Multilayer Nanofiber Composite Separator for Lithium-Ion Batteries with High Safety
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of APEAP Membranes
2.2.1. Fabrication of PE Membrane
2.2.2. Fabrication of PI and AP Electrospun Membrane
2.2.3. Fabrication of APEAP Membrane
2.3. Characterization
3. Results and Discussion
3.1. Nanofiber Morphology and Electrospun Solution Property
3.2. Wettability
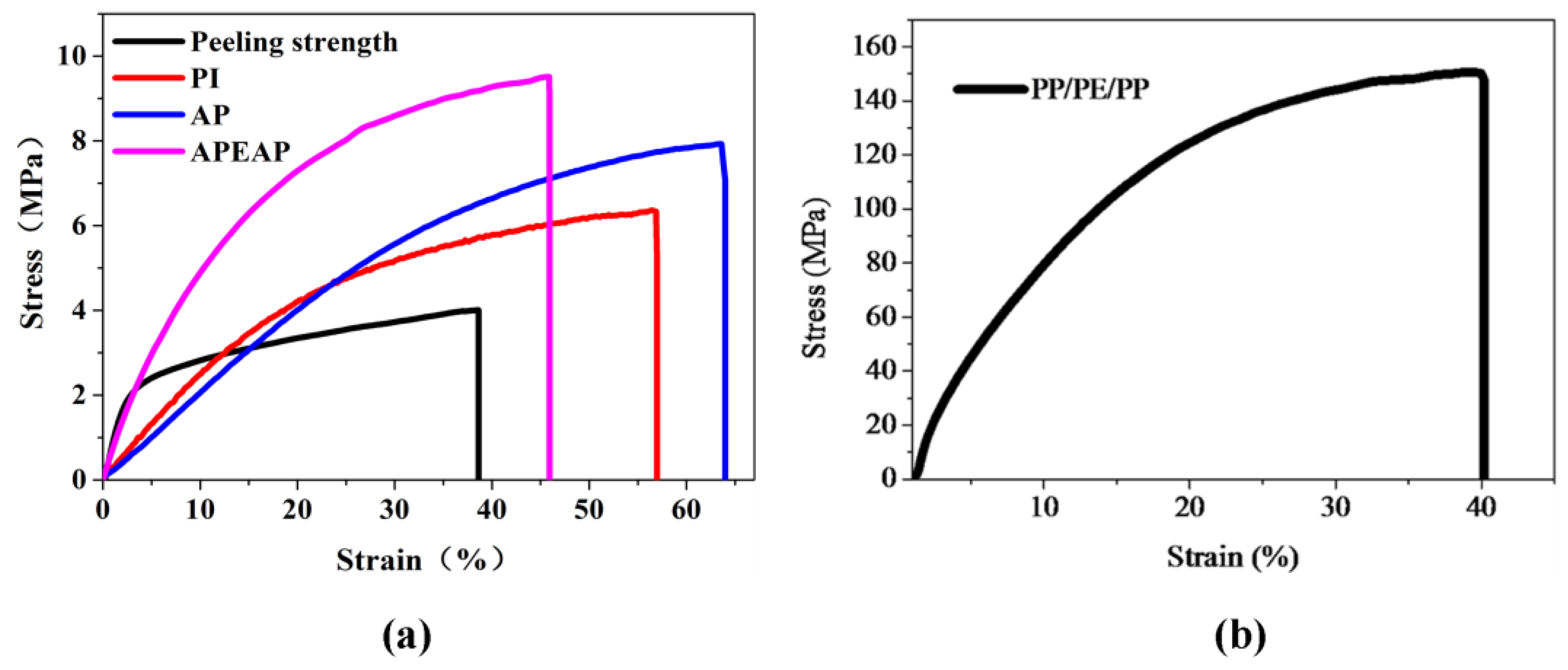
3.3. Mechanical Performance
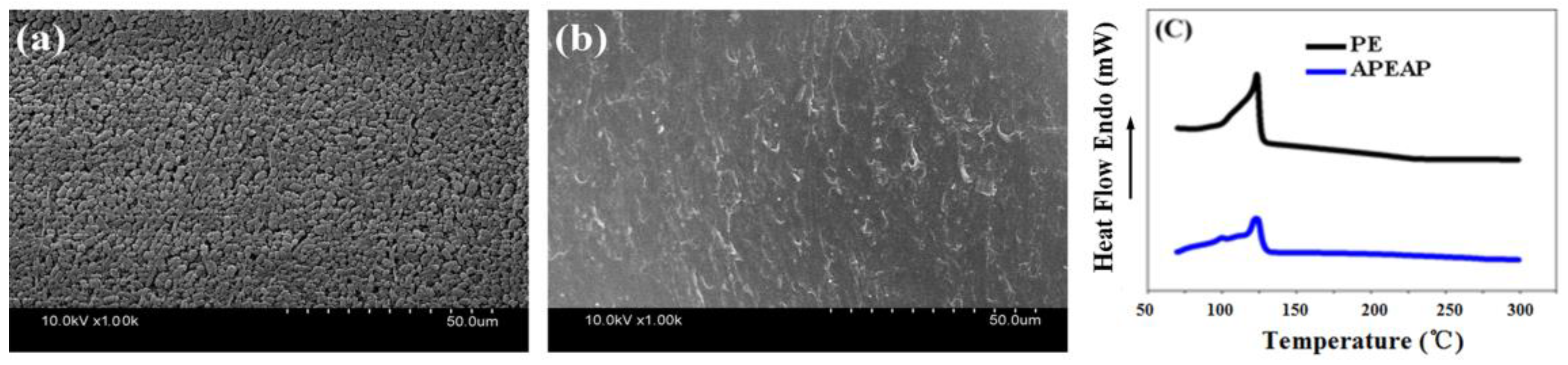
3.4. Thermal-Dimensional Stability
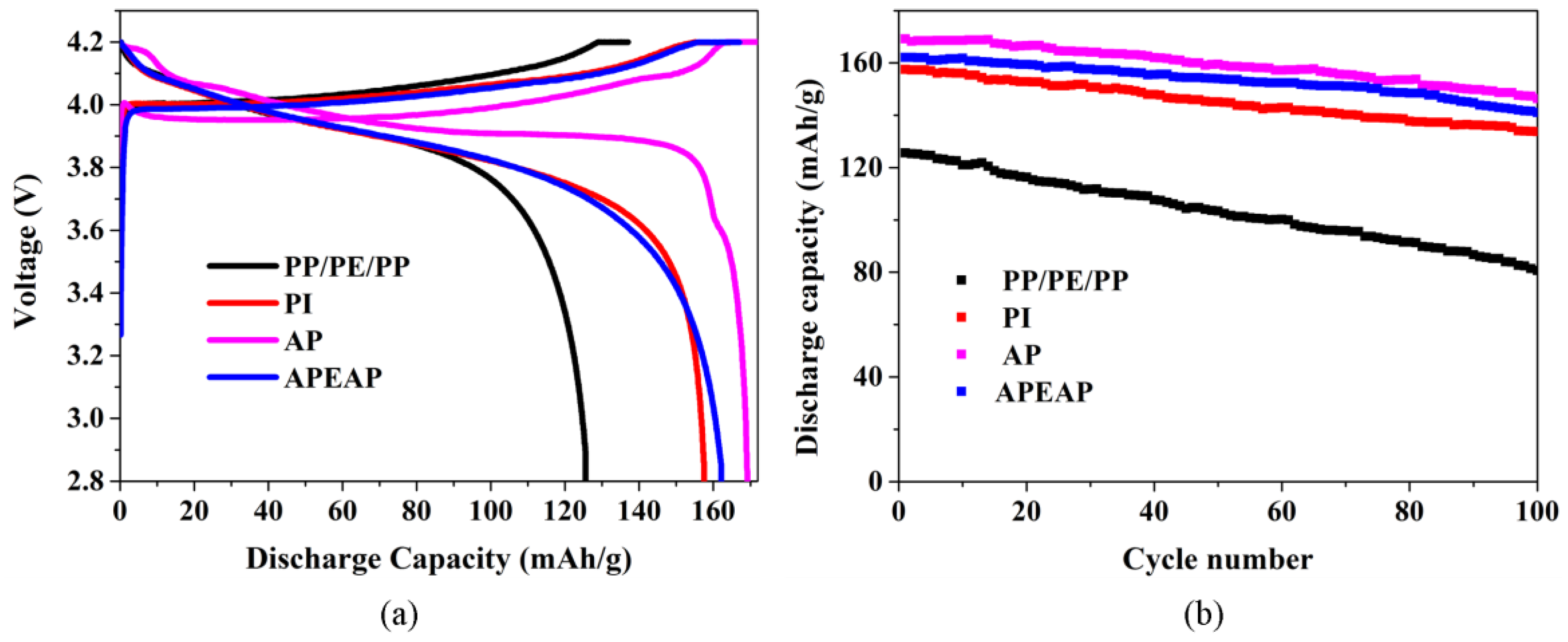
3.5. Electrochemical Performance
3.6. Battery Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lee, M.J.; Hwang, J.K.; Kim, J.H.; Lim, H.S.; Sun, Y.K.; Suh, K.D.; Lee, Y.M. Electrochemical performance of a thermally rearranged polybenzoxazole nanocomposite membrane as a separator for lithium-ion batteries at elevated temperature. J. Power Sources 2016, 305, 259–266. [Google Scholar] [CrossRef]
- Pan, R.; Sun, R.; Wang, Z.; Lindh, J.; Edström, K.; Strömme, M.; Nyholm, L. Sandwich-structured nano/micro fiber-based separators for lithium metal batteries. Nano Energy 2019, 55, 316–326. [Google Scholar] [CrossRef]
- Sun, Y.; Radke, C.J.; McCloskey, B.D.; Prausnitz, J.M. Wetting behavior of four polar organic solvents containing one of three lithium salts on a lithium-ion-battery separator. J. Colloid Interface Sci. 2018, 529, 582–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Liu, Y.; Yang, W.; Ren, Q.; Li, F.; Huang, Z. Lithium ion battery separator with high performance and high safety enabled by tri-layered SiO2@ PI/m-PE/SiO2@ PI nanofiber composite membrane. J. Power Sources 2018, 396, 265–275. [Google Scholar] [CrossRef]
- Liu, X.; Song, K.; Lu, C.; Huang, Y.; Duan, X.; Li, S.; Ding, Y. Electrospun PU@ GO separators for advanced lithium ion batteries. J. Membr. Sci. 2018, 555, 1–6. [Google Scholar] [CrossRef]
- Tan, J.; Kong, L.; Qiu, Z.; Yan, Y. Flexible, high-wettability and thermostable separator based on fluorinated polyimide for lithium-ion battery. J. Solid State Electrochem. 2018, 11, 3363–3373. [Google Scholar] [CrossRef]
- Cui, Z.; Shi, H.; Ding, J.; Zhang, J.; Wang, H.; Wang, H. Fabrication of poly (vinylidene fluoride) separator with better thermostability and electrochemical performance for lithium ion battery by blending polyester. Mater. Lett. 2018, 228, 466–469. [Google Scholar] [CrossRef]
- Boriboon, D.; Vongsetskul, T.; Limthongkul, P.; Kobsiriphat, W.; Tammawat, P. Cellulose ultrafine fibers embedded with titania particles as a high performance and eco-friendly separator for lithium-ion batteries. Carbohydr. Polym. 2018, 189, 145–151. [Google Scholar] [CrossRef]
- Li, J.; Niu, X.; Song, J.; Li, Y.; Li, X.; Hao, W.; He, T. Harvesting vapor by hygroscopic acid to create pore: Morphology, crystallinity and performance of poly (ether ether ketone) lithium ion battery separator. J. Membr. Sci. 2019, 577, 1–11. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Liang, H.Q.; Gu, L.; Yu, Y.; Huang, Y.Q.; Xu, Z.K. PVDF/PAN blend separators via thermally induced phase separation for lithium ion batteries. Polymer 2016, 107, 54–60. [Google Scholar] [CrossRef]
- Hong, X.J.; Song, C.L.; Yang, Y.; Tan, H.C.; Li, G.H.; Cai, Y.P.; Wang, H. Cerium Based Metal-Organic Frameworks as an Efficient Separator Coating Catalyzing the Conversion of Polysulfides for High Performance Lithium-Sulfur Batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef]
- Ali, S.; Tan, C.; Waqas, M.; Lv, W.; Wei, Z.; Wu, S.; Goodenough, J.B. Highly Efficient PVDF-HFP/Colloidal Alumina Composite Separator for High-Temperature Lithium-Ion Batteries. Adv. Mater. Interfaces 2018, 5, 1701147. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Farooqui, U.R.; Hamid, N.A. Porous (PVDF-HFP/PANI/GO) ternary hybrid polymer electrolyte membranes for lithium-ion batteries. RSC Adv. 2018, 8, 25725–25733. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Kim, B.; Nam, S.; Lee, H.J.; Chung, H.; Cho, S.; Kang, C. Cross-linked poly (vinylidenefluoride-co-hexafluoropropene)(PVDF-co-HFP) gel polymer electrolyte for flexible Li-ion battery integrated with organic light emitting diode (OLED). Materials 2018, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Ma, Y.; Yang, W. Improved performance of lithium ion battery separator enabled by co-electrospinnig polyimide/poly (vinylidene fluoride-co-hexafluoropropylene) and the incorporation of TiO2-(2-hydroxyethyl methacrylate). J. Power Sources 2015, 273, 1127–1135. [Google Scholar] [CrossRef]
- Rana, M.; Li, M.; Huang, X.; Luo, B.; Gentle, I.; Knibbe, R. Recent advances in separators to mitigate technical challenges associated with re-chargeable lithium sulfur batteries. J. Mater. Chem. A 2019, 7, 6596–6615. [Google Scholar] [CrossRef]
- Hwang, K.; Sohn, H.; Yoon, S. Mesostructured niobium-doped titanium oxide-carbon (Nb-TiO2-C) composite as an anode for high-performance lithium-ion batteries. J. Power Sources 2018, 378, 225–234. [Google Scholar] [CrossRef]
- Yang, C.L.; Liu, H.Y.; Xia, Q.L.; Li, Z.H.; Xiao, Q.Z.; Lei, G.T. Effects of SiO2 Nanoparticles and Diethyl Carbonate on the Electrochemical Properties of a Fibrous Nanocomposite Polymer Electrolyte for Rechargeable Lithium Batteries. Arab. J. Sci. Eng. 2014, 39, 6711–6720. [Google Scholar] [CrossRef]
- Lee, J.; Lee, C.L.; Park, K.; Kim, I.D. Synthesis of an Al2O3-coated polyimide nanofiber mat and its electrochemical characteristics as a separator for lithium ion batteries. J. Power Sources 2014, 248, 1211–1217. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ahn, C.H.; Lee, M.B.; Choi, M.S. Characteristics of electrospun PVDF/SiO2 composite nanofiber membranes as polymer electrolyte. Mater. Chem. Phys. 2011, 127, 137–142. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, X.; Cao, C.; Li, J.; Zhu, Y. Poly (vinylidene fluoride)/SiO2 composite membranes prepared by electrospinning and their excellent properties for nonwoven separators for lithium-ion batteries. J. Power Sources 2014, 251, 423–431. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Zhang, L.; Cao, H.; Wang, Y.; Yao, J. Optimal spinneret layout in Von Koch curves of fractal theory based needleless electrospinning process. AIP ADV 2016, 6, 230. [Google Scholar] [CrossRef]
- Pi, J.K.; Wu, G.P.; Yang, H.C.; Arges, C.G.; Xu, Z.K. Separators with biomineralized zirconia coatings for enhanced thermo- and electro-performance of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 21971–21978. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Ma, Y.; Liu, J.; Liu, X. Improved performance of PVdF-HFP/PI nanofiber membrane for lithium ion battery separator prepared by a bicomponent cross-electrospinning method. Mater. Lett. 2014, 133, 67–70. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Ultrafine polyacrylonitrile/silica composite fibers via electrospinning. Mater. Lett. 2008, 62, 2161–2164. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Chen, C.; Zhang, X. Fabrication and characterization of SiO2/PVDF composite nanofiber-coated PP nonwoven separators for lithium-ion batteries. J. Polym. Sci. Polym. Phys. 2013, 51, 1719–1726. [Google Scholar] [CrossRef]
- Jung, H.R.; Ju, D.H.; Lee, W.J.; Zhang, X.; Kotek, R. Electrospun hydrophilic fumed silica/polyacrylonitrile nanofiber-based composite electrolyte membranes. Electrochim. Acta 2009, 54, 3630–3637. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Huang, S.; He, X.; Yang, P.; Wu, D.; Zhao, J. Functional separator consisted of polyimide nonwoven fabrics and polyethylene coating layer for lithium-ion batteries. J. Power Sources 2015, 298, 158–165. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, N.; Cao, Q.; Jing, B.; Wang, X.; Wang, Q.; Kuang, H. A novel electrospun PVDF/PMMA gel polymer electrolyte with in situ TiO2 for Li-ion batteries. Solid State Ion. 2013, 249, 93–97. [Google Scholar] [CrossRef]
- Wang, Q.; Song, W.L.; Wang, L.; Song, Y.; Shi, Q.; Fan, L.Z. Electrospun polyimide-based fiber membranes as polymer electrolytes for lithium-ion batteries. Electrochim. Acta 2014, 132, 538–544. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Jin, X.; Huang, Z.; Kang, F. The high performances of SiO2/Al2O3-coated electrospun polyimide fibrous separator for lithium-ion battery. J. Membr. Sci. 2015, 493, 1–7. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Dirican, M.; Fu, K.; Zhang, X. Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques. J. Membr. Sci. 2014, 456, 57–65. [Google Scholar] [CrossRef]
- Liu, W.; Chen, J.; Chen, Z.; Liu, K.; Zhou, G.; Sun, Y.; Cui, Y. Stretchable Lithium-ion batteries enabled by device-scaled wavy structure and elastic-sticky separator. Adv. Energy Mater. 2017, 7, 1701076. [Google Scholar] [CrossRef]
- Yang, C.; Tong, H.; Luo, C.; Yuan, S.; Chen, G.; Yang, Y. Boehmite particle coating modified microporous polyethylene membrane: A promising separator for lithium ion batteries. J. Power Sources 2017, 348, 80–86. [Google Scholar] [CrossRef]
- Kundu, M.; Costa, C.M.; Dias, J.; Maceiras, A.; Vilas, J.L.; Lanceros-Méndez, S. On the relevance of the polar β-phase of poly (vinylidene fluoride) for high performance lithium-ion battery separators. J. Phys. Chem. C 2017, 121, 26216–26225. [Google Scholar] [CrossRef]
- Li, Z.; Cao, T.; Zhang, Y.; Han, Y.; Xu, S.; Xu, Z. Novel lithium ion battery separator based on hydroxymethyl functionalized poly (ether ether ketone). J. Membr. Sci. 2017, 540, 422–429. [Google Scholar] [CrossRef]


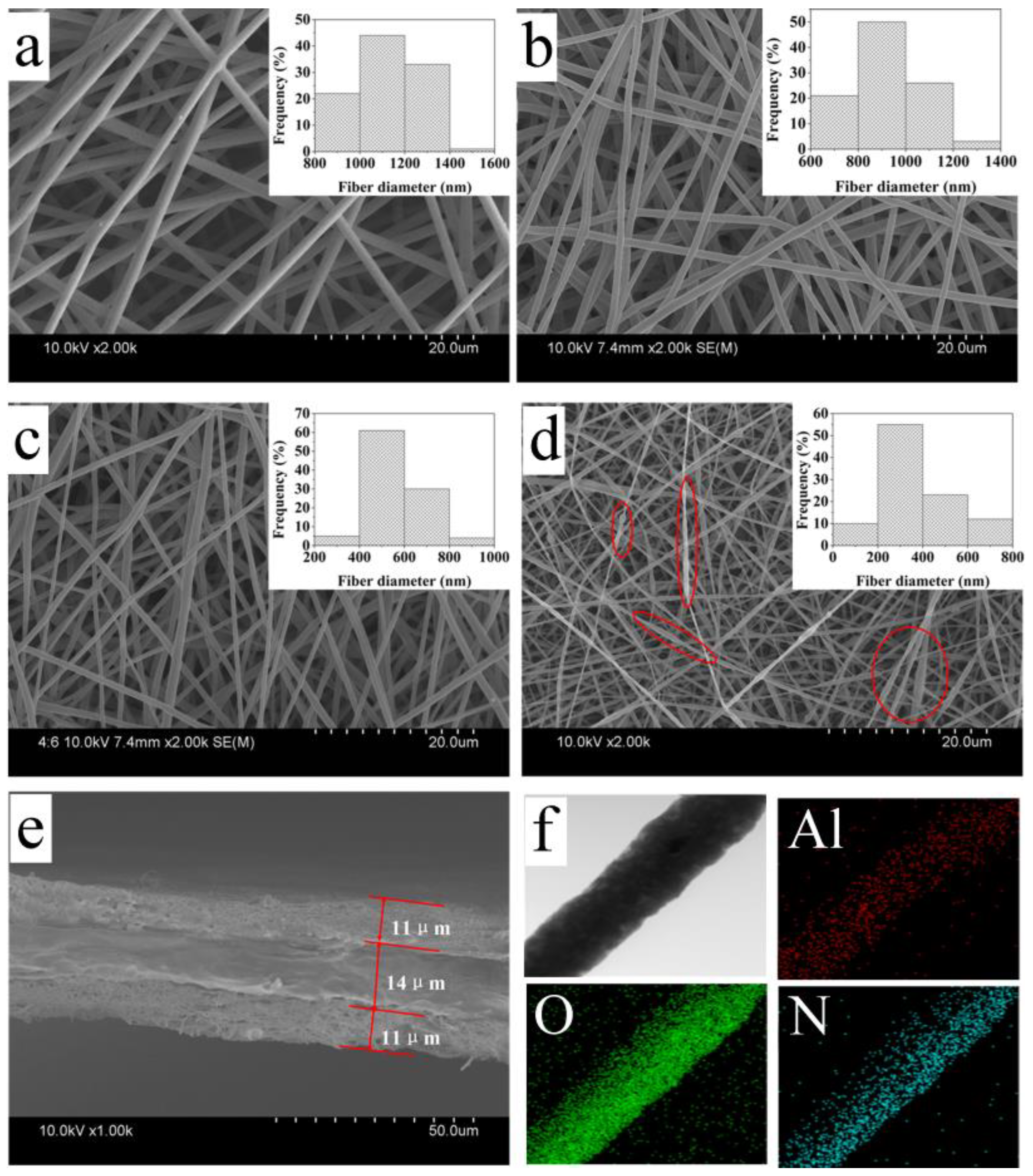


| Al2O3 % Against PI (by wt.) | Conductivity (μm/cm) | Viscosity (Pa·s) | Nanofiber Diameter | |
|---|---|---|---|---|
| Average (nm) | CV % | |||
| 0 | 1.37 | 1.29 | 1125 | 12.17 |
| 1 | 15.68 | 0.92 | 914 | 14.17 |
| 2 | 19.49 | 0.59 | 524 | 20.45 |
| 3 | 35.82 | 0.25 | 401 | 51.56 |
| Sample ID | PP/PE/PP | PI | AP | APEAP |
|---|---|---|---|---|
| Porosity (%) | 39 | 64 | 78 | 72 |
| Electrolyte uptake (%) | 92 | 467 | 611 | 592 |
| Contact angle (°) | 53 | 24 | 19 | 19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Liu, Y.; Hu, X.; Yao, J.; Chen, Z.; Hao, M.; Tian, W.; Huang, Z.; Li, F. Multilayer Nanofiber Composite Separator for Lithium-Ion Batteries with High Safety. Polymers 2019, 11, 1671. https://doi.org/10.3390/polym11101671
Yang W, Liu Y, Hu X, Yao J, Chen Z, Hao M, Tian W, Huang Z, Li F. Multilayer Nanofiber Composite Separator for Lithium-Ion Batteries with High Safety. Polymers. 2019; 11(10):1671. https://doi.org/10.3390/polym11101671
Chicago/Turabian StyleYang, Wenxiu, Yanbo Liu, Xuemin Hu, Jinbo Yao, Zhijun Chen, Ming Hao, Wenjun Tian, Zheng Huang, and Fangying Li. 2019. "Multilayer Nanofiber Composite Separator for Lithium-Ion Batteries with High Safety" Polymers 11, no. 10: 1671. https://doi.org/10.3390/polym11101671




