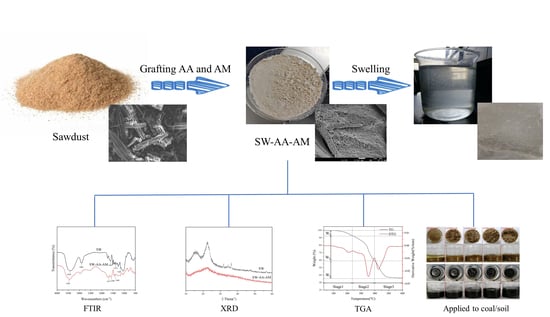
Preparation and Characterization of Superabsorbent Polymers Based on Sawdust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SW-AA-AM Polymer
2.3. Characterization
2.3.1. FTIR Analysis
2.3.2. XRD Analysis
2.3.3. SEM Analysis
2.3.4. Thermal Stability Analysis
2.4. Swelling Performance
2.5. Reusability
2.6. Water Retention Capacity
2.7. Water Retention Performance in Soil or Coal
3. Results and Discussion
3.1. Characterization
3.1.1. FTIR Analysis
3.1.2. XRD Analysis
3.1.3. SEM Analysis
3.1.4. Thermal Stability Analysis
3.2. Mechanism of SW-AA-AM Polymer Production
3.3. Swelling Performance
3.3.1. Effect of MBA Content on the Swelling Rate
3.3.2. Effect of AA Content on the Swelling Rate
3.3.3. Effect of AM Content on the Swelling Rate
3.3.4. Effect of APS Content on the Swelling Rate
3.3.5. Effect of the Neutralization of AA on the Swelling Rate
3.3.6. Effect of Temperature on the Swelling Rate
3.4. Water Retention Capacity
3.5. Reusability
3.6. Water Retention Performance in Soil or Coal
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shen, J.; Cui, C.; Li, J.; Wang, L. In Situ Synthesis of a Silver-Containing Superabsorbent Polymer via a Greener Method Based on Carboxymethyl Celluloses. Molecules 2018, 23, 2483. [Google Scholar] [CrossRef]
- Shen, J.; Li, B.; Zhan, X.; Wang, L. A one pot method for preparing an antibacterial superabsorbent hydrogel with a Semi-IPN structure based on tara gum and polyquaternium-7. Polymers 2018, 10, 696. [Google Scholar] [CrossRef]
- Olad, A.; Pourkhiyabi, M.; Gharekhani, H.; Doustdar, F. Semi-IPN superabsorbent nanocomposite based on sodium alginate and montmorillonite: Reaction parameters and swelling characteristics. Carbohydr. Polym. 2018, 190, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogels. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Luo, M.T.; Li, H.L.; Huang, C.; Zhang, H.R.; Xiong, L.; Chen, X.F.; Chen, X. De Cellulose-based absorbent production from bacterial cellulose and acrylic acid: Synthesis and performance. Polymers 2018, 10, 702. [Google Scholar] [CrossRef]
- Yang, S.T.; Park, Y.S. Release pattern of dexamethasone after administration through an implant-mediated drug delivery device with an active plunger of super absorbent polymer. Drug Deliv. Transl. Res. 2018, 8, 702–707. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, Z.; Guo, C.; Li, S. Influence of super absorbent polymer on soil water retention, seed germination and plant survivals for rocky slopes eco-engineering. Ecol. Eng. 2014, 62, 27–32. [Google Scholar] [CrossRef]
- Alharbi, K.; Ghoneim, A.; Ebid, A.; El-Hamshary, H.; El-Newehy, M.H. Controlled release of phosphorous fertilizer bound to carboxymethyl starch-g-polyacrylamide and maintaining a hydration level for the plant. Int. J. Biol. Macromol. 2018, 116, 224–231. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Mobarak, F. Green nanotechnology: A short cut to beneficiation of natural fibers. Int. J. Biol. Macromol. 2011, 48, 134–136. [Google Scholar] [CrossRef]
- Liu, X.; Yang, R.; Xu, M.; Ma, C.; Li, W.; Yin, Y.; Huang, Q.; Wu, Y.; Li, J.; Liu, S. Hydrothermal Synthesis of Cellulose Nanocrystal-Grafted-Acrylic Acid Aerogels with Superabsorbent Properties. Polymers 2018, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.M.; Sokker, H.H.; Zayed, E.M.; Nour Eldien, F.A.; Abd Alrahman, N.M. Removal of hazardous pollutants using bifunctional hydrogel obtained from modified starch by grafting copolymerization. Int. J. Biol. Macromol. 2018, 120, 2188–2199. [Google Scholar] [CrossRef] [PubMed]
- Riyazi, S.; Kevern, J.T.; Mulheron, M. Super absorbent polymers (SAPs) as physical air entrainment in cement mortars. Constr. Build. Mater. 2017, 147, 669–676. [Google Scholar] [CrossRef]
- Shen, D.; Shi, H.; Tang, X.; Ji, Y.; Jiang, G. Effect of internal curing with super absorbent polymers on residual stress development and stress relaxation in restrained concrete ring specimens. Constr. Build. Mater. 2016, 120, 309–320. [Google Scholar] [CrossRef]
- Zhang, J.; Li, A.; Wang, A. Synthesis and characterization of multifunctional poly(acrylic acid-co-acrylamide)/sodium humate superabsorbent composite. React. Funct. Polym. 2006, 66, 747–756. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Wang, A. Synthesis and characterization of chitosan-g-poly(acrylic acid)/attapulgite superabsorbent composites. Carbohydr. Polym. 2007, 68, 367–374. [Google Scholar] [CrossRef]
- Song, W.; Xin, J.; Zhang, J. One-pot synthesis of soy protein (SP)-poly(acrylic acid) (PAA) superabsorbent hydrogels via facile preparation of SP macromonomer. Ind. Crops Prod. 2017, 100, 117–125. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Roh, H.G.; Oh, S.; Kim, S.; Kim, M.; Kim, D.; Park, J. Preparation and characterization of superabsorbent polymers based on starch aldehydes and carboxymethyl cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef]
- Razali, M.A.A.; Ismail, H.; Ariffin, A. Graft copolymerization of polyDADMAC to cassava starch: Evaluation of process variables via central composite design. Ind. Crops Prod. 2015, 65, 535–545. [Google Scholar] [CrossRef]
- Álvarez-Castillo, E.; Del Toro, A.; Aguilar, J.M.; Guerrero, A.; Bengoechea, C. Optimization of a thermal process for the production of superabsorbent materials based on a soy protein isolate. Ind. Crops Prod. 2018, 125, 573–581. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Zhang, X.; Wang, Y. Slow-release nitrogen and boron fertilizer from a functional superabsorbent formulation based on wheat straw and attapulgite. Chem. Eng. J. 2011, 167, 342–348. [Google Scholar] [CrossRef]
- Xie, L.; Liu, M.; Ni, B.; Wang, Y. New environment-friendly use of wheat straw in slow-release fertilizer formulations with the function of superabsorbent. Ind. Eng. Chem. Res. 2012, 51, 3855–3862. [Google Scholar] [CrossRef]
- Cheng, W.M.; Hu, X.M.; Wang, D.M.; Liu, G.H. Preparation and characteristics of corn straw-Co-AMPS-Co-AA superabsorbent hydrogel. Polymers 2015, 7, 2431–2445. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Xu, X.; Zhong, Q. Synthesis and characterization of a novel super-absorbent based on wheat straw. Bioresour. Technol. 2011, 102, 2853–2858. [Google Scholar] [CrossRef]
- Fang, J.M.; Sun, R.C.; Tomkinson, J. Isolation and characterization of hemicelluloses and cellulose from rye straw by alkaline peroxide extraction. Cellulose 2000, 7, 87–107. [Google Scholar] [CrossRef]
- Wan, T.; Huang, R.; Zhao, Q.; Xiong, L.; Qin, L.; Tan, X.; Cai, G. Synthesis of wheat straw composite superabsorbent. J. Appl. Polym. Sci. 2013, 130, 3404–3410. [Google Scholar] [CrossRef]
- Jin, S.; Chen, J.; Mao, J.; Yue, G.; Han, Y.; Yu, X. A novel superabsorbent from raw corn straw and poly(acrylic acid). Polym. Compos. 2017, 38, 1353–1362. [Google Scholar] [CrossRef]
- Dai, D.; Fan, M. Preparation of bio-composite from wood sawdust and gypsum. Ind. Crops Prod. 2015, 74, 417–424. [Google Scholar] [CrossRef]
- Jung, C.H.; Choi, J.H.; Lim, Y.M.; Jeun, J.P.; Kang, P.H.; Nho, Y.C. Preparation and characterization of polypropylene nanocomposites containing polystyrene-grafted alumina nanoparticles. J. Ind. Eng. Chem. 2006, 12, 900–904. [Google Scholar]
- Rani, G.U.; Mishra, S.; Pathak, G.; Jha, U.; Sen, G. Synthesis and applications of poly(2-hydroxyethylmethacrylate) grafted agar: A microwave based approach. Int. J. Biol. Macromol. 2013, 61, 276–284. [Google Scholar] [CrossRef]
- Li, Y.N.; Sun, Y.; Deng, X.H.; Yang, Q.; Bai, Z.Y.; Xu, Z. Bin Graft polymerization of acrylic acid onto polyphenylene sulfide nonwoven initiated by low temperature plasma. J. Appl. Polym. Sci. 2006, 102, 5884–5889. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, C.Y.; Moon, S.Y.; Choi, J.H.; Chang, B.J.; Kim, J.H. Surface-attached brush-type CO2-philic poly(PEGMA)/PSf composite membranes by UV/ozone-induced graft polymerization: Fabrication, characterization, and gas separation properties. J. Memb. Sci. 2019, 589, 117214. [Google Scholar] [CrossRef]
- Zhuo, J.; Sun, G. Light-induced surface graft polymerizations initiated by an anthraquinone dye on cotton fibers. Carbohydr. Polym. 2014, 112, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Wang, K.; Dong, Y.; Yan, Y.; Zhang, W.; Qi, C.; Han, C.; Li, J.; Zhang, S. Highly hydrophobic and self-cleaning bulk wood prepared by grafting long-chain alkyl onto wood cell walls. Wood Sci. Technol. 2017, 51, 395–411. [Google Scholar] [CrossRef]
- Qiu, H.; Yang, S.; Han, Y.; Shen, X.; Fan, D.; Li, G.; Chu, F. Improvement of the Performance of Plantation Wood by Grafting Water-Soluble Vinyl Monomers onto Cell Walls. ACS Sustain. Chem. Eng. 2018, 6, 14450–14459. [Google Scholar] [CrossRef]
- Cabane, E.; Keplinger, T.; Merk, V.; Hass, P.; Burgert, I. Renewable and functional wood materials by grafting polymerization within cell walls. ChemSusChem 2014, 7, 1020–1025. [Google Scholar] [CrossRef]
- Suo, A.L.; Qian, J.M.; Yao, Y.; Zhang, W.G. Synthesis and properties of carboxymethyl cellulose-graft-poly (acrylic acid-co-acrylamide) as a novel cellulose-based superabsorbent. J. Appl. Polym. Sci. 2007, 103, 1382–1388. [Google Scholar] [CrossRef]
- Zheng, M.; Lian, F.; Zhu, Y.; Zhang, Y.; Liu, B.; Zhang, L.; Zheng, B. pH-responsive poly (xanthan gum-g-acrylamide-g-acrylic acid) hydrogel: Preparation, characterization, and application. Carbohydr. Polym. 2019, 210, 38–46. [Google Scholar] [CrossRef]
- Liu, Z.; Miao, Y.; Wang, Z.; Yin, G. Synthesis and characterization of a novel super-absorbent based on chemically modified pulverized wheat straw and acrylic acid. Carbohydr. Polym. 2009, 77, 131–135. [Google Scholar] [CrossRef]
- Wan, T.; Huang, R.; Xiong, L.; Zhao, Q.; Luo, L.; Zhang, H.; Cai, G. Swelling behaviors and gel strength studies of wheat straw-composite superabsorbent. J. Compos. Mater. 2014, 48, 2341–2348. [Google Scholar] [CrossRef]
- Li, J. Wood Spectroscopy, 1st ed.; Science Press: Beijing, China, 2009; pp. 104–116. [Google Scholar]
- Baki, M.; Abedi-Koupai, J. Preparation and characterization of a superabsorbent slow-release fertilizer with sodium alginate and biochar. J. Appl. Polym. Sci. 2018, 135, 45966. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Xing, R.; Chen, X.; Liu, S.; Qin, Y.; Li, K.; Wang, X.; Li, R.; Li, P. Synthesis of superabsorbent polymers based on chitosan derivative graft acrylic acid-co-acrylamide and its property testing. Int. J. Biol. Macromol. 2019, 132, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Mondal, M.K. Exhaustive characterization on chemical and thermal treatment of sawdust for improved biogas production. Biomass Convers. Biorefinery 2018, 8, 991–1003. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Han, H.; Qiu, Z.; Achal, V. Stimulatory effect of in-situ detoxification on bioethanol production by rice straw. Energy 2017, 135, 32–39. [Google Scholar] [CrossRef]
- Phitsuwan, P.; Sakka, K.; Ratanakhanokchai, K. Structural changes and enzymatic response of Napier grass (Pennisetum purpureum) stem induced by alkaline pretreatment. Bioresour. Technol. 2016, 218, 247–256. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Luan, S.; Li, W. Utilization of waste hemicelluloses lye for superabsorbent hydrogel synthesis. Int. J. Biol. Macromol. 2019, 132, 954–962. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Slow-release potassium silicate fertilizer with the function of superabsorbent and water retention. Ind. Eng. Chem. Res. 2007, 46, 6494–6500. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Samadi, M.; Ghasemzadeh, H. Fast-swelling superabsorbent hydrogels from poly(2-hydroxy ethyl acrylate-co-sodium acrylate) grafted on starch. Starch/Staerke 2008, 60, 79–86. [Google Scholar] [CrossRef]
- Zhang, B.; Cui, Y.; Yin, G.; Li, X.; Liao, L.; Cao, X. Synthesis and swelling properties of protein-poly(acrylic acid-co-acrylamide) superabsorbent composite. Polym. Compos. 2011, 32, 683–691. [Google Scholar] [CrossRef]
- Bao, Q.; Nie, W.; Liu, C.; Liu, Y.; Zhang, H.; Wang, H.; Jin, H. Preparation and characterization of a binary-graft-based, water-absorbing dust suppressant for coal transportation. J. Appl. Polym. Sci. 2019, 136, 47065. [Google Scholar] [CrossRef]
- Yang, W.; Guo, S.; Li, P.; Song, R.; Yu, J. Foliar antitranspirant and soil superabsorbent hydrogel affect photosynthetic gas exchange and water use efficiency of maize grown under low rainfall conditions. J. Sci. Food Agric. 2019, 99, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.Z.; Liu, Y.R.; Zheng, Y.M. Effects of super absorbent polymers on soil microbial properties and Chinese cabbage (Brassica chinensis) growth. J. Soils Sediments 2013, 13, 711–719. [Google Scholar] [CrossRef]









| Group | MBA/AA (%) | AA (g) | AM:AA | APS/AA (%) | Neutralization Value (%) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | 0.2 | 6 | 1:6 | 0.6 | 50 | 60 |
| 2 | 0.4 | 6 | 1:6 | 0.6 | 50 | 60 |
| 3 | 0.6 | 6 | 1:6 | 0.6 | 50 | 60 |
| 4 | 0.8 | 6 | 1:6 | 0.6 | 50 | 60 |
| 5 | 1.0 | 6 | 1:6 | 0.6 | 50 | 60 |
| 6 | 0.6 | 6 | 1:6 | 0.6 | 50 | 60 |
| 7 | 0.6 | 7 | 1:6 | 0.6 | 50 | 60 |
| 8 | 0.6 | 8 | 1:6 | 0.6 | 50 | 60 |
| 9 | 0.6 | 9 | 1:6 | 0.6 | 50 | 60 |
| 10 | 0.6 | 10 | 1:6 | 0.6 | 50 | 60 |
| 11 | 0.6 | 8 | 1:4 | 0.6 | 50 | 60 |
| 12 | 0.6 | 8 | 1:5 | 0.6 | 50 | 60 |
| 13 | 0.6 | 8 | 1:6 | 0.6 | 50 | 60 |
| 14 | 0.6 | 8 | 1:7 | 0.6 | 50 | 60 |
| 15 | 0.6 | 8 | 1:8 | 0.6 | 50 | 60 |
| 16 | 0.6 | 8 | 1:6 | 0.1 | 50 | 60 |
| 17 | 0.6 | 8 | 1:6 | 0.2 | 50 | 60 |
| 18 | 0.6 | 8 | 1:6 | 0.3 | 50 | 60 |
| 19 | 0.6 | 8 | 1:6 | 0.4 | 50 | 60 |
| 20 | 0.6 | 8 | 1:6 | 0.5 | 50 | 60 |
| 21 | 0.6 | 8 | 1:6 | 0.3 | 30 | 60 |
| 22 | 0.6 | 8 | 1:6 | 0.3 | 40 | 60 |
| 23 | 0.6 | 8 | 1:6 | 0.3 | 50 | 60 |
| 24 | 0.6 | 8 | 1:6 | 0.3 | 60 | 60 |
| 25 | 0.6 | 8 | 1:6 | 0.3 | 70 | 60 |
| 26 | 0.6 | 8 | 1:6 | 0.3 | 50 | 40 |
| 27 | 0.6 | 8 | 1:6 | 0.3 | 50 | 50 |
| 28 | 0.6 | 8 | 1:6 | 0.3 | 50 | 60 |
| 29 | 0.6 | 8 | 1:6 | 0.3 | 50 | 70 |
| 30 | 0.6 | 8 | 1:6 | 0.3 | 50 | 80 |
| Repeat Times | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| SR (g/g) | 738.12 | 676.71 | 537.86 | 396.59 | 327.36 |
| r | 1 | 0.92 | 0.73 | 0.54 | 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhang, S.; Chen, Z.; Wang, M.; Cao, J.; Wang, R. Preparation and Characterization of Superabsorbent Polymers Based on Sawdust. Polymers 2019, 11, 1891. https://doi.org/10.3390/polym11111891
Zhang M, Zhang S, Chen Z, Wang M, Cao J, Wang R. Preparation and Characterization of Superabsorbent Polymers Based on Sawdust. Polymers. 2019; 11(11):1891. https://doi.org/10.3390/polym11111891
Chicago/Turabian StyleZhang, Mingchang, Shaodi Zhang, Zhuoran Chen, Mingzhi Wang, Jinzhen Cao, and Ruoshui Wang. 2019. "Preparation and Characterization of Superabsorbent Polymers Based on Sawdust" Polymers 11, no. 11: 1891. https://doi.org/10.3390/polym11111891
APA StyleZhang, M., Zhang, S., Chen, Z., Wang, M., Cao, J., & Wang, R. (2019). Preparation and Characterization of Superabsorbent Polymers Based on Sawdust. Polymers, 11(11), 1891. https://doi.org/10.3390/polym11111891