A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Thin Films for Optical Measurements and PLQYs Setup
2.3. Synthesis of Ligands LR, LG and LB
2.4. Synthesis of Metallopolymers PR, PG, PB and PW
2.5. Conductivity Measurements
3. Results and Discussion
3.1. Ligands LR, LG and LB
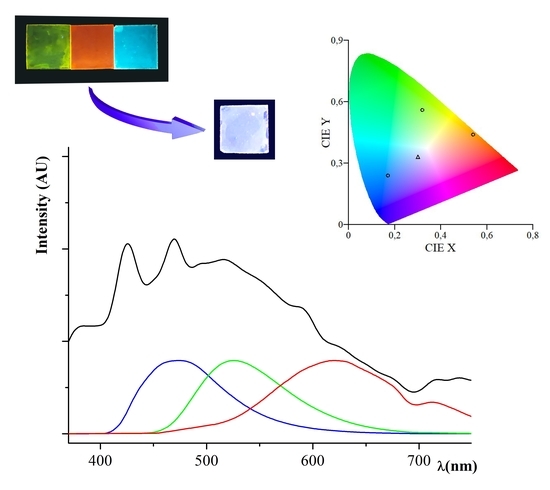
3.2. Polymers PR, PG, PB and PW
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Frohleiks, J.; Wepfer, S.; Bacher, G.; Nannen, E. Realization of red iridium-based ionic transition metal complex light-emitting electrochemical cells (iTMC-LECs) by interface-induced color shift. Acs Appl. Mater. Inter. 2019, 11, 22612–22620. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.R.; Koh, C.Y.; Slinker, J.D.; Flores-Torres, S.; Abruña, H.D.; Malliaras, G.G. Addition of a Phosphorescent Dopant in Electroluminescent Devices from Ionic Transition Metal Complexes. Chem. Mater. 2005, 17, 6114–6116. [Google Scholar] [CrossRef]
- Carlson, B.; Phelan, G.D.; Jiang, X.; Kaminsky, W.; Jen, A.K.Y.; Dalton, L.R. Organic light emitting devices based upon divalent osmium complexes: Part 1: design, synthesis, and characterization of osmium complexes. In Organic Light-Emitting Materials and Devices VI; International Society for Optics and Photonics: Seattle, WA, USA, 2003; Volume 4800, pp. 93–104. [Google Scholar] [CrossRef]
- Tian, M.; Li, P.; Wang, Z.; Li, Z.; Cheng, J.; Sun, Y.; Wang, C.; Teng, X.; Yang, Z.; Teng, F. Controlling multi luminescent centers via anionic polyhedron substitution to achieve single Eu2+ activated high-color-rendering white light/tunable emissions in single-phased Ca2(BO3)1−x(PO4) Cl phosphors for ultraviolet converted LEDs. Chem. Eng. J. 2017, 326, 667–679. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, S.; Rajendar, V.; Park, S.-H. Fabrication of ZnO thin films by sol–gel spin coating and their UV and white-light emission properties. J. Electron. Mater. 2017, 46, 6029–6037. [Google Scholar] [CrossRef]
- He, Z.; Zhao, W.; Lam, J.W.Y.; Peng, Q.; Ma, H.; Liang, G.; Shuai, Z.; Tang, B.Z. White light emission from a single organic molecule with dual phosphorescence at room temperature. Nat. Commun. 2017, 8, 416. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Holmes, R.J.; Forrest, S.R. Efficient organic electrophosphorescent White-Light-Emitting Device with a Triple Doped Emissive Layer. Adv. Mater. 2004, 16, 624–628. [Google Scholar] [CrossRef]
- Gong, X.; Ma, W.; Ostrowski, J.C.; Bazan, G.C.; Moses, D.; Heeger, A.J. White electrophosphorescence from Semiconducting Polymer Blends. Adv. Mater. 2004, 16, 615–619. [Google Scholar] [CrossRef]
- Cheng, G.; Li, F.; Duan, Y.; Feng, J.; Liu, S.; Qiu, S.; Lin, D.; Ma, Y.; Lee, S.T. White organic light-emitting devices using a phosphorescent sensitizer. Appl. Phys. Lett. 2003, 82, 4224–4226. [Google Scholar] [CrossRef]
- Gill, R.E.; van Hutten, P.F.; Meetsma, A.; Hadziioannou, G. Synthesis and Crystal Structure of a Cyano-Substituted Oligo(p-phenylenevinylene). Chem. Mater. 1996, 8, 1341–1346. [Google Scholar] [CrossRef]
- Caruso, U.; Diana, R.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Facile synthesis of new Pd(II) and Cu(II) based metallomesogens from ligands containing thiophene rings. Inorg. Chem. Commun. 2009, 12, 1135–1138. [Google Scholar] [CrossRef]
- Xin, Y.; Shen, W.; Deng, Z.; Zhang, J. Highly Emissive and Color-Tunable Perovskite Cross-linkers for Luminescent Polymer Networks. ACS Appl. Mater. Interfaces 2018, 10, 28971–28978. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Luo, H.; Shi, G.; Yang, J.; Chi, Z.; Ma, Y. Luminescent network film deposited electrochemically from a carbazole functionalized AIE molecule and its application for OLEDs. J. Mater. Chem. C 2015, 3, 3752–3759. [Google Scholar] [CrossRef]
- Li, M.; Tang, S.; Shen, F.; Liu, M.; Xie, W.; Xia, H.; Liu, L.; Tian, L.; Xie, Z.; Lu, P.; et al. Highly luminescent network films from electrochemical deposition of peripheral carbazole functionalized fluorene oligomer and their applications for light-emitting diodes. Chem. Commun. 2006, 32, 3393–3395. [Google Scholar] [CrossRef] [PubMed]
- Roviello, A.; Borbone, F.; Carella, A.; Diana, R.; Roviello, G.; Panunzi, B.; Ambrosio, A.; Maddalena, P. High quantum yield photoluminescence of new polyamides containing oligo-PPV amino derivatives and related oligomers. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2677–2689. [Google Scholar] [CrossRef]
- Costa, R.D.; Orti, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Panunzi, B.; Borbone, F.; Capobianco, A.; Concilio, S.; Diana, R.; Peluso, A.; Piotto, S.; Tuzi, A.; Velardo, A.; Caruso, U. Synthesis, spectroscopic properties and DFT calculations of a novel multipolar azo dye and its zinc(II) complex. Inorg. Chem. Commun. 2017, 84, 103–108. [Google Scholar] [CrossRef]
- Kwon, S.K.; Lee, Y.K.; Park, T.J.; Uchiike, H.; Kwon, J.H.; Jang, J.; Jin, J.K.; Shin, D.C.; You, H. High-Efficiency White Polymer Light-Emitting Diodes Based on Blended RGB Polymers. Mol. Cryst. Liq. Cryst. 2006, 458, 263–272. [Google Scholar] [CrossRef]
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)‒Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716. [Google Scholar] [CrossRef]
- Di Marcantonio, M.; Vollkommer, F.; Bacher, G.; Nannen, E. A light-emitting electrochemical cell (LEC) containing a hole-blocking layer of TmPyPB. J. Mater. Chem. C 2018, 6, 9742–9748. [Google Scholar] [CrossRef]
- Diana, R.; Caruso, U.; Concilio, S.; Piotto, S.; Tuzi, A.; Panunzi, B. A real-time tripodal colorimetric/fluorescence sensor for multiple target metal ions. Dyes Pigm. 2018, 155, 249–257. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Failla, S.; Malandrino, G.; Bella, S.D. New molecular architectures by aggregation of tailored zinc(ii) Schiff-base complexes. New J. Chem. 2011, 35, 2826–2831. [Google Scholar] [CrossRef]
- Nishikitani, Y.; Cho, T.; Uchida, S.; Nishimura, S.; Oyaizu, K.; Nishide, H. Polymer-based white-light-emitting electrochemical cells with very high color-rendering index based on blue-green fluorescent polyfluorenes and red-phosphorescent iridium complexes. ChemPlusChem 2018, 83, 463–469. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Piotto, S.; Shikler, R.; Tuzi, A.; Panunzi, B. From cadmium(II)-aroylhydrazone complexes to metallopolymers with enhanced photoluminescence. A structural and DFT study. Inorg. Chim. Acta 2017, 458, 129–137. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Lin, Z.; Ling, Q. White-light hydrotalcite-like compound emission from the incorporation of red-, green-, and blue-emitting metal complexes. Opt. Mater. Express 2012, 3, 105–113. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, W.; Chen, R.; Wang, Z.; Yan, Q.; Miao, Y.; Jia, H.; Xu, B.; Wong, W.-Y. Program controlling the emission color of blend polymer phosphors containing Eu(III), Tb(III), Be(II) ions for WLEDs. Opt. Mater. 2019, 89, 250–260. [Google Scholar] [CrossRef]
- Farinella, F.; Maini, L.; Mazzeo, P.P.; Fattori, V.; Monti, F.; Braga, D. White luminescence achieved by a multiple thermochromic emission in a hybrid organic-inorganic compound based on 3-picolylamine and copper(i) iodide. Dalton Trans. 2016, 45, 17939–17947. [Google Scholar] [CrossRef]
- Lin, R.B.; Liu, S.Y.; Ye, J.W.; Li, X.Y.; Zhang, J.P. Photoluminescent metal-organic frameworks for gas sensing. Adv. Sci. 2016, 3, 1500434. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5513–5560. [Google Scholar] [CrossRef] [Green Version]
- Mendiratta, S.; Lee, C.H.; Usman, M.; Lu, K.L. Metal-organic frameworks for electronics: emerging second order nonlinear optical and dielectric materials. Sci. Technol. Adv. Mater. 2015, 16, 054204. [Google Scholar] [CrossRef] [PubMed]
- Di Serio, M.; Carotenuto, G.; Cucciolito, M.E.; Lega, M.; Ruffo, F.; Tesser, R.; Trifuoggi, M. Shiff base complexes of zinc(II) as catalysts for biodiesel production. J. Mol. Catal. A Chem. 2012, 353, 106–110. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Panunzi, B.; Piotto, S.; Shikler, R.; Tuzi, A. Mono-, Di-, and Polymeric Pyridinoylhydrazone ZnII Complexes: Structure and Photoluminescent Properties. Eur. J. Inorg. Chem. 2016, 2016, 818–825. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Palma, S.D.; Fusco, S.; Nabha, S.; Panunzi, B.; Shikler, R. High solid state photoluminescence quantum yields and effective color tuning in polyvinylpyridine based zinc(II) metallopolymers. Macromol. Chem. Phys. 2015, 216, 1516–1522. [Google Scholar] [CrossRef]
- Meier, S.B.; Tordera, D.; Pertegás, A.; Roldán-Carmona, C.; Ortí, E.; Bolink, H.J. Light-emitting electrochemical cells: recent progress and future prospects. Mater. Today 2014, 17, 217–223. [Google Scholar] [CrossRef]
- Fresta, E.; Costa, R.D. Beyond traditional light-emitting electrochemical cells—A review of new device designs and emitters. J. Mater. Chem. C 2017, 5, 5643–5675. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, L.; Liang, J.; Gao, H.; Chou, S.; Pei, Q. Efficient white polymer light-emitting electrochemical cells. Mater. Horiz. 2015, 2, 338–343. [Google Scholar] [CrossRef]
- Vak, D.; Oh, S.-H.; Kim, D.-Y. Efficient single-component light-emitting electrochemical cells with an ion-conducting water-soluble polyfluorene. Appl. Phys. Lett. 2009, 94, 243305. [Google Scholar] [CrossRef]
- Mandal, S.S.; Varshney, U.; Bhattacharya, S. Role of the central metal ion and ligand charge in the DNA binding and modification by metallosalen complexes. Bioconjugate Chem. 1997, 8, 798–812. [Google Scholar] [CrossRef]
- Piotto, S.; Concilio, S.; Sessa, L.; Diana, R.; Torrens, G.; Juan, C.; Caruso, U.; Iannelli, P. Synthesis and Antimicrobial Studies of New Antibacterial Azo-Compounds Active against Staphylococcus aureus and Listeria monocytogenes. Molecules 2017, 22, 1372. [Google Scholar] [CrossRef]
- Oliveri, I.P.; Failla, S.; Colombo, A.; Dragonetti, C.; Righetto, S.; Di Bella, S. Synthesis, characterization, optical absorption/fluorescence spectroscopy, and second-order nonlinear optical properties of aggregate molecular architectures of unsymmetrical Schiff-base zinc(II) complexes. Dalton Trans. 2014, 43, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B.; Tuzi, A.; Piotto, S.; Concilio, S.; Caruso, U. An amphiphilic pyridinoyl-hydrazone probe for colorimetric and fluorescence pH sensing. Molecules 2019, submitted. [Google Scholar]
- Feng, W.-X.; Yin, S.-Y.; Pan, M.; Wang, H.-P.; Fan, Y.-N.; Lü, X.-Q.; Su, C.-Y. PMMA-copolymerized color tunable and pure white-light emitting Eu3+–Tb3+ containing Ln-metallopolymers. J. Mater. Chem. C 2017, 5, 1742–1750. [Google Scholar] [CrossRef]
- Argeri, M.; Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Color tuning and noteworthy photoluminescence quantum yields in crystalline mono-/dinuclear ZnII complexes. Eur. J. Inorg. Chem. 2014, 2014, 5916–5924. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Causà, M.; Fusco, S.; Panunzi, B.; Roviello, A.; Shikler, R.; Tuzi, A. Series of O,N,O-tridentate ligands zinc(II) complexes with high solid-state photoluminescence quantum yield. Eur. J. Inorg. Chem. 2014, 2014, 2695–2703. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Tuzi, A.; Piotto, S.; Caruso, U. Fluorescence pH-dependent sensing of Zn(II) by a tripodal ligand. A comparative X-ray and DFT study. J. Lumin. 2019, 212, 200–206. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Tuzi, A.; Caruso, U. Two tridentate pyridinyl-hydrazone zinc(II) complexes as fluorophores for blue emitting layers. J. Mol. Struct. 2019, 1197, 672–680. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, A.; Tingoli, M.; Tuzi, A. Two aminobenzothiazole derivatives for Pd(II) and Zn(II) coordination: Synthesis, characterization and solid state fluorescence. Inorg. Chem. Commun. 2011, 14, 46–48. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: from mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef] [Green Version]
- Taraba, L.; Krizek, T.; Kozlik, P.; Hodek, O.; Coufal, P. Protonation of polyaniline-coated silica stationary phase affects the retention behavior of neutral hydrophobic solutes in reversed-phase capillary liquid chromatography. J. Sep. Sci. 2018, 41, 2886–2894. [Google Scholar] [CrossRef]
- Hou, S.-S.; Fan, N.-S.; Tseng, Y.-C.; Jan, J.-S. Self-assembly and hydrogelation of coil–sheet poly(l-lysine)-block-poly(l-threonine) Block Copolypeptides. Macromolecules 2018, 51, 8054–8063. [Google Scholar] [CrossRef]
- Shu, W.; Guan, C.; Guo, W.; Wang, C.; Shen, Y. Conjugated poly(aryleneethynylenesiloles) and their application in detecting explosives. J. Mater. Chem. 2012, 22, 3075–3081. [Google Scholar] [CrossRef]
- Sivalingam, S.; Debsharma, K.; Dasgupta, A.; Sankararaman, S.; Prasad, E. Effect of Slip-Stack Self-Assembly on Aggregation-Induced Emission and Solid-State Luminescence in 1,3-Diarylpropynones. ChemPlusChem 2019, 84, 392–402. [Google Scholar] [CrossRef]
- Borbone, F.; Tuzi, A.; Panunzi, B.; Piotto, S.; Concilio, S.; Shikler, R.; Nabha, S.; Centore, R. On-off mechano-responsive switching of ESIPT luminescence in polymorphic n-salicylidene-4-amino-2-methylbenzotriazole. Cryst. Growth Des. 2017, 17, 5517–5523. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers 2019, 11, 1379. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Caruso, U.; Piotto, S. Solid-state highly efficient dr mono and poly-dicyano-phenylenevinylene fluorophores. Molecules 2018, 23, 1505. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Piotto, S. AIE/ACQ effects in two DR/NIR emitters: A structural and DFT comparative analysis. Molecules 2018, 23, 1947. [Google Scholar] [CrossRef]
- Liu, C.-F.; Jiu, Y.; Wang, J.; Yi, J.; Zhang, X.-W.; Lai, W.-Y.; Huang, W. Star-shaped single-polymer systems with simultaneous RGB emission: design, synthesis, saturated shite electroluminescence, and amplified spontaneous emission. Macromolecules 2016, 49, 2549–2558. [Google Scholar] [CrossRef]
- Fan, C.; Li, Y.; Yang, C.; Wu, H.; Qin, J.; Cao, Y. Phosphoryl/sulfonyl-substituted iridium complexes as blue phosphorescent emitters for single-layer blue and white organic light-Emitting diodes by solution process. Chem. Mater. 2012, 24, 4581–4587. [Google Scholar] [CrossRef]
- Arasu, V.; Jo, D.; Chae, H.; Chung, H.K.; Park, S.H. Configuration- and concentration-dependent hybrid white light generation using red, green, and blue quantum dots embedded in DNA thin films. Nanoscale Adv. 2019, 1, 602–612. [Google Scholar] [CrossRef]
- Gautier, R.; Massuyeau, F.; Galnon, G.; Paris, M. Lead halide post-perovskite-type chains for high-efficiency white-light emission. Adv. Mater. 2019, 31, 1807383. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Chen, S.; Wang, T.; Yu, D.; Peng, C.; Liu, H.; Hu, Y. Density, viscosity and electrical conductivity of 1-butyl-3-methylimidazolium hexafluorophosphate + monoethanolamine and + N, N-dimethylethanolamine. J. Mol. Liq. 2008, 143, 100–108. [Google Scholar] [CrossRef]
- Chandran, M.; Kwon, Y.; Choe, Y. Light Emitting Electrochemical Cells Based on Ionic Iridium Complexes and Ionic Conductor Blend as the Active Layer. Mol. Cryst. Liq. Cryst. 2014, 601, 173–181. [Google Scholar] [CrossRef]
- Shanmugasundaram, K.; Subeesh, M.S.; Sunesh, C.D.; Chitumalla, R.K.; Jang, J.; Choe, Y. Synthesis and photophysical characterization of an ionic fluorene derivative for blue light-emitting electrochemical cells. Org. Electron. 2015, 24, 297–302. [Google Scholar] [CrossRef]
- Di Marcantonio, M.; Namanga, J.E.; Smetana, V.; Gerlitzki, N.; Vollkommer, F.; Mudring, A.V.; Bacher, G.; Nannen, E. Green-yellow emitting hybrid light emitting electrochemical cell. J. Mater. Chem. C 2017, 5, 12062–12068. [Google Scholar] [CrossRef]
- Sakanoue, T.; Li, J.; Tanaka, H.; Ito, R.; Ono, S.; Kuroda, S.I.; Takenobu, T. High Current Injection into Dynamic p-n Homojunction in Polymer Light-Emitting Electrochemical Cells. Adv. Mater. 2017, 29, 1606392. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Epstein, A.J. Interface Control of Light-Emitting Devices Based on Pyridine-Containing Conjugated Polymers. Acc. Chem. Res. 1999, 32, 217–224. [Google Scholar] [CrossRef]
- Maruyama, S.; Prastiawan, I.B.H.; Toyabe, K.; Higuchi, Y.; Koganezawa, T.; Kubo, M.; Matsumoto, Y. Ionic conductivity in ionic liquid nano thin films. ACS Nano 2018, 12, 10509–10517. [Google Scholar] [CrossRef]
- Watanabe, M.; Mizumura, T. Conductivity study on ionic liquid/polymer complexes. Solid State Ionics 1996, 86, 353–356. [Google Scholar] [CrossRef]




| Compound | Mp (°C) (a) | Tg (°C) (b) | Td (°C) (c) | %ZnOt (d) | %ZnOex (e) |
|---|---|---|---|---|---|
| LB | 262 | - | 310 | - | - |
| LG | 290 | - | 302 | - | - |
| LR | 263 | - | 300 | - | - |
| PB | - | 172 | 340 | 1.66 | 1.70 |
| PG | - | 188 | 335 | 1.64 | 1.66 |
| PR | - | 171 | 330 | 1.57 | 1.59 |
| PW | - | 168 | 330 | 1.63 | 1.60 |
| Compound | λabs.sol(nm) (a) | λem.sol(nm) (b) | λabs.film(nm) (c) | λem.film(nm) (d) | PLQY% (e) |
|---|---|---|---|---|---|
| LB | 329 | 411(490) | 330 | 501 | 3.0 ± 0.2 |
| LG | 339 | 512 | 340 | 521 | 5.1 ± 0.2 |
| LR | 346 | 479 | 380 | 570 | 0.7 ± 0.1 |
| PB | 344 | 492 | (316)386 | 474 | 71 ± 2 |
| PG | 343 | 516 | (318)430 | 526 | 82 ± 2 |
| PR | 435 | 502 | (329)435 | 620 | 25 ± 2 |
| PW | 354(broad) | 400–600(weak) | 251(315, 387) | 350–700 | 57 ± 2 |
| Compound | BMIM-PF6 (% wt.) | Conductivity (μS/m) |
|---|---|---|
| PB | 0 | 1.9·10−4 |
| PG | 0 | 4.4·10−3 |
| PR | 0 | 1.5·10−4 |
| PW | 0 | 2.3·10−2 |
| PB | 10 | 5.7·10−2 |
| PG | 10 | 1.3·10−1 |
| PR | 10 | 3.4·10−2 |
| PW | 10 | 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panunzi, B.; Diana, R.; Caruso, U. A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach. Polymers 2019, 11, 1712. https://doi.org/10.3390/polym11101712
Panunzi B, Diana R, Caruso U. A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach. Polymers. 2019; 11(10):1712. https://doi.org/10.3390/polym11101712
Chicago/Turabian StylePanunzi, Barbara, Rosita Diana, and Ugo Caruso. 2019. "A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach" Polymers 11, no. 10: 1712. https://doi.org/10.3390/polym11101712
APA StylePanunzi, B., Diana, R., & Caruso, U. (2019). A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach. Polymers, 11(10), 1712. https://doi.org/10.3390/polym11101712