pathogen deactivation. Carbohyd. Polym. 2016, 135, 94–100. [Google Scholar] [CrossRef]
- Li, Y.; Cai, P.; Tong, Z.; Xiao, H.; Pan, Y. Preparation of copolymer-based nanoparticles with broad-spectrum antimicrobial activity. Polymers 2017, 9, 71712. [Google Scholar] [CrossRef]
- Pan, Y.; Xue, Y.; Snow, J.; Xiao, H. Tailor-made antimicrobial/antiviral star polymer via ATRP of cyclodextrin and guanidine-based macromonomer. Macromol. Chem. Phys. 2015, 216, 511–518. [Google Scholar] [CrossRef]
- Exley, S.E.; Paslay, L.C.; Sahukhal, G.S.; Abel, B.A.; Brown, T.D.; McCormick, C.L.; Heinhorst, S.; Koul, V.; Choudhary, V.; Elasri, M.O.; et al. Antimicrobial peptide mimicking primary amine and guanidine containing methacrylamide copolymers prepared by raft polymerization. Biomacromolecules 2015, 16, 3845–3852. [Google Scholar] [CrossRef]
- Lienkamp, K.; Madkour, A.E.; Musante, A.; Nelson, C.F.; Nusslein, K.; Tew, G.N. Antimicrobial polymers prepared by romp with unprecedented selectivity: A molecular construction kit approach. J. Am. Ceram. Soc. 2008, 130, 9836–9843. [Google Scholar] [CrossRef]
- Wang, X.; Lu, P.; Li, Y.; Xiao, H.; Liu, X. Antibacterial activities and mechanisms of fluorinated graphene and guanidine-modified graphene. RSC Adv. 2016, 6, 8763–8772. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, M.; Xu, Z.; Jiang, C.; Shen, C.; Meng, Q. Guanidyl-functionalized graphene/polysulfone mixed matrix ultrafiltration membrane with superior permselective, antifouling and antibacterial properties for water treatment. J. Colloid Interface Sci. 2019, 540, 295–305. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, H.; Zhao, G.; He, B. Antimicrobial and thermal-responsive layer-by-layer assembly based on ionic-modified guanidine polymer and pva. Polym. Bull. 2008, 61, 541–551. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, A.; Xiao, H. Properties of a novel thermal sensitive polymer based on poly(vinyl alcohol) and its layer-by-layer assembly. Polym. Adv. Technol. 2007, 18, 335–345. [Google Scholar] [CrossRef]
- Qian, L.; Guan, Y.; He, B.; Xiao, H. Synergy of wet strength and antimicrobial activity of cellulose paper induced by a novel polymer complex. Mater. Lett. 2008, 62, 3610–3612. [Google Scholar] [CrossRef]
- Sun, S.; An, Q.; Li, X.; Qian, L.; He, B.; Xiao, H. Synergistic effects of chitosan-guanidine complexes on enhancing antimicrobial activity and wet-strength of paper. Bioresour. Technol. 2010, 101, 5693–5700. [Google Scholar] [CrossRef]
- Qian, L.; Guan, Y.; Xiao, H. Preparation and characterization of inclusion complexes of a cationic beta-cyclodextrin polymer with butylparaben or triclosan. Int. J. Pharm. 2008, 357, 244–251. [Google Scholar] [CrossRef]
- Qian, L.; Dong, C.; Liang, X.; He, B.; Xiao, H. Polyelectrolyte complex containing antimicrobial guanidine-based polymer and its adsorption on cellulose fibers. Holzforschung 2014, 68, 103–111. [Google Scholar] [CrossRef]
- Kukharenko, O.; Bardeau, J.; Zaets, I.; Ovcharenko, L.; Tarasyuk, O.; Porhyn, S.; Mischenko, I.; Vovk, A.; Rogalsky, S.; Kozyrovska, N. Promising low cost antimicrobial composite material based on bacterial cellulose and polyhexamethylene guanidine hydrochloride. Eur. Polym. J. 2014, 60, 247–254. [Google Scholar] [CrossRef]
- Li, J.S.; Xiao, H.N.; Li, J.H.; Zhong, Y.P. Drug carrier systems based on water-soluble cationic beta-cyclodextrin polymers. Int. J. Pharm. 2004, 278, 329–342. [Google Scholar] [CrossRef]
- Guan, Y.; Qian, L.; Xiao, H. Novel anti-microbial host-guest complexes based on cationic beta-cyclodextrin polymers and triclosan/butylparaben. Macromol. Rapid Commun. 2007, 28, 2244–2248. [Google Scholar] [CrossRef]
- Qian, L.; Guan, Y.; Ziaee, Z.; He, B.; Zheng, A.; Xiao, H. Rendering cellulose fibers antimicrobial using cationic beta-cyclodextrin-based polymers included with antibiotics. Cellulose 2009, 16, 309–317. [Google Scholar] [CrossRef]
- Qian, L.; Xiao, H.; Zhao, G.; He, B. Synthesis of modified guanidine-based polymers and their antimicrobial activities revealed by afm and clsm. ACS Appl. Mater. Interfaces 2011, 3, 1895–1901. [Google Scholar] [CrossRef]
- Ng, V.W.L.; Chanb, J.M.W.; Sardonb, H.; Onob, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef]
- Thoma, L.M.; Boles, B.R.; Kuroda, K. Cationic methacrylate polymers as topical antimicrobial agents against staphylococcus aureus nasal colonization. Biomacromolecules 2014, 15, 2933–2943. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Zheng, A.; Xiao, H. Soil burial biodegradation of antimicrobial biodegradable pbat films. Polym. Degrad. Stab. 2015, 116, 14–22. [Google Scholar] [CrossRef]
- Wang, H.; Wei, D.; Ziaee, Z.; Xiao, H.; Zheng, A.; Zhao, Y. Preparation and properties of nonleaching antimicrobial linear low-density polyethylene films. Ind. Eng. Chem. Res. 2015, 54, 1824–1831. [Google Scholar] [CrossRef]
- Wei, D.; Wang, H.; Ziaee, Z.; Chibante, F.; Zheg, A.; Xiao, H. Non-leaching antimicrobial biodegradable pbat films through a facile and novel approach. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 986–991. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Tarach, I.; Richert, A.; Cichosz, M.; Koter, I.; Nowaczyk, J. Physicochemical and barrier properties of polylactide films including antimicrobial additives. Mater. Chem. Phys. 2019, 230, 299–307. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Walczak, M.; Jankiewicz, U.; Pejchalová, M. Antimicrobial activity of polyhexamethylene guanidine derivatives introduced into polycaprolactone. J. Polym. Environ. 2018, 26, 589–595. [Google Scholar] [CrossRef]
- Heydarifard, S.; Pan, Y.; Xiao, H.; Nazhad, M.M.; Shipin, O. Water-resistant cellulosic filter containing non-leaching antimicrobial starch for water purification and disinfection. Carbohydr. Polym. 2017, 163, 146–152. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Y.; Gai, J. Guanidinium-functionalized nanofiltration membranes integrating anti-fouling and antimicrobial effects. J. Mater. Chem. A 2018, 6, 6442–6454. [Google Scholar] [CrossRef]
- Menger, F.M.; Keiper, J.S. Gemini surfactants. Angew. Chem. Int. Ed. 2000, 39, 1906–1920. [Google Scholar] [CrossRef]
- Zhu, B.; Jia, L.; Guo, X.; Yin, J.; Zhao, Z.; Chen, N.; Chen, S.; Jia, Y. Controllable assembly of a novel cationic gemini surfactant containing a naphthalene and amide spacer with β-cyclodextrin. Soft Mater. 2019, 15, 3198–3207. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Guo, J.; Ma, J.; Lu, Y. Application of green cationic silicon-based gemini surfactants to improve antifungal properties, fiber dispersion and dye absorption of sheepskin. J. Clean. Prod. 2019, 206, 430–437. [Google Scholar] [CrossRef]
- Tan, H.; Xiao, H. Synthesis and antimicrobial characterization of novel l-lysine gemini surfactants pended with reactive groups. Tetrahedron Lett. 2008, 49, 1759–1761. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, M.; Zhou, L.; Tan, H.; Li, J.; Xiao, H.; Li, J.; Snow, J. Synthesis and antibacterial characterization of gemini surfactant monomers and copolymers. Polym. Chem. 2012, 3, 907–913. [Google Scholar] [CrossRef]
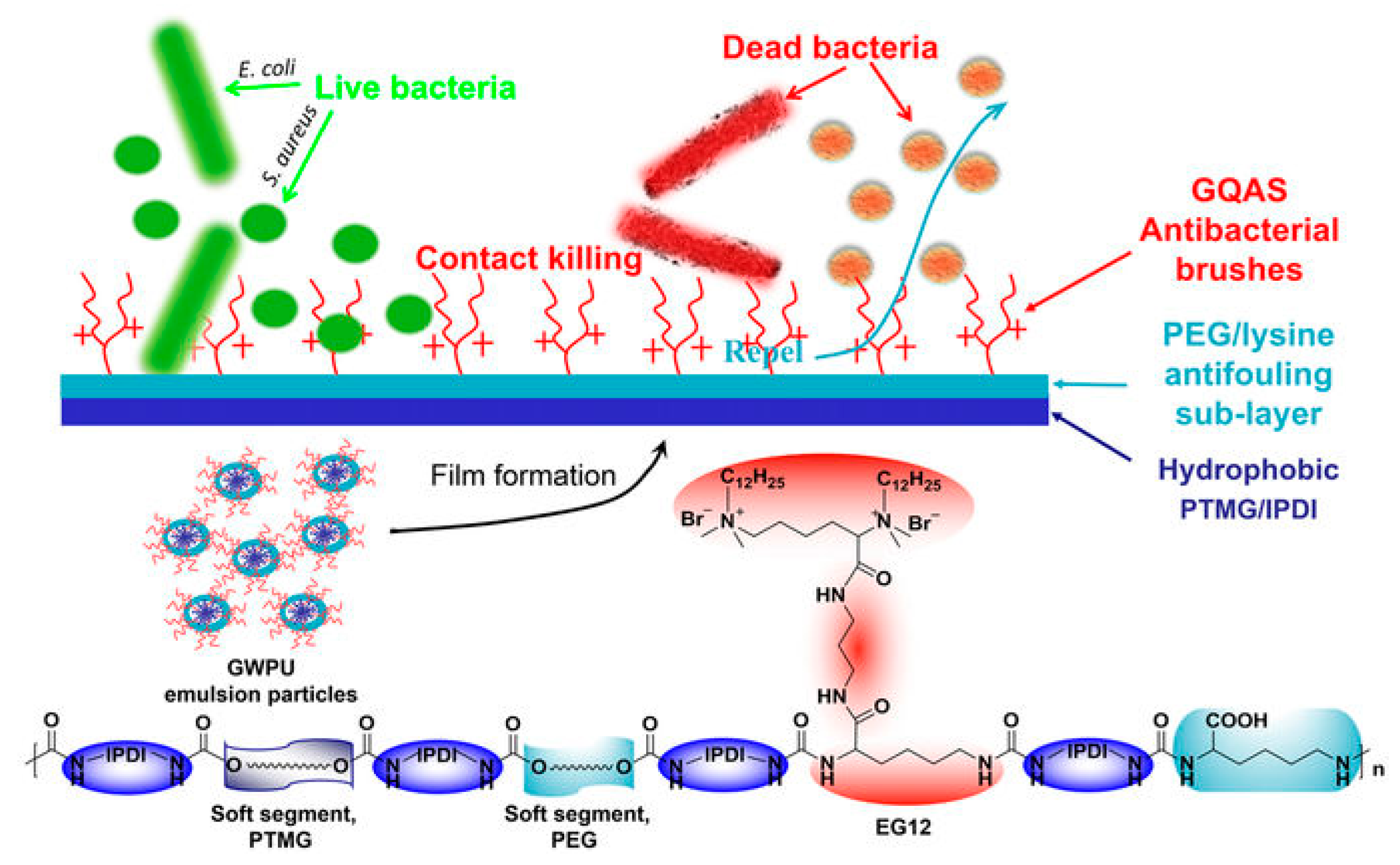
- Zhang, Y.; Li, Y.; Li, J.; Gao, Y.; Tan, H.; Wang, K.; Li, J.; Fu, Q. Synthesis and antibacterial characterization of waterborne polyurethanes with gemini quaternary ammonium salt. Sci. Bull. 2015, 60, 1114–1121. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Li, J.; Gao, Y.; Luo, F.; Tan, H.; Wang, K.; Fu, Q. A novel surface structure consisting of contact-active antibacterial upper-layer and antifouling sub-layer derived from gemini quaternary ammonium salt polyurethanes. Sci. Rep. 2016, 6, 32140. [Google Scholar] [CrossRef]
- He, M.; Xiao, H.; Zhou, Y.; Lu, P. Synthesis, characterization and antimicrobial activities of water-soluble amphiphilic copolymers containing ciprofloxacin and quaternary ammonium salts. J. Mater. Chem. B 2015, 3, 3704–3713. [Google Scholar] [CrossRef]
- Xu, X.; Xiao, H.; Ziaee, Z.; Wang, H.; Guan, Y.; Zheng, A. Novel comb-like ionenes with aliphatic side chains: Synthesis and antimicrobial properties. J. Mater. Sci. 2013, 48, 1162–1171. [Google Scholar] [CrossRef]
- Uppu, D.S.S.M.; Samaddar, S.; Hoque, J.; Konai, M.M.; Krishnamoorthy, P.; Shome, B.R.; Haldar, J. Side chain degradable cationic—Amphiphilic polymers with tunable hydrophobicity show in vivo activity. Biomacromolecules 2016, 17, 3094–3102. [Google Scholar] [CrossRef]
- Akarca, G. Composition and antibacterial effect on food borne pathogens of hibiscus surrattensis l. Calyces essential oil. Ind. Crops Prod. 2019, 137, 285–289. [Google Scholar] [CrossRef]
- Rauf, A.; Ye, J.; Zhang, S.; Shi, L.; Akram, M.A.; Ning, G. Synthesis, structure and antibacterial activity of a copper(II) coordination polymer based on thiophene-2,5-dicarboxylate ligand. Polyhedron 2019, 166, 130–136. [Google Scholar] [CrossRef]
- Chrom, C.L.; Renn, L.M.; Caputo, G.A. Characterization and antimicrobial activity of amphiphilic peptide ap3 and derivative sequences. Antibiotics 2019, 8, 20. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Luo, T.; Zhao, Q.; Cui, K.; Huang, J.; Jiang, T.; Ma, Z. Synthesis of novel guanidine-based aba triblock copolymers and their antimicrobial honeycomb films. Polym. Chem. 2018, 9, 3922–3930. [Google Scholar] [CrossRef]
- Thakur, A.; Ranote, S.; Kumar, D.; Bhardwaj, K.K.; Gupta, R.; Chauhan, G.S. Synthesis of a pegylated dopamine ester with enhanced antibacterial and antifungal activity. ACS Omega 2018, 3, 7925–7933. [Google Scholar] [CrossRef]
- Ng, V.W.L.; Tan, J.P.K.; Leong, J.; Voo, Z.X.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial polycarbonates: Investigating the impact of nitrogen-containing heterocycles as quaternizing agents. Macromolecules 2014, 47, 1285–1291. [Google Scholar] [CrossRef]








| Antimicrobial Polymer | MIC (μg/mL) | Reference | |
|---|---|---|---|
| Escherichia coli | Staphylococcus aureus | ||
| Chitosan | 1280 | [39] | |
| Hibiscus surrattensis L. calyces essential oil | 38 ± 10 | 54 ± 40 | [66] |
| Epichlorohydrin-crosslinked PHGH | 7.8 | [46] | |
| Copper(II) coordination polymer | 250 | 250 | [67] |
| Amphiphilic Peptide AP3 | 8.3 | 4.15 | [68] |
| Star Polymer with guanidine arms | 1.56 | [31] | |
| PFQ copolymer containing gemini | 54 | 54 | [60] |
| Poly(methacryl guanidine hydrochloride) | 113 | - | [69] |
| PEGylated dopamine ester | 3120 | 3120 | [70] |
| Quaternized polycarbonates with propyl and hexyl side chains | 31 | 4 | [71] |
| (6,7,4) ammonium polyionenes (6,7,12) ammonium polyionenes | 8 62.5 | [64] | |
| Guanidine copolymer-based nanoparticles | 8 | 2 | [30] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; Xia, Q.; Xiao, H. Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview. Polymers 2019, 11, 1283. https://doi.org/10.3390/polym11081283
Pan Y, Xia Q, Xiao H. Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview. Polymers. 2019; 11(8):1283. https://doi.org/10.3390/polym11081283
Chicago/Turabian StylePan, Yuanfeng, Qiuyang Xia, and Huining Xiao. 2019. "Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview" Polymers 11, no. 8: 1283. https://doi.org/10.3390/polym11081283
APA StylePan, Y., Xia, Q., & Xiao, H. (2019). Cationic Polymers with Tailored Structures for Rendering Polysaccharide-Based Materials Antimicrobial: An Overview. Polymers, 11(8), 1283. https://doi.org/10.3390/polym11081283