Side Chain Effect of Hydroxypropyl Cellulose Derivatives on Reflection Properties
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
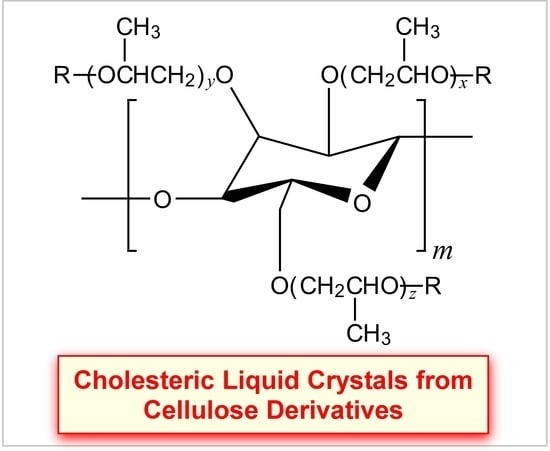
3.1. Syntheses of HPC Derivatives
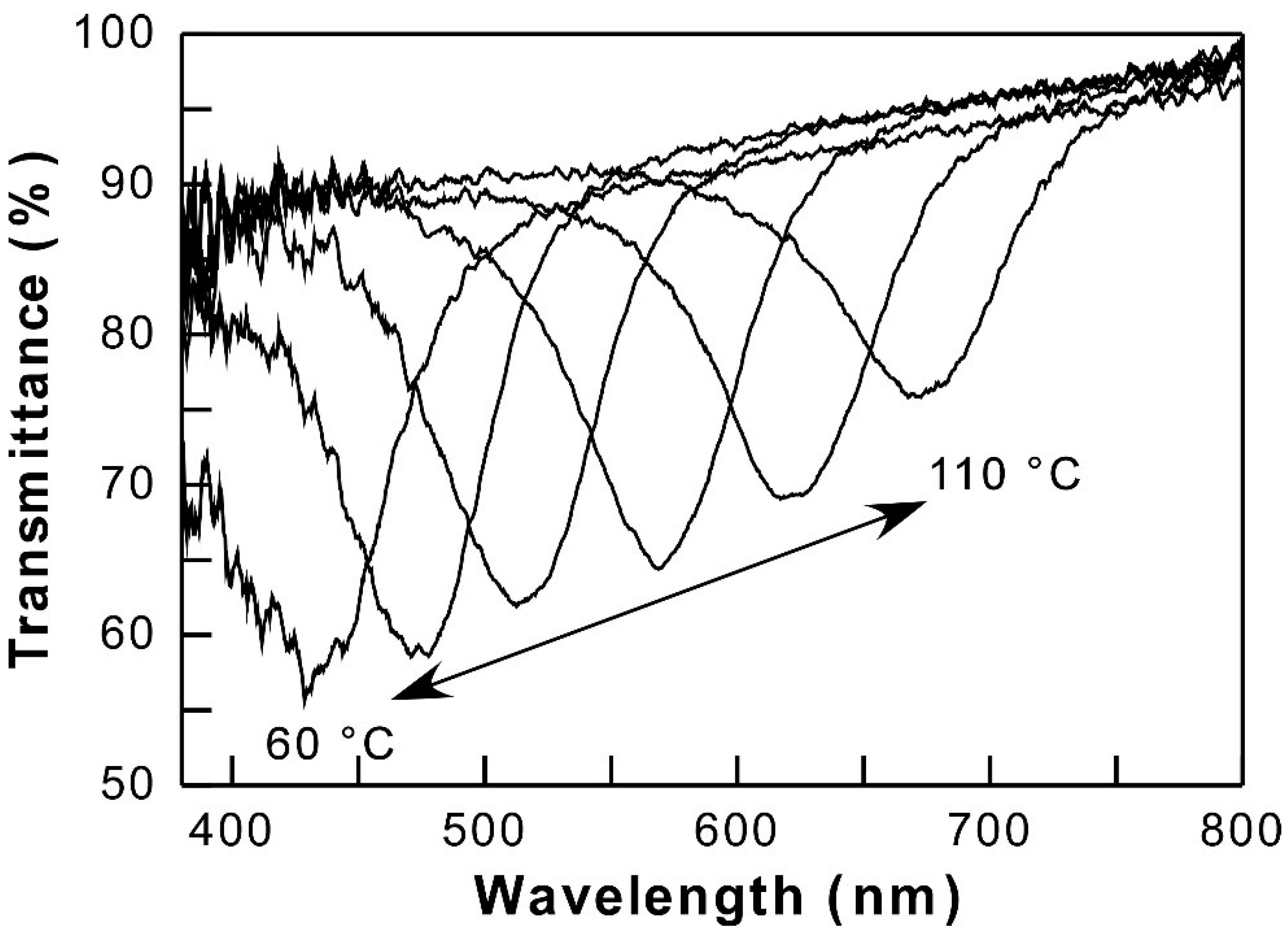
3.2. Reflection Properties of Thermotropic HPC Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Robinson, C. Liquid‒Crystalline Structures in Solutions of a Polypeptide. Trans. Faraday Soc. 1956, 52, 571–592. [Google Scholar] [CrossRef]
- Gray, G.W.; McDonnell, D.G. The Relationship between Helical Twist Sense, Absolute Configuration and Molecular Structure for Non-Sterol Cholesteric Liquid Crystals. Mol. Cryst. Liq. Cryst. 1976, 34, 211–217. [Google Scholar] [CrossRef]
- De Vries, H. Rotatory Power and Other Optical Properties of Certain Liquid Crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef]
- Furumi, S. Recent Progress in Chiral Photonic Band-Gap Liquid Crystals for Laser Applications. Chem. Rec. 2010, 10, 394–408. [Google Scholar] [CrossRef]
- Tseng, S.-L.; Laivins, G.V.; Gray, D.G. Propanoate Ester of (2-Hydroxypropyl)cellulose: A Thermotropic Cholesteric Polymer That Reflects Visible Light at Ambient Temperatures. Macromolecules 1982, 15, 1262–1264. [Google Scholar] [CrossRef]
- Bhadani, S.N.; Tseng, S.-L.; Gray, D.G. Lyotropic and Thermotropic Liquid-Crystalline Phase Formation from Fractions of a Semiflexible Cellulosic Polymer. Makromol. Chem. 1983, 184, 1727–1740. [Google Scholar] [CrossRef]
- Bhadani, S.N.; Gray, D.G. Cellulose-Based Liquid Crystalline Polymers; Esters of (Hydroxypropyl) Cellulose. Mol. Cryst. Liq. Cryst. 1983, 99, 29–38. [Google Scholar] [CrossRef]
- Steinmeier, H.; Zugenmaier, P. Formation of Liquid-Crystalline Phases by Two Phenyl-Alkanoyl Esters of o-(Hydroxypropyl)cellulose and the (3-Chlorophenyl)urethane of Cellulose. Carbphydr. Res. 1988, 173, 75–88. [Google Scholar] [CrossRef]
- Ritcey, A.M.; Gray, D.G. Circular Reflectivity from the Cholesteric Liquid Crystalline Phase of (2-Ethoxyprpoyl)cellulose. Macromolecules 1988, 21, 1251–1255. [Google Scholar] [CrossRef]
- Yamagishi, T.A.; Guittard, F.; Godinho, M.H.; Martins, A.F.; Cambon, A.; Sixou, P. Comparison of Thermal and Cholesteric Mesophase Properties among the Three Kind of Hydroxypropylcellulose (HPC) Derivatives. Polym. Bull. 1994, 32, 47–54. [Google Scholar] [CrossRef]
- Kosho, H.; Hiramatsu, S.; Nishi, T.; Tanaka, Y.; Kawaguchi, S.; Watanabe, J. Thermotropic Cholesteric Liquid Crystals in Ester Derivatives of Hydroxypropylcellulose. High. Perform. Polym. 1999, 11, 41–48. [Google Scholar] [CrossRef]
- Yamagishi, T.A.; Nakamoto, Y.; Sixou, P. Preparation and Cholesteric Mesophase Properties of (Butyl-co-pentyl)propylcellulose. Cellulose 2006, 12, 205–211. [Google Scholar] [CrossRef]
- Huang, B.; Ge, J.J.; Li, Y.; Hou, H. Aliphatic Acid Esters of (2-Hydroxypropyl)cellulose‒Effect of Side Chain Length on Properties of Cholesteric Liquid Crystals. Polymer 2007, 48, 264–269. [Google Scholar] [CrossRef]
- Ishizaki, T.; Uenuma, S.; Furumi, S. Thermotropic Properties of Cholesteric Liquid Crystal from Hydroxypropyl Cellulose Mixed Esters. Kobunshi Ronbunshu 2015, 72, 737–745. (In Japanese) [Google Scholar] [CrossRef]
- Kawaguchi, A.; Aoki, R.; Hayata, K.; Furukawa, M.; Fukawa, M.; Furumi, S. Fabrication of Human-Friendly Liquid Crystal Materials with α-Ionone. J. Photopolym. Sci. Technol. 2019, 32, 639–643. [Google Scholar]
- Aoki, R.; Fukawa, M.; Furumi, S. Preparation of the Color Films from Cellulose Derivatives in a Diacrylate Liquid. J. Photopolym. Sci. Technol. 2019, 32, 651–656. [Google Scholar]
- Fukawa, M.; Kawaguchi, A.; Hayata, K.; Aoki, R.; Furukawa, M.; Furumi, S. Syntheses and Properties of Cellulosic Derivatives for Reflection Color Films. J. Photopolym. Sci. Technol. 2019, 32, 633–637. [Google Scholar] [Green Version]
- Hayata, K.; Suzuki, T.; Fukawa, M.; Furumi, S. Thermotropic Cholesteric Liquid Crystals from Cellulose Derivatives with Ester and Carbamate Groups. J. Photopolym. Sci. Technol. 2019, 32, 645–649. [Google Scholar] [Green Version]
- Miyagi, K.; Teramoto, Y. Function Extension of Dual-Mechanochromism of Acylated Hydroxypropylcellulose/Synthetic Polymer Composites Achieved by “Moderate” Compatibility as well as Hydrogen Bonding. Polymer 2019, 174, 150–158. [Google Scholar] [CrossRef]
- Müller, M.; Zentel, R. Cholesteric Phases and Films from Cellulose Derivatives. Macromol. Chem. Phys. 2000, 201, 2055–2063. [Google Scholar]
- Ho, F.F.L.; Kohler, R.R.; Ward, G.A. Determination of Molar Substitution and Degree of Substitution of Hydroxypropyl Cellulose by Nuclear Magnetic Resonance Spectrometry. Anal. Chem. 1972, 44, 178–181. [Google Scholar] [CrossRef]


| HPC Derivatives | Modification Degrees (PrE:TFMPC) | k (nm/°C) |
|---|---|---|
| HPC-PrE | 2.98:0 | 6.96 |
| HPC-PrE/m-TFMPC | 2.62:0.31 | 5.70 |
| HPC-PrE/m-TFMPC | 2.36:0.56 | 5.21 |
| HPC-PrE/p-TFMPC | 2.59:0.32 | 4.76 (5.65) * |
| HPC-PrE/p-TFMPC | 2.49:0.48 | 4.99 |
| HPC-PrE/b-TFMPC | 2.71:0.29 | 5.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayata, K.; Furumi, S. Side Chain Effect of Hydroxypropyl Cellulose Derivatives on Reflection Properties. Polymers 2019, 11, 1696. https://doi.org/10.3390/polym11101696
Hayata K, Furumi S. Side Chain Effect of Hydroxypropyl Cellulose Derivatives on Reflection Properties. Polymers. 2019; 11(10):1696. https://doi.org/10.3390/polym11101696
Chicago/Turabian StyleHayata, Kenichiro, and Seiichi Furumi. 2019. "Side Chain Effect of Hydroxypropyl Cellulose Derivatives on Reflection Properties" Polymers 11, no. 10: 1696. https://doi.org/10.3390/polym11101696