Shape-Memory Metallopolymer Networks Based on a Triazole–Pyridine Ligand
Abstract
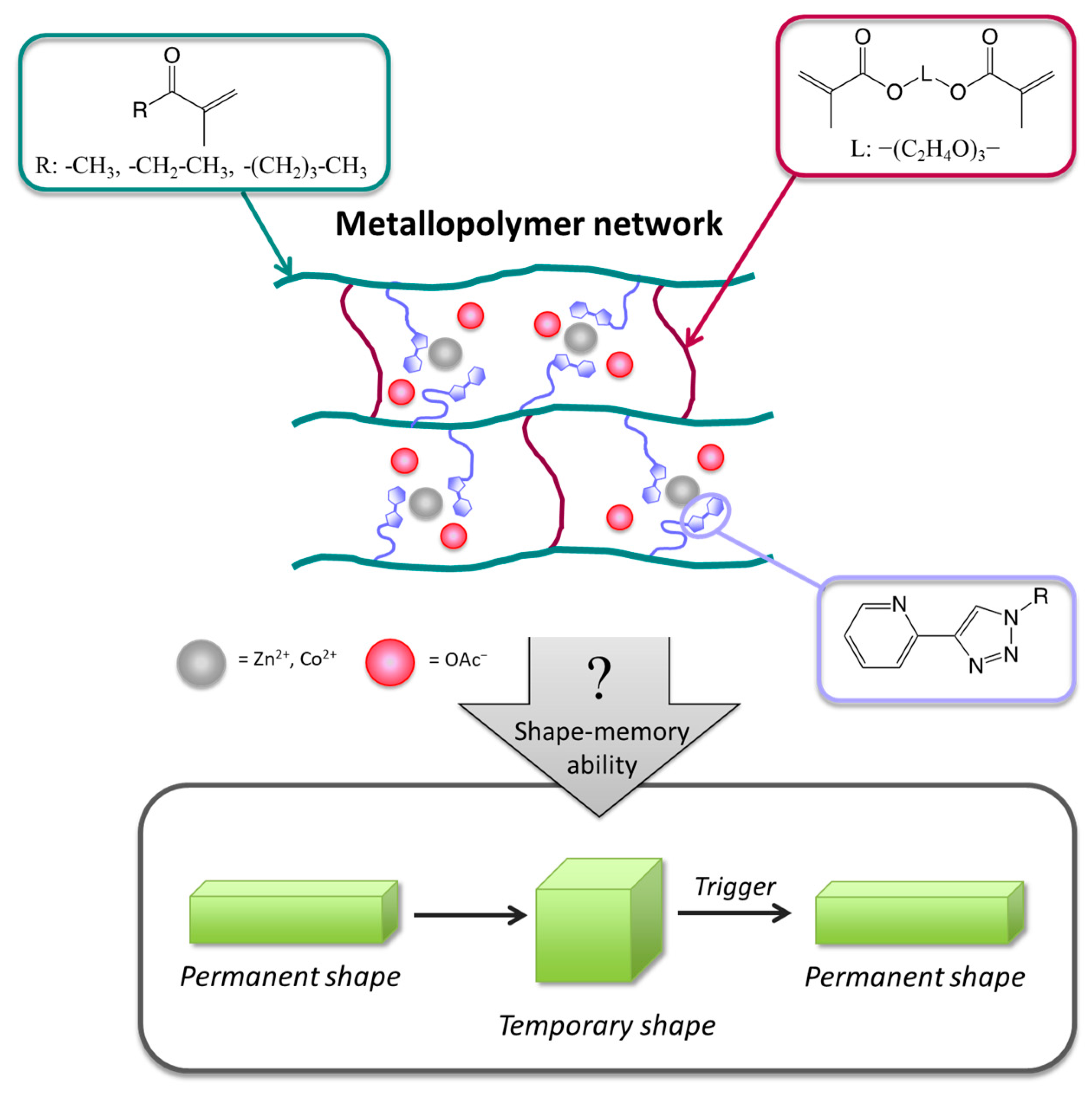
:1. Introduction
2. Experimental Part
2.1. Materials and Methods
2.2. Synthesis of the Monomer and Model System
2.2.1. 11-[4-(Pyridine-2-yl)-1H-1,2,3-triazol-1-yl]undecane-1-ol (1)
2.2.2. 11-(4-(Pyridine-2-yl)-1H-1,2,3-triazol-1-yl)undecanyl-methacrylate (2)
2.2.3. 11-(4-(Pyridine-2-yl)-1H-1,2,3-triazol-1-yl)undecanyl-acetate (3)
2.3. Synthesis of Model Complexes
2.4. Synthesis of the Polymer Networks
2.5. Synthesis of the Metallo Polymer Networks
3. Results and Discussion
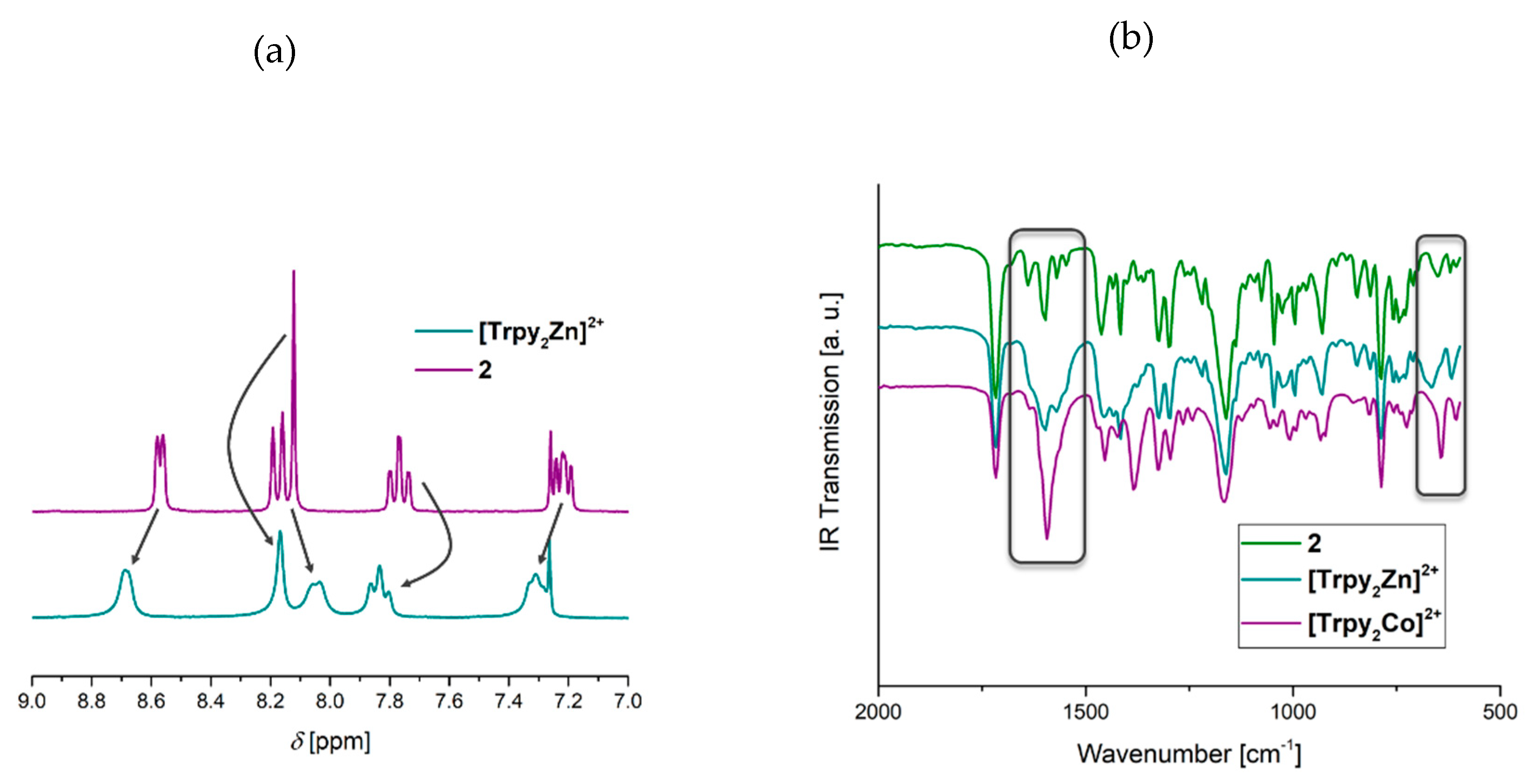
3.1. Synthesis of the Triazole-Pyridine Monomer and Model System
3.2. Isothermal Titration Calorimetry Investigations (ITC)
3.3. Model Complexes
3.4. Polymer Networks
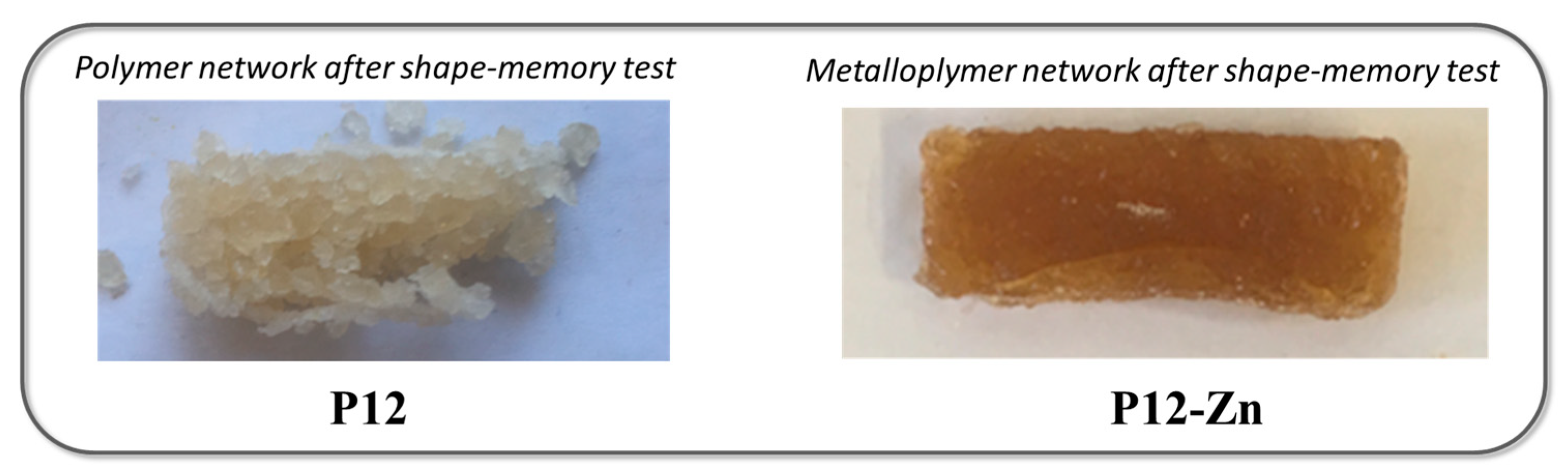
3.5. Metallopolymer Networks
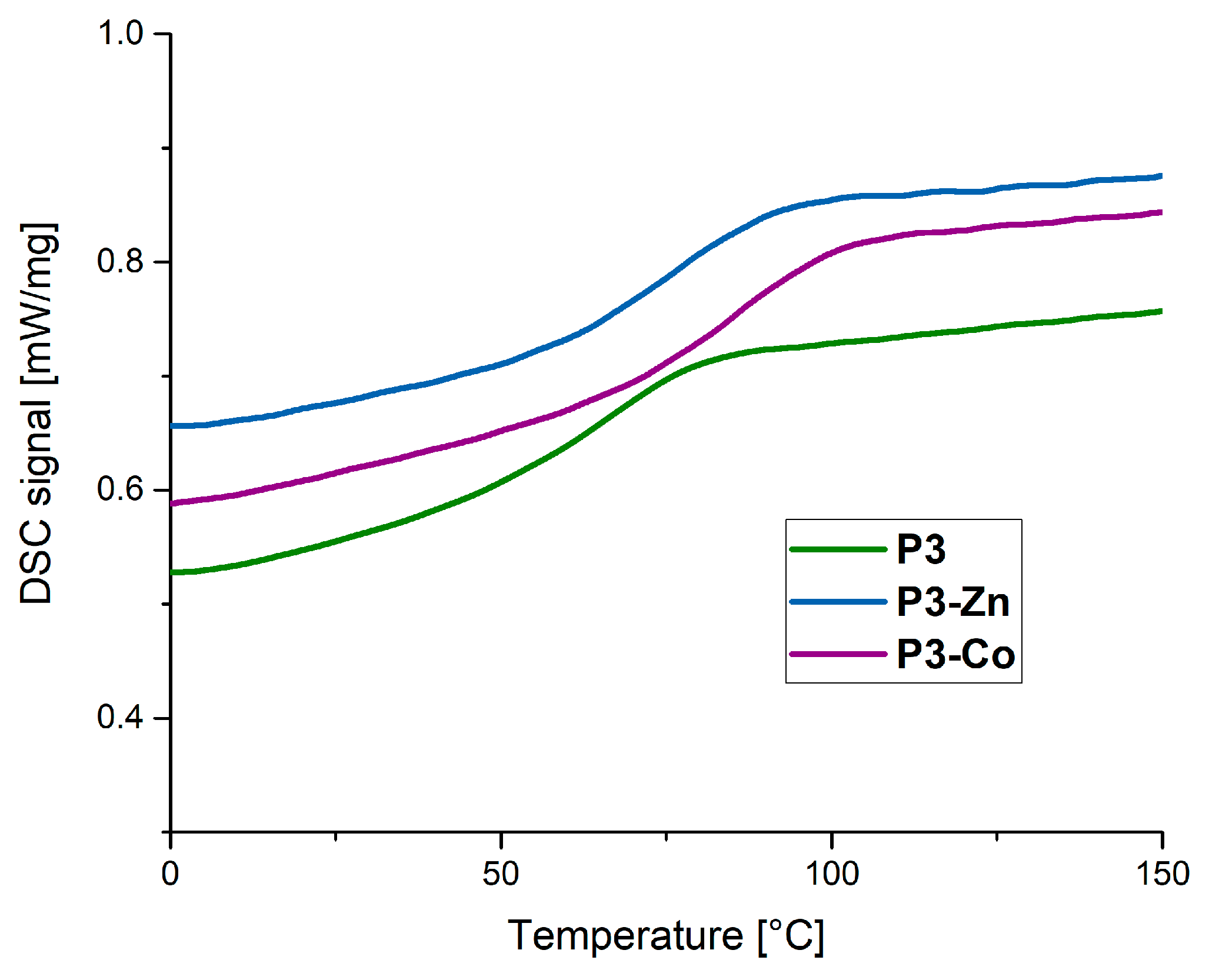
3.6. Thermal Behaviour of the Polymer and Metallopolymer Networks
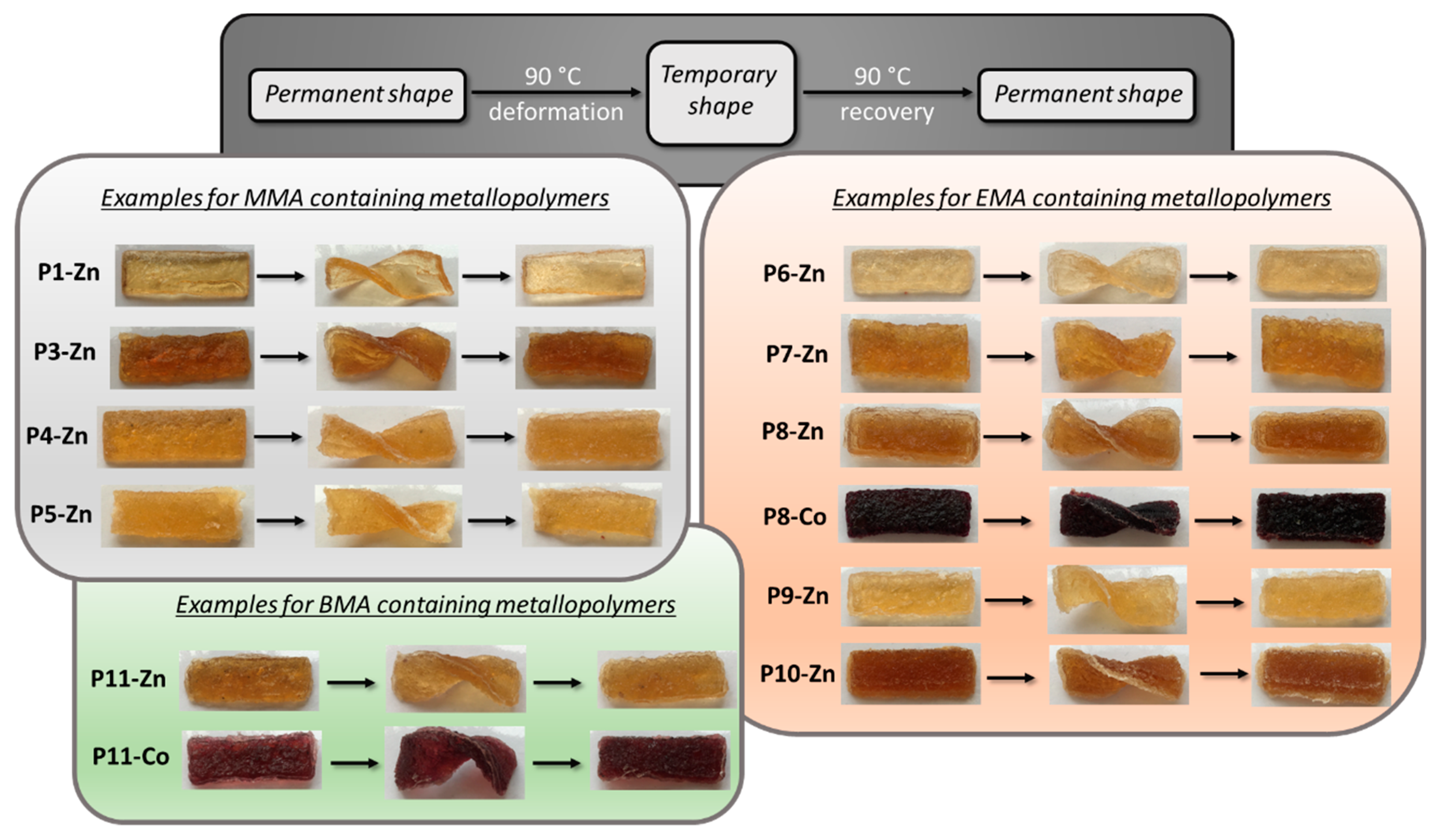
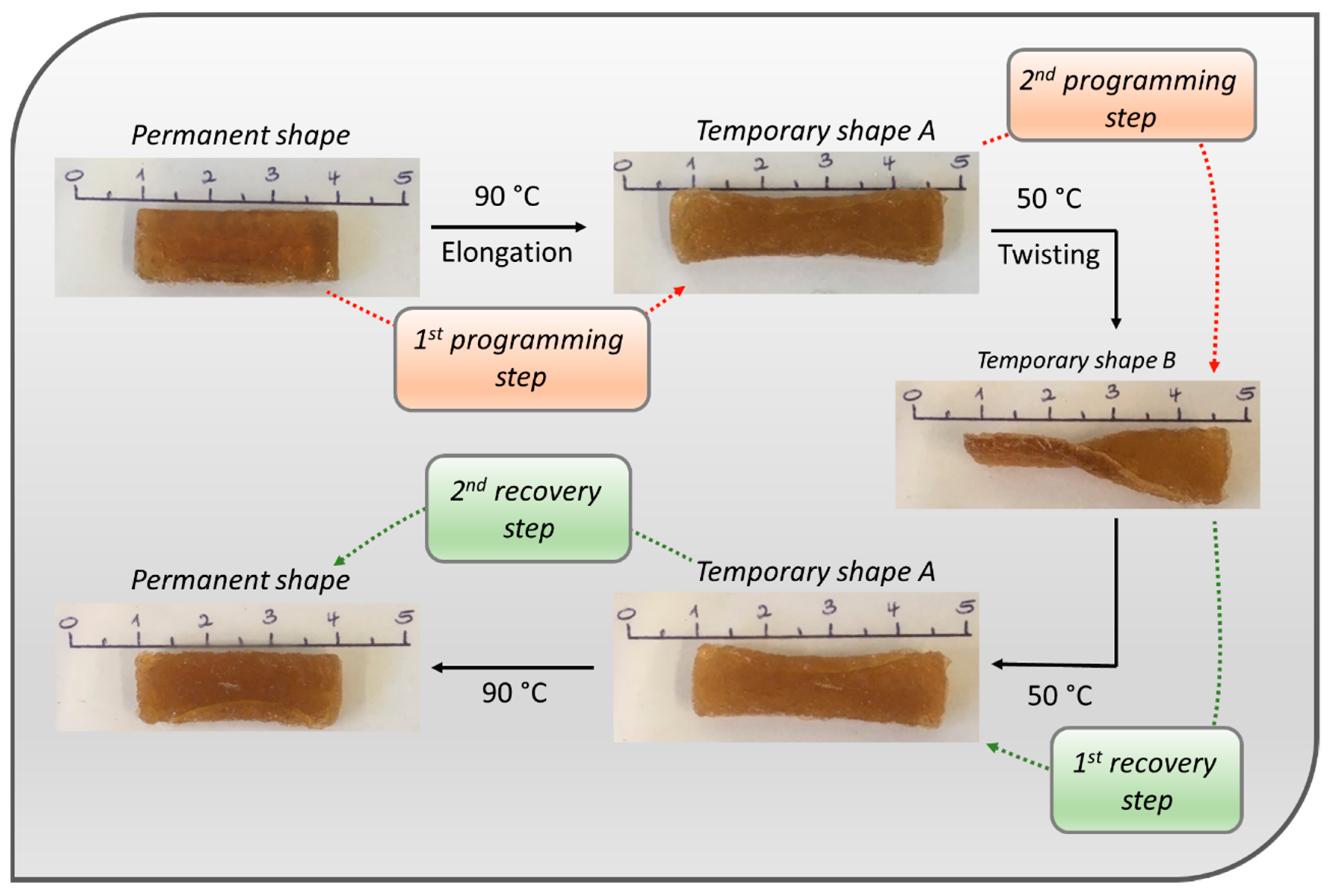
3.7. Shape-Memory Ability of the Metallopolymer Networks
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chung, T.; Romo-Uribe, A.; Mather, P.T. Two-way reversible shape memory in a semicrystalline network. Macromolecules 2008, 41, 184–192. [Google Scholar] [CrossRef]
- Galaev, I.Y.; Mattiasson, B. ‘Smart’ polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999, 17, 335–340. [Google Scholar] [CrossRef]
- Kumar, A.; Srivastava, A.; Galaev, I.Y.; Mattiasson, B. Smart polymers: Physical forms and bioengineering applications. Prog. Polym. Sci. 2007, 32, 1205–1237. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Schattling, P.; Jochum, F.D.; Theato, P. Multi-stimuli responsive polymers—The all-in-one talents. Polym. Chem. 2014, 5, 25–36. [Google Scholar] [CrossRef]
- De Las Heras Alarcon, C.; Pennadam, S.; Alexander, C. Stimuli responsive polymers for biomedical applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hoffman, A.S. Graft copolymers that exhibit temperature-induced phase transitions over a wide range of pH. Nature 1995, 373, 49–52. [Google Scholar] [CrossRef]
- Crespy, D.; Rossi, R.M. Temperature-responsive polymers with LCST in the physiological range and their applications in textiles. Polymer Int. 2007, 56, 1461–1468. [Google Scholar] [CrossRef]
- Weber, C.; Hoogenboom, R.; Schubert, U.S. Temperature responsive bio-compatible polymers based on poly(ethylene oxide) and poly(2-oxazoline)s. Prog. Polym. Sci. 2012, 37, 686–714. [Google Scholar] [CrossRef]
- Lynn, D.M.; Amiji, M.M.; Langer, R. Ph-responsive polymer microspheres: Rapid release of encapsulated material within the range of intracellular pH. Angew. Chem. Int. Ed. 2001, 40, 1707–1710. [Google Scholar] [CrossRef]
- Dai, S.; Ravi, P.; Tam, K.C. Ph-responsive polymers: Synthesis, properties and applications. Soft Matter 2008, 4, 435. [Google Scholar] [CrossRef]
- Jochum, F.D.; zur Borg, L.; Roth, P.J.; Theato, P. Thermo- and light-responsive polymers containing photoswitchable azobenzene end groups. Macromolecules 2009, 42, 7854–7862. [Google Scholar] [CrossRef]
- Jochum, F.D.; Theato, P. Temperature- and light-responsive smart polymer materials. Chem. Soc. Rev. 2013, 42, 7468–7483. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nishio, I.; Sun, S.-T.; Ueno-Nishio, S. Collapse of gels in an electric field. Science 1982, 218, 467. [Google Scholar] [CrossRef]
- Liu, F.; Urban, M.W. Recent advances and challenges in designing stimuli-responsive polymers. Prog. Polym. Sci. 2010, 35, 3–23. [Google Scholar] [CrossRef]
- Dahlke, J.; Zechel, S.; Hager, M.D.; Schubert, U.S. How to design a self-healing polymer: General concepts of dynamic covalent bonds and their application for intrinsic healable materials. Adv. Mater. Interf. 2018, 5, 1800051. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing applications: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267. [Google Scholar] [CrossRef]
- Liu, C.; Qin, H.; Mather, P.T. Review of progress in shape-memory polymers. J. Mater. Chem. 2007, 17, 1543. [Google Scholar] [CrossRef]
- Lendlein, A.; Kelch, S. Shape-memory polymers. Angew. Chem. Int. Ed. 2002, 41, 2034–2057. [Google Scholar] [CrossRef]
- Behl, M.; Lendlein, A. Shape-memory polymers. Kirk Othmer Encycl. Chem. Technol. 2011, 1–16. [Google Scholar] [CrossRef]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Tadaki, T.; Otsuka, K.; Shimizu, K. Shape memory alloys. Annu. Rev. Mater. Sci. 1988, 18, 25–45. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.; Jünger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef]
- Huang, W.M.; Yang, B.; Zhao, Y.; Ding, Z. Thermo-moisture responsive polyurethane shape-memory polymer and composites: A review. J. Mater. Chem. 2010, 20, 3367. [Google Scholar] [CrossRef]
- Rivero, G.; Nguyen, L.-T.T.; Hillewaere, X.K.D.; Du Prez, F.E. One-pot thermo-remendable shape memory polyurethanes. Macromolecules 2014, 47, 2010–2018. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Wang, H.-Q.; Lai, J.-C.; Li, C.-H. A self-healing and shape memory polymer that functions at body temperature. Molecules 2019, 24, 3224. [Google Scholar] [CrossRef]
- Baer, G.; Wilson, T.; Maitland, D.; Matthews, D. 483 shape memory polymer neurovascular stents. J. Invest. Med. 2006, 54, S162. [Google Scholar] [CrossRef]
- Santo, L.; Quadrini, F.; Accettura, A.; Villadei, W. Shape memory composites for self-deployable structures in aerospace applications. Proc. Eng. 2014, 88, 42–47. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Ye, J.; Zheng, N.; Kohli, P.; Choi, D.; Zhang, Y.; Xie, Z.; Zhang, Q.; Luan, H.; et al. Freestanding 3D mesostructures, functional devices, and shape-programmable systems based on mechanically induced assembly with shape memory polymers. Adv. Mater. 2019, 31, 1805615. [Google Scholar] [CrossRef]
- Dalton, E.; Chai, Q.; Shaw, M.W.; McKenzie, T.J.; Mullins, E.S.; Ayres, N. Hydrogel-coated polyurethane/urea shape memory polymer foams. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1389–1395. [Google Scholar] [CrossRef]
- Guo, Y.; Lv, Z.; Huo, Y.; Sun, L.; Chen, S.; Liu, Z.; He, C.; Bi, X.; Fan, X.; You, Z. A biodegradable functional water-responsive shape memory polymer for biomedical applications. J. Mater. Chem. B 2019, 7, 123–132. [Google Scholar] [CrossRef]
- Lendlein, A.; Schmidt, A.M.; Schroeter, M.; Langer, R. Shape-memory polymer networks from oligo (ϵ-caprolactone) dimethacrylates. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1369–1381. [Google Scholar] [CrossRef]
- Kim, B.K.; Lee, S.Y.; Xu, M. Polyurethanes having shape memory effects. Polymer 1996, 37, 5781–5793. [Google Scholar] [CrossRef]
- Alteheld, A.; Feng, Y.; Kelch, S.; Lendlein, A. Biodegradable, amorphous copolyester-urethane networks having shape-memory properties. Angew. Chem. Int. Ed. 2005, 44, 1188–1192. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Morshedian, J.; Khonakdar, H.A.; Mehrabzadeh, M.; Eslami, H. Preparation and properties of heat-shrinkable cross-linked low-density polyethylene. Adv. Polym. Tech. 2003, 22, 112–119. [Google Scholar] [CrossRef]
- Maksimkin, A.; Kaloshkin, S.; Zadorozhnyy, M.; Tcherdyntsev, V. Comparison of shape memory effect in UHMWPE for bulk and fiber state. J. Alloy. Compd. 2014, 586, S214–S217. [Google Scholar] [CrossRef]
- Heuwers, B.; Quitmann, D.; Katzenberg, F.; Tiller, J.C. Stress-induced melting of crystals in natural rubber: A new way to tailor the transition temperature of shape memory polymers. Macromol. Rapid Commun. 2012, 33, 1517–1522. [Google Scholar] [CrossRef]
- Burke, K.A.; Mather, P.T. Soft shape memory in main-chain liquid crystalline elastomers. J. Mater. Chem. 2010, 20, 3449. [Google Scholar] [CrossRef]
- Xie, T.; Rousseau, I.A. Facile tailoring of thermal transition temperatures of epoxy shape memory polymers. Polymer 2009, 50, 1852–1856. [Google Scholar] [CrossRef]
- Yakacki, C.M.; Shandas, R.; Safranski, D.; Ortega, A.M.; Sassaman, K.; Gall, K. Strong, tailored, biocompatible shape-memory polymer networks. Adv. Funct. Mater. 2008, 18, 2428–2435. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Yamashiro, M.; Iji, M. Recyclable shape-memory polymer: Poly (lactic acid) crosslinked by a thermoreversible Diels-Alder reaction. J. Appl. Polym. Sci. 2009, 112, 876–885. [Google Scholar] [CrossRef]
- Li, J.; Viveros, J.A.; Wrue, M.H.; Anthamatten, M. Shape-memory effects in polymer networks containing reversibly associating side-groups. Adv. Mater. 2007, 19, 2851–2855. [Google Scholar] [CrossRef]
- Kumpfer, J.R.; Rowan, S.J. Thermo-, photo-, and chemo-responsive shape-memory properties from photo-cross-linked metallo-supramolecular polymers. J. Am. Chem. Soc. 2011, 133, 12866–12874. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.; Cao, Y.; Peng, Y.; Xu, J.; Chen, A.S.C. Complex of polyelectrolyte network with surfactant as novel shape memory networks. Chem. Commun. 2001, 1694–1695. [Google Scholar] [CrossRef]
- Zhang, P.; Behl, M.; Peng, X.; Balk, M.; Lendlein, A. Chemoresponsive shape-memory effect of rhodium–phosphine coordination polymer networks. Chem. Mater. 2019, 31, 5402–5407. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, G.; Zheng, N.; Zhao, Q.; Xie, T. A metallosupramolecular shape-memory polymer with gradient thermal plasticity. Angew. Chem. Int. Ed. 2017, 56, 12599–12602. [Google Scholar] [CrossRef]
- Whittell, G.R.; Hager, M.D.; Schubert, U.S.; Manners, I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nat. Mater. 2011, 10, 176–188. [Google Scholar] [CrossRef]
- Happ, B.; Friebe, C.; Winter, A.; Hager, M.D.; Hoogenboom, R.; Schubert, U.S. 2-(1 H-1,2,3-Triazol-4-yl)-pyridine ligands as alternatives to 2,2′-bipyridines in ruthenium(II) complexes. Chem. Asian J. 2009, 4, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Happ, B.; Pavlov, G.M.; Perevyazko, I.; Hager, M.D.; Winter, A.; Schubert, U.S. Induced charge effect by co(II) complexation on the conformation of a copolymer containing a bidentate 2-(1,2,3-Triazol-4-yl)pyridine chelating unit. Macromol. Chem. Phys. 2012, 213, 1339–1348. [Google Scholar] [CrossRef]
- Hannewald, N.; Enke, M.; Nischang, I.; Zechel, S.; Hager, M.D.; Schubert, U.S. Mechanical activation of terpyridine metal complexes in polymers. J. Inorgan. Organometall. Polym. Mater. 2019, 1–13. [Google Scholar] [CrossRef]
- Fleischhaker, F.; Haehnel, A.P.; Misske, A.M.; Blanchot, M.; Haremza, S.; Barner-Kowollik, C. Glass-transition-, melting-, and decomposition temperatures of tailored polyacrylates and polymethacrylates: General trends and structure–property relationships. Macromol. Chem. Phys. 2014, 215, 1192–1200. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Mozhdehi, D.; Neal, J.A.; Grindy, S.C.; Cordeau, Y.; Ayala, S.; Holten-Andersen, N.; Guan, Z. Tuning dynamic mechanical response in metallopolymer networks through simultaneous control of structural and temporal properties of the networks. Macromolecules 2016, 49, 6310–6321. [Google Scholar] [CrossRef]





| Educts | m [mg] | n [mmol] | Yield [mg] | |
|---|---|---|---|---|
| [Trpy2Zn]2+ | 2 Zn(OAc)2 2H2O | 247 71 | 0.642 0.321 | 318 (100%) |
| [Trpy2Co]2+ | 2 Co(OAc)2 4H2O | 220 71 | 0.572 0.286 | 291 (100%) |
| Metal Salt | Kd | Ka | n |
|---|---|---|---|
| Zn(OAc)2 | 2.95 × 10−3 | 3.39 × 102 | 1.8 |
| Co(OAc)2 | 3.74 × 10−4 | 2.68 × 103 | 2.4 |
| Main Monomer | Theoretical Units of the TEGDMA | Theoretical Units of the Ligand | Units of the Main Monomer (MMA, EMA or BMA) | |||
|---|---|---|---|---|---|---|
| Theoretical | Found by NMR | Found by EA | ||||
| P1 | MMA | 1 | 1 | 20 | 18.3 | 14.6 |
| P2 | 1 | 2 | 20 | 19.7 | 18.5 | |
| P3 | 1 | 4 | 20 | 20.3 | 22.0 | |
| P4 | 2 | 2 | 20 | 21.7 | 22.7 | |
| P5 | 2 | 4 | 20 | 16.7 | 21.1 | |
| P6 | EMA | 1 | 1 | 20 | 20.0 | 19.7 |
| P7 | 1 | 2 | 20 | 16.0 | 16.1 | |
| P8 | 1 | 4 | 20 | 21.0 | 21.3 | |
| P9 | 2 | 2 | 20 | 18.0 | 18.2 | |
| P10 | 2 | 4 | 20 | 20.5 | 21.5 | |
| P11 | BMA | 1 | 1 | 20 | 21.0 | 21.6 |
| P12 | 1 | 2 | 20 | 21.0 | 19.7 | |
| P13 | 2 | 2 | 20 | 19.5 | 21.0 | |
| Sample | Cycle | Rf [%] | Rr [%] |
|---|---|---|---|
| P1-Zn (MMA, 5% crosslinker, 5% ligand) | 1 | 99.8 | 84.4 |
| 2 | 100 | 85.5 | |
| 3 | 100 | 81.8 | |
| Average | 99.9 | 83.9 | |
| P6-Zn (EMA, 5% crosslinker, 5% ligand) | 1 | 98.7 | 95.1 |
| 2 | 98.7 | 95.0 | |
| 3 | 98.7 | 96.2 | |
| Average | 98.7 | 95.5 | |
| P7-Zn (EMA, 5% crosslinker, 10% ligand) | 1 | 98.5 | 95.2 |
| 2 | 98.4 | 95.4 | |
| 3 | 98.4 | 95.4 | |
| Average | 98.4 | 95.3 | |
| P8-Zn (EMA, 5% crosslinker, 10% ligand) | 1 | 98.5 | 96.3 |
| 2 | 98.0 | 97.5 | |
| 3 | 98.0 | 98.2 | |
| Average | 98.2 | 97.3 | |
| P11-Zn (BMA, 5% crosslinker, 5% ligand) | 1 | 99.1 | 96.6 |
| 2 | 99.1 | 95.8 | |
| 3 | 100.0 | 96.6 | |
| Average | 99.4 | 96.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meurer, J.; Hniopek, J.; Zechel, S.; Enke, M.; Vitz, J.; Schmitt, M.; Popp, J.; Hager, M.D.; Schubert, U.S. Shape-Memory Metallopolymer Networks Based on a Triazole–Pyridine Ligand. Polymers 2019, 11, 1889. https://doi.org/10.3390/polym11111889
Meurer J, Hniopek J, Zechel S, Enke M, Vitz J, Schmitt M, Popp J, Hager MD, Schubert US. Shape-Memory Metallopolymer Networks Based on a Triazole–Pyridine Ligand. Polymers. 2019; 11(11):1889. https://doi.org/10.3390/polym11111889
Chicago/Turabian StyleMeurer, Josefine, Julian Hniopek, Stefan Zechel, Marcel Enke, Jürgen Vitz, Michael Schmitt, Jürgen Popp, Martin D. Hager, and Ulrich S. Schubert. 2019. "Shape-Memory Metallopolymer Networks Based on a Triazole–Pyridine Ligand" Polymers 11, no. 11: 1889. https://doi.org/10.3390/polym11111889
APA StyleMeurer, J., Hniopek, J., Zechel, S., Enke, M., Vitz, J., Schmitt, M., Popp, J., Hager, M. D., & Schubert, U. S. (2019). Shape-Memory Metallopolymer Networks Based on a Triazole–Pyridine Ligand. Polymers, 11(11), 1889. https://doi.org/10.3390/polym11111889










