Study on Preparation and Separation and Adsorption Performance of Knitted Tube Composite β-Cyclodextrin/Chitosan Porous Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Knitted Tube Composite β-CD/CS Porous Membranes
2.3. Characterization
2.3.1. FTIR Analysis
2.3.2. SEM Analysis
2.3.3. Mechanical Properties Test
2.3.4. Contact Angle Test
2.3.5. Water Flux Test
2.3.6. Rejection Rate Test
2.4. Adsorption Experiments
2.5. Adsorption Isotherms
2.6. Adsorption Kinetics
3. Results and Discussion
3.1. FTIR Results
3.2. Structure Characterization of β-CD/CS Porous Membranes
3.3. Mechanical Properties
3.4. Influence of Crosslinking Time on the Hydrophilicity
3.5. Effect of Crosslinking Time on Separation Performance
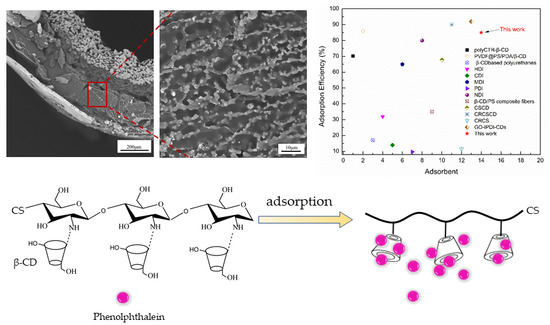
3.6. Analysis of Adsorption Performance of PP by β-CD/CS Porous Membranes
3.6.1. PP Standard Curve
3.6.2. Effect of Crosslinking Time on Adsorption Rate
3.6.3. Effect of Adsorption Time
3.6.4. Effect of Initial Concentration
3.7. Adsorption Isotherm
3.8. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Tong, X.; Zhang, B.; Zhang, C.; Zhang, H.; Chen, Y. Enhanced permeation and antifouling performance of polyvinyl chloride (PVC) blend Pluronic F127 ultrafiltration membrane by using salt coagulation bath (SCB). J. Membr. Sci. 2018, 548, 32–41. [Google Scholar] [CrossRef]
- Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polymers 2019, 11, 1418. [Google Scholar] [CrossRef] [PubMed]
- Tabriz, A.; Alvi, M.; Niazi, M.B.K.; Batool, M.; Bhatti, M.F.; Khan, A.L.; Khan, A.U.; Jamil, T.; Ahmad, N.M. Quaternized trimethyl functionalized chitosan based antifungal membranes for drinking water treatment. Carbo. Polym. 2019, 207, 17–25. [Google Scholar] [CrossRef]
- Wang, M.; Qu, R.; Sun, C.; Niu, Y.; Zhang, Y.; Gao, J.; Cai, H.; Song, X. Synthesis, characterization, and adsorption properties of m-aramid and chitosan hybrid composite films with the ratio of 100/0, 85/15, 65/35, 50/50, and 35/65 toward Hg(II) ions. Desalin. Water Treat. 2019, 146, 197–209. [Google Scholar] [CrossRef]
- Rahmi; Marlina; Nisfayati. Comparison of cadmium adsorption onto chitosan and epichlorohydrin crosslinked chitosan/eggshell composite. IOP Conf. Ser. Mater. Sci. Eng. 2018, 352, 012047. [Google Scholar] [CrossRef]
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions; a review. Int. J. Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Tang, N.; Song, B.; Chen, X.; Cheng, X. Sulfamic acid modified hydrochar derived from sawdust for removal of benzotriazole and Cu(II) from aqueous solution: Adsorption behavior and mechanism. Bioresour. Technol. 2019, 290, 11–14. [Google Scholar] [CrossRef]
- Bandforuzi, S.R.; Hadjmohammadi, M.R. Modified magnetic chitosan nanoparticles based on mixed hemimicelle of sodium dodecyl sulfate for enhanced removal and trace determination of three organophosphorus pesticides from natural waters. Anal. Chim. Acta 2019, 1078, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Qu, L.; Tian, M.; Zhu, S.; Zhang, X.; Tang, X.; Sun, K. Chitosan/Graphene Oxide Composite as an Effective Adsorbent for Reactive Red Dye Removal. Water Environ. Res. 2016, 88, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Wang, Y.; Zhang, Q.; Chen, L.; Zhao, Y. In situ formation of poly (thiolated chitosan-co-alkylated β-cyclodextrin) hydrogels using click cross-linking for sustained drug release. J. Mater. Sci. 2019, 54, 1677–1691. [Google Scholar] [CrossRef]
- 11. Gu Haixin. Study on the Adsorption of Chromium and Cadmium in Water by Chitosan Cross-Linked β-Cyclodextrin. Master’s Thesis, University of Shanghai, Shanghai, China, 2014.
- Vieira, R.S.; Beppu, M.M. Interaction of natural and crosslinked chitosan membranes with Hg(II) ions. Colloid Surface A 2006, 279, 196–207. [Google Scholar] [CrossRef]
- Qin, Z.P.; Sun, B.; Guo, C.Y.; Guo, H.X. Preparation and multifunctional separation of chitosan porous membrane. Membr. Sci. Tech. 2019, 39, 48–53. [Google Scholar]
- Sikder, M.T.; Rahman, M.M.; Jakariya, M.; Hosokawa, T.; Kurasaki, M.; Saito, T. Remediation of water pollution with native cyclodextrins and modified cyclodextrins: A comparative overview and perspectives. Chem. Eng. J. 2019, 355, 920–941. [Google Scholar] [CrossRef]
- Wang, H.-D.; Chu, L.-Y.; Song, H.; Yang, J.-P.; Xie, R.; Yang, M. Preparation and enantiomer separation characteristics of chitosan/β-cyclodextrin composite membranes. J. Membr. Sci. 2007, 297, 262–270. [Google Scholar] [CrossRef]
- Azmeera, V.; Tungala, K.; Adhikary, P.; Kumar, K.; Krishnamoorthi, S. Solution and microwave assisted synthesis of β-Cyclodextrin grafted polyacrylamide: Water treatment and In-vitro drug release study. Int. J. Biol. Macromol. 2017, 104, 1204–1211. [Google Scholar] [CrossRef]
- Tan, P.; Hu, Y. Improved synthesis of graphene/β-cyclodextrin composite for highly efficient dye adsorption and removal. J. Mol. Liq. 2017, 242, 181–189. [Google Scholar] [CrossRef]
- Ying Hwa, G.; Yih Hui, B.; Jairaj Moses, E.; Shahriman, M.S.; Md Yusoff, M.; Yahaya, N.; Sambasevam, K.P.; Surikumaran, H.; Chandrasekaram, K.; Raoov, M. Smart combination of β-cyclodextrin polymer-conjugated magnetic nanosorbent for potential adsorption of deoxyribonucleic acid. Sep. Sci. Technol. 2019, 54, 902–915. [Google Scholar] [CrossRef]
- Junthip, J. Water-insoluble cyclodextrin polymer crosslinked with citric acid for paraquat removal from water. J. Macromol. Sci. A 2019, 56, 555–563. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Lin, J.-C.; Hai, W.; Tan, H.-W.; Luo, Y.-W.; Xie, X.-L.; Cao, Y.; He, F.-A. A novel crosslinked β-cyclodextrin-based polymer for removing methylene blue from water with high efficiency. Colloid Surface A 2019, 560, 59–68. [Google Scholar] [CrossRef]
- Preethi, J.; Meenakshi, S. Fabrication of La3+ Impregnated Chitosan/β-Cyclodextrin Biopolymeric Materials for Effective Utilization of Chromate and Fluoride Adsorption in Single Systems. J. Chem. Eng. Data 2018, 63, 723–731. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Yin, D.; Chen, L.; Kalliola, S.; Tang, J.; Iakovleva, E.; Tam, K.C.; Sillanpää, M. One-pot synthesis of trifunctional chitosan-EDTA-β-cyclodextrin polymer for simultaneous removal of metals and organic micropollutants. Sci. Rep. 2017, 7, 15811. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Hasan, S.W. Nucleophilic-functionalized β-cyclodextrin-polyethersulfone structures from facile lamination process as nanoporous membrane active layers for wastewater post-treatment: Molecular implications. J. Membr. Sci. 2018, 563, 914–925. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, B.; Xu, J.; Pan, K.; Hou, H.; Hu, J.; Yang, J. Cross-linked chitosan/β-cyclodextrin composite for selective removal of methyl orange: Adsorption performance and mechanism. Carbohyd. Polym. 2018, 182, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Shan, M.X.; Matsuyama, H.; Teramoto, M.; Okuno, J.; Lloyd, D.R.; Kubota, N. Effect of diluent on poly(ethylene-co-vinyl alcohol) hollow-fiber membrane formation via thermally induced phase separation. J. Appl. Polym. Sci. 2005, 95, 219–225. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural Characterization of Thin-Film Polyamide Reverse Osmosis Membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Wilson, L.D.; Pratt, D.Y.; Kozinski, J.A. Preparation and sorption studies of β-cyclodextrin-chitosan–glutaraldehyde terpolymers. J. Colloid Interface Sci. 2013, 393, 271–277. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Fourmentin, M.; Fourmentin, S.; Torri, G.; Crini, G. Synthesis of silica materials containing cyclodextrin and their applications in wastewater treatment. Environ. Chem. Lett. 2019, 17, 683–696. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, C.; Yan, X.; Xiao, R.; Wang, T.; Li, M. β-Cyclodextrin Modified Natural Chitosan as a Green Inhibitor for Carbon Steel in Acid Solutions. Ind. Eng. Chem. Res. 2015, 54, 5664–5672. [Google Scholar] [CrossRef]
- Junthip, J. Coating of PET Textiles with Anionic Cyclodextrin Polymer for Paraquat Removal from Aqueous Solution. Fiber Polym. 2018, 19, 2335–2343. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Correction to Synthesis of β-Cyclodextrin-Based Electrospun Nanofiber Membranes for Highly Efficient Adsorption and Separation of Methylene Blue. ACS Appl. Mater. Interfaces 2017, 9, 4279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Guo, J.; Zhang, W.; Zheng, Z. Adsorption properties of crosslinked amino starch to methylene blue dye. J. Text. 2018, 39, 103–110. [Google Scholar]
- Wu, N. Preparation of Chitosan/β-Cyclodextrin/Titanium Dioxide Porous Adsorption Membrane and Treatment of Copper-Containing Wastewater. Master’s Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2015. [Google Scholar]
- Liu, Q.; Zhong, L.-B.; Zhao, Q.-B.; Frear, C.; Zheng, Y.-M. Synthesis of Fe3O4/Polyacrylonitrile Composite Electrospun Nanofiber Mat for Effective Adsorption of Tetracycline. ACS Appl. Mater. Interfaces 2015, 7, 14573–14583. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf. Environ. Prot. 1998, 76, 332–340. [Google Scholar] [CrossRef]
- Gao, S.; Jiang, J.-Y.; Liu, Y.-Y.; Fu, Y.; Zhao, L.-X.; Li, C.-Y.; Ye, F. Enhanced Solubility, Stability, and Herbicidal Activity of the Herbicide Diuron by Complex Formation with β-Cyclodextrin. Polymers 2019, 11, 1396. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D. Kinetics and mechanisms of metal sorption at the mineral/water interface. In Mineral-Water Interfacial Reactions; American Chemical Society: Washington, DC, USA, 1999; pp. 108–135. [Google Scholar]
- Liu, X.; Zhou, M.; Zhou, X.; Wang, L.; Liu, X. Functionalized Poly(arylene ether nitrile) Porous Membrane with High Pb(II) Adsorption Performance. Polymers 2019, 11, 1412. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, F.; Pei, H.; Li, J.; Cui, Z.; He, B. Antibacterial and environmentally friendly chitosan/polyvinyl alcohol blend membranes for air filtration. Carbohydr. Polym. 2018, 198, 241–248. [Google Scholar] [CrossRef]
- Danquah, M.K.; Aruei, R.C.; Wilson, L.D. Phenolic Pollutant Uptake Properties of Molecular Templated Polymers Containing β-Cyclodextrin. J. Phys. Chem. B 2018, 122, 4748–4757. [Google Scholar] [CrossRef]
- Shang, C.; Huang, P.M.; Stewart, J.W.B. Kinetics of adsorption of organic and inorganic phosphates by short-range ordered precipitate of aluminum. Can. J. Soil Sci. 1990, 70, 461–470. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Mekatel, H.; Amokrane, S.; Bellal, B.; Trari, M.; Nibou, D. Photocatalytic reduction of Cr(VI) on nanosized Fe2O3 supported on natural Algerian clay: Characteristics, kinetic and thermodynamic study. Chem. Eng. J. 2012, 200, 611–618. [Google Scholar] [CrossRef]
- Gao, N.; Yang, J.; Wu, Y.; Yue, J.; Cao, G.; Zhang, A.; Ye, L.; Feng, Z. β-Cyclodextrin functionalized coaxially electrospun poly(vinylidene fluoride) @ polystyrene membranes with higher mechanical performance for efficient removal of phenolphthalein. React. Funct. Polym. 2019, 141, 100–111. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Wilson, L.D.; Headley, J.V.; Peru, K.M. Investigation of the sorption properties of β-cyclodextrin-based polyurethanes with phenolic dyes and naphthenates. J. Colloid Interface Sci. 2011, 356, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.; Wilson, L. Kinetic Uptake Studies of Powdered Materials in Solution. Nanomaterials 2015, 5, 969–980. [Google Scholar] [CrossRef]
- Uyar, T.; Havelund, R.; Nur, Y.; Hacaloglu, J.; Besenbacher, F.; Kingshott, P. Molecular filters based on cyclodextrin functionalized electrospun fibers. J. Membr. Sci. 2009, 332, 129–137. [Google Scholar] [CrossRef]
- Jie, Y.; Yao, Z.; Qiu, F.; Hao, Z.; Yang, D.; Jie, W.; Wen, W. Kinetic, isotherm and thermodynamic studies for removal of methyl orange using a novel β-cyclodextrin functionalized graphene oxide-isophorone diisocyanate composites. Chem. Eng. Res. Des. 2016, 106, 168–177. [Google Scholar]













| Membranes | Tensile Strength/MPa | Elongation at Break/% |
|---|---|---|
| M-K | 30.77 | 30.30 |
| M-CK | 33.97 | 32.42 |
| M-B | 0.13 | 17.36 |
| M-CB | 0.19 | 21.53 |
| Parameters | Langmuir | Freundlich | Temkin |
|---|---|---|---|
| Constants | qm = 0.7242 | n = 1.5716 | bT = 15821 |
| bL = 0.5313 | k = 0.2417 | A = 5.3069 | |
| R2 | 0.9510 | 0.9305 | 0.9015 |
| Equation | Parameters | Co (mg/L) | ||||
|---|---|---|---|---|---|---|
| 2.5 | 3 | 3.5 | 4 | 4.5 | ||
| Pseudo-first-order Equation | a | 0.0039 | 0.0047 | 0.0049 | 0.0048 | 0.0067 |
| b | −2.2744 | −2.1400 | −2.0622 | −1.5346 | −1.5286 | |
| R2 | 0.7594 | 0.5231 | 0.6970 | 0.8101 | 0.9807 | |
| Pseudo-second-order Equation | a | 11.1047 | 13.7550 | 11.5965 | 5.9720 | 6.4760 |
| b | 7.9696 | 6.5067 | 6.0742 | 3.6564 | 3.4184 | |
| R2 | 0.8936 | 0.9613 | 0.9081 | 0.7814 | 0.6053 | |
| Double Constant Equation | a | 0.0973 | 0.1180 | 0.1272 | 0.1244 | 0.1489 |
| b | −2.4566 | −1.7519 | −2.8375 | −2.2967 | −1.7872 | |
| R2 | 0.9992 | 0.9526 | 0.8716 | 0.9374 | 0.8748 | |
| Elovich Equation | a | 0.0110 | 0.0164 | 0.0177 | 0.0398 | 0.0289 |
| b | 0.0822 | 0.0860 | 0.0938 | 0.1450 | 0.1618 | |
| R2 | 0.9821 | 0.8905 | 0.9496 | 0.8310 | 0.9469 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Li, N.; Lu, Q.; Wang, X.; Zhu, Y. Study on Preparation and Separation and Adsorption Performance of Knitted Tube Composite β-Cyclodextrin/Chitosan Porous Membrane. Polymers 2019, 11, 1737. https://doi.org/10.3390/polym11111737
Tang Q, Li N, Lu Q, Wang X, Zhu Y. Study on Preparation and Separation and Adsorption Performance of Knitted Tube Composite β-Cyclodextrin/Chitosan Porous Membrane. Polymers. 2019; 11(11):1737. https://doi.org/10.3390/polym11111737
Chicago/Turabian StyleTang, Qian, Nana Li, Qingchen Lu, Xue Wang, and Yaotian Zhu. 2019. "Study on Preparation and Separation and Adsorption Performance of Knitted Tube Composite β-Cyclodextrin/Chitosan Porous Membrane" Polymers 11, no. 11: 1737. https://doi.org/10.3390/polym11111737
APA StyleTang, Q., Li, N., Lu, Q., Wang, X., & Zhu, Y. (2019). Study on Preparation and Separation and Adsorption Performance of Knitted Tube Composite β-Cyclodextrin/Chitosan Porous Membrane. Polymers, 11(11), 1737. https://doi.org/10.3390/polym11111737