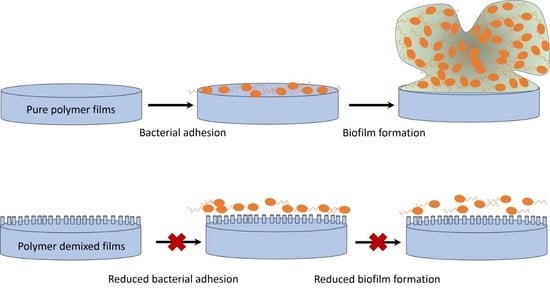
Effect of Polymer Demixed Nanotopographies on Bacterial Adhesion and Biofilm Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polymer Demixed Thin Films
2.2. Contact Angle Analysis
2.3. Fourier-Transform Infrared Spectroscopy Analysis
2.4. X-ray Photoelectron Spectroscopy Analysis
2.5. Atomic Force Microscopy Analysis
2.6. Adhered Cell Colony Forming Unit (CFU) Assay
2.7. Statistical Analysis
3. Results
3.1. Wettability: Contact Angle
3.2. Surface Chemistry: FTIR
3.3. Surface Chemistry: XPS
3.4. Surface Topography: AFM
3.5. Bacterial Response: Adhered Cell CFU Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations—The Review on Antimicrobial Resistance Chaired by Jim O’Neill; Wellcome Trust and HM Government: London, UK, 2016. [Google Scholar]
- Angulo, F.J.; Baker, N.L.; Olsen, S.J.; Anderson, A.; Barrett, T.J. Antimicrobial use in agriculture: Controlling the transfer of antimicrobial resistance to humans. Semin. Pediatr. Infect. Dis. 2004, 15, 78–85. [Google Scholar] [CrossRef]
- Thanner, S.; Drissner, D.; Walsh, F. Antimicrobial resistance in agriculture. mBio 2016, 7. [Google Scholar] [CrossRef]
- Witte, W. Medical consequences of antibiotic use in agriculture. Am. Assoc. Adv. Sci. 1998, 279, 996–997. [Google Scholar]
- McGowan, J.E., Jr. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev. Infect. Dis. 1983, 5, 1033–1048. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277. [Google Scholar]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Ann. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef]
- Maki, D.G.; Stolz, S.M.; Wheeler, S.; Mermel, L.A. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter: A randomized, controlled trial. Ann. Intern. Med. 1997, 127, 257–266. [Google Scholar] [CrossRef]
- Elliott, T.; Moss, H.; Tebbs, S.; Wilson, I.; Bonser, R.; Graham, T.; Burke, L.; Faroqui, M. Novel approach to investigate a source of microbial contamination of central venous catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1997, 16, 210–213. [Google Scholar] [CrossRef]
- Stickler, D. Bacterial biofilms and the encrustation of urethral catheters. Biofouling 1996, 9, 293–305. [Google Scholar] [CrossRef]
- Reid, G.; Denstedt, J.D.; Kang, Y.S.; Lam, D.; Nause, C. Microbial adhesion and biofilm formation on ureteral stents in vitro and in vivo. J. Urol. 1992, 148, 1592–1594. [Google Scholar] [CrossRef]
- Busscher, H.; Rinastiti, M.; Siswomihardjo, W.; Van der Mei, H. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef]
- Garvin, K.L.; Hanssen, A.D. Infection after total hip arthroplasty. Past, present, and future. JBJS 1995, 77, 1576–1588. [Google Scholar] [CrossRef]
- Sanderson, P. Infection in orthopaedic implants. J. Hosp. Infect. 1991, 18, 367–375. [Google Scholar] [CrossRef]
- Anwar, H.; Costerton, J. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 1990, 34, 1666–1671. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Moskowitz, S.M.; Foster, J.M.; Emerson, J.; Burns, J.L. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 2004, 42, 1915–1922. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Fluid Drag Reduction with Shark-Skin Riblet Inspired Microstructured Surfaces. Adv. Funct. Mater. 2013, 23, 4507–4528. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- D’Sa, R.A.; Raj, J.; Dickinson, P.J.; McCabe, F.; Meenan, B.J. Human fetal osteoblast response on poly (methyl methacrylate)/polystyrene demixed thin film blends: Surface chemistry vs topography effects. ACS Appl. Mater. Interfaces 2015, 8, 14920–14931. [Google Scholar] [CrossRef]
- Dalby, M.; Giannaras, D.; Riehle, M.; Gadegaard, N.; Affrossman, S.; Curtis, A. Rapid fibroblast adhesion to 27 nm high polymer demixed nano-topography. Biomaterials 2004, 25, 77–83. [Google Scholar] [CrossRef]
- Dalby, M.J. Cellular response to low adhesion nanotopographies. Int. J. Nanomed. 2007, 2, 373. [Google Scholar]
- Dalby, M.J.; Yarwood, S.J.; Riehle, M.O.; Johnstone, H.J.; Affrossman, S.; Curtis, A.S. Increasing fibroblast response to materials using nanotopography: Morphological and genetic measurements of cell response to 13-nm-high polymer demixed islands. Exp. Cell Res. 2002, 276, 1–9. [Google Scholar] [CrossRef]
- Khattak, M.; Pu, F.; Curran, J.M.; Hunt, J.A.; D’Sa, R.A. Human mesenchymal stem cell response to poly (ε-caprolactone/poly (methyl methacrylate) demixed thin films. J. Mater. Sci. Mater. Med. 2015, 26, 178. [Google Scholar] [CrossRef]
- Lim, J.Y.; Hansen, J.C.; Siedlecki, C.A.; Runt, J.; Donahue, H.J. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J. R. Soc. Interface 2005, 2, 97–108. [Google Scholar] [CrossRef]
- Coombes, A.; Rizzi, S.; Williamson, M.; Barralet, J.; Downes, S.; Wallace, W. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325. [Google Scholar] [CrossRef]
- Kim, S.B.; Kim, Y.J.; Yoon, T.L.; Park, S.A.; Cho, I.H.; Kim, E.J.; Kim, I.A.; Shin, J.-W. The characteristics of a hydroxyapatite–chitosan–PMMA bone cement. Biomaterials 2004, 25, 5715–5723. [Google Scholar] [CrossRef]
- Klawitter, J.J.; Weinstein, A.M.; Peterson, L.J. Fabrication and characterization of porous-rooted polymethylmethacrylate (PMMA) dental implants. J. Dent. Res. 1977, 56, 385–393. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Sharma, C.P. Absorbable polymeric surgical sutures: Chemistry, production, properties, biodegradability, and performance. J. Biomater. Appl. 2010, 25, 291–366. [Google Scholar] [CrossRef]
- Terrada, C.; Julian, K.; Cassoux, N.; Prieur, A.-M.; Debre, M.; Quartier, P.; LeHoang, P.; Bodaghi, B. Cataract surgery with primary intraocular lens implantation in children with uveitis: Long-term outcomes. J. Cataract Refract. Surg. 2011, 37, 1977–1983. [Google Scholar] [CrossRef]
- Lee, D.G.; Urbach, J.M.; Wu, G.; Liberati, N.T.; Feinbaum, R.L.; Miyata, S.; Diggins, L.T.; He, J.; Saucier, M.; Déziel, E. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 2006, 7, 90. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.; Irwin, J. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- Mohamed, A.; Gordon, S.H.; Biresaw, G. Polycaprolactone/polystyrene bioblends characterized by thermogravimetry, modulated differential scanning calorimetry and infrared photoacoustic spectroscopy. Polym. Degrad Stabil 2007, 92, 1177–1185. [Google Scholar] [CrossRef]
- Ton-That, C.; Shard, A.; Daley, R.; Bradley, R. Effects of annealing on the surface composition and morphology of PS/PMMA blend. Macromolecules 2000, 33, 8453–8459. [Google Scholar] [CrossRef]
- Heriot, S.Y.; Jones, R.A.L. An interfacial instability in a transient wetting layer leads to lateral phase separation in thin spin-cast polymer-blend films. Nat. Mater. 2005, 4, 782–786. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry Ithaca; Cornell University: New York, NY, USA, 1953. [Google Scholar]
- Flory, P.J. Thermodynamics of high polymer solutions. J. Chem. Phys. 1942, 10, 51–61. [Google Scholar] [CrossRef]
- Huggins, M.L. Solutions of long chain compounds. J. Chem. Phys. 1941, 9, 440. [Google Scholar] [CrossRef]
- Ton-That, C.; Shard, A.G.; Teare, D.O.H.; Bradley, R.H. XPS and AFM surface studies of solvent-cast PS/PMMA blends. Polymer 2001, 42, 1121–1129. [Google Scholar] [CrossRef]
- Ma, M.; He, Z.; Yang, J.; Wang, Q.; Chen, F.; Wang, K.; Zhang, Q.; Deng, H.; Fu, Q. Vertical Phase Separation and Liquid–Liquid Dewetting of Thin PS/PCL Blend Films during Spin Coating. Langmuir 2011, 27, 1056–1063. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahara, A.; Kajiyama, T. Surface molecular aggregation structure and surface molecular motions of high-molecular-weight polystyrene/low-molecular-weight poly (methyl methacrylate) blend films. Macromolecules 1998, 31, 863–869. [Google Scholar] [CrossRef]
- Wool, R.P. Polymer Interfaces: Structure and Strength; Hanser: Cincinnati, OH, USA, 1995. [Google Scholar]
- Lewin, M.; Mey-Marom, A.; Frank, R. Surface free energies of polymeric materials, additives and minerals. Polym. Adv. Technol. 2005, 16, 429–441. [Google Scholar] [CrossRef]
- Lins, L.; Bugatti, V.; Livi, S.; Gorrasi, G. Ionic liquid as surfactant agent of hydrotalcite: Influence on the final properties of polycaprolactone matrix. Polymers 2018, 10, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldarelli, C.; Jain, R.K.; Ivanov, I.B.; Ruckenstein, E. Stability of symmetric and unsymmetric thin liquid films to short and long wavelength perturbations. J. Colloid Interface Sci. 1980, 78, 118–143. [Google Scholar] [CrossRef] [Green Version]
- De Silva, J.; Geoghegan, M.; Higgins, A.; Krausch, G.; David, M.-O.; Reiter, G. Switching layer stability in a polymer bilayer by thickness variation. Phys. Rev. Lett. 2007, 98, 267802. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, W.; Weng, Y.; Chen, X.; Cheng, Y.; Zhou, P. Fabrication of PDMS surfaces with micro patterns and the effect of pattern sizes on bacteria adhesion. Food Control 2016, 68, 344–351. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef]







| Contact Angle (o) | |||
|---|---|---|---|
| PS:PCL | PS:PMMA | PCL:PMMA | |
| 0:100 | 76.2 ± 1.2 a | 68.9 ± 1.6 a,f | 68.9 ± 1.6 a |
| 25:75 | 71.8 ± 3.6 b | 70.0 ± 1.0 b,c | 67.2 ± 2.4 b,c |
| 50:50 | 72.9 ± 3.8 c,d | 68.6 ± 2.4 d,e | 71.4 ± 1.2 |
| 75:25 | 81.4 ± 2.3 b,c | 81.1 ± 5.3 b,d,f | 72.7 ± 2.8 b |
| 100:0 | 83.8 ± 1.5 a,b,d | 83.8 ± 1.5 a,c,e | 76.2 ± 1.2 a,c |
| C 1s Component at.% (Binding Energy, eV) | |||||
|---|---|---|---|---|---|
| C–C/C–H Aliphatic (285.0) | β-shifted C (C–C/C–H Aromatic) a (285.5) | C–O (286.3) | C=O (289.1) | π–π* (291.6) | |
| PCL100 | 56.0 ± 3.9 | 15.9 ± 0.7 | 8.1 ± 2.5 | 15.7 ± 0.6 | - |
| PS25PCL75 | 54.4 ± 4.4 | 21.7 ± 4.7 | 13.1 ± 0.6 | 10.2 ± 0.3 | 0.7 ± 0.2 |
| PS50PCL50 | 69.3 ± 7.5 | 15.7 ± 7.5 | 8.4 ± 0.5 | 5.6 ± 0.5 | 1.3 ± 0.4 |
| PS75PCL25 | 55.9 ± 2.9 | 34.0 ± 2.9 | 4.6 ± 0.5 | 1.6 ± 0.7 | 3.7 ± 0.7 |
| PS100 | 69.7 ± 0.6 | 24.1 ± 0.3 a | - | - | 6.2 ± 0.3 |
| PMMA100 | 38.0 ± 1.3 | 21.0 ± 1.8 | 21.4 ± 2.0 | 19.6 ± 1.0 | - |
| PS25PMMA75 | 43.5 ± 3.1 | 23.0 ± 4.2 | 18.3 ± 1.0 | 15.2 ± 1.0 | - |
| PS50PMMA50 | 54.7 ± 4.5 | 18.0 ± 5.7 | 15.1 ± 1.5 | 10.8 ± 0.6 | 1.2 ± 0.1 |
| PS75PMMA25 | 62.2 ± 3.7 | 20.2 ± 1.3 | 9.1 ± 3.5 | 6.5 ± 2.2 | 2.0 ± 0.6 |
| PS100 | 69.7 ± 0.6 | 24.1 ± 0.3a | - | - | 6.2 ± 0.3 |
| PMMA100 | 38.0 ± 1.3 | 21.0 ± 1.8 | 21.4 ± 2.0 | 19.6 ± 1.0 | - |
| PCL25PMMA75 | 44.5 ± 1.8 | 18.3 ± 0.6 | 19.1 ± 0.6 | 18.4 ± 0.2 | - |
| PCL50PMMA50 | 44.5 ± 1.8 | 17.7 ± 0.8 | 20.0 ± 3.0 | 17.8 ± 0.4 | - |
| PCL75PMMA25 | 42.2 ± 4.8 | 21.4 ± 4.2 | 19.2 ± 0.7 | 17.3 ± 0.2 | - |
| PCL100 | 56.0 ± 3.9 | 15.9 ± 0.7 | 8.1 ± 2.5 | 15.7 ± 0.6 | - |
| Topography | Feature Height/Depth (nm) | Feature Diameter (nm) | Feature Spacing (nm) | Rq (nm) | Ra (nm) | |
|---|---|---|---|---|---|---|
| PCL100 | flat | - | - | - | 5.2 ± 1.4 | 3.7 ± 0.9 |
| PS25PCL75 | islands | 48 ± 20 | 185 ± 53 | 197 ± 69 | 17.3 ± 5.5 | 14.4 ± 4.5 |
| PS50PCL50 | ribbons | 65 ± 14 | 609 ± 185 | 381 ± 237 | 21.3 ± 4.3 | 18.7 ± 4.1 |
| PS75PCL25 | pits | 72 ± 19 | 711 ± 239 | 248 ± 152 | 23.9 ± 4.4 | 18.9 ± 4.4 |
| PS100 | flat | - | - | - | 0.3 ± 0.0 | 0.2 ± 0.0 |
| PMMA100 | flat | - | - | - | 0.3 ± 0.1 | 0.2 ± 0.0 |
| PS25PMMA75 | islands | 7 ± 2 | 160 ± 42 | 200 ± 69 | 2.6 ± 0.4 | 2.2 ± 0.4 |
| PS50PMMA50 | pits | 8 ± 5 | 118 ± 39 | 60 ± 33 | 3.2 ± 1.2 | 2.5 ± 0.9 |
| PS75PMMA25 | pits | 11 ± 4 | 190 ± 57 | 88 ± 28 | 3.5 ± 0.9 | 2.7 ± 0.7 |
| PS100 | flat | - | - | - | 0.3 ± 0.0 | 0.2 ± 0.0 |
| PMMA100 | flat | - | - | - | 0.3 ± 0.1 | 0.2 ± 0.0 |
| PCL25PMMA75 | pitted islands | 29 ± 12 | 232 ± 100 | 145 ± 101 | 9.8 ± 5.4 | 8.4 ± 5.0 |
| PCL50PMMA50 | islands | 30 ± 20 | 154 ± 82 | 191 ± 113 | 9.6 ± 5.9 | 7.9 ± 4.7 |
| PCL75PMMA25 | islands | 16 ± 5 | 162 ± 57 | 186 ± 92 | 3.9 ± 1.0 | 2.9 ± 0.8 |
| PCL100 | flat | - | - | - | 5.2 ± 1.4 | 3.7 ± 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fleming, G.; Aveyard, J.; Fothergill, J.L.; McBride, F.; Raval, R.; D’Sa, R.A. Effect of Polymer Demixed Nanotopographies on Bacterial Adhesion and Biofilm Formation. Polymers 2019, 11, 1921. https://doi.org/10.3390/polym11121921
Fleming G, Aveyard J, Fothergill JL, McBride F, Raval R, D’Sa RA. Effect of Polymer Demixed Nanotopographies on Bacterial Adhesion and Biofilm Formation. Polymers. 2019; 11(12):1921. https://doi.org/10.3390/polym11121921
Chicago/Turabian StyleFleming, George, Jenny Aveyard, Joanne L. Fothergill, Fiona McBride, Rasmita Raval, and Raechelle A. D’Sa. 2019. "Effect of Polymer Demixed Nanotopographies on Bacterial Adhesion and Biofilm Formation" Polymers 11, no. 12: 1921. https://doi.org/10.3390/polym11121921
APA StyleFleming, G., Aveyard, J., Fothergill, J. L., McBride, F., Raval, R., & D’Sa, R. A. (2019). Effect of Polymer Demixed Nanotopographies on Bacterial Adhesion and Biofilm Formation. Polymers, 11(12), 1921. https://doi.org/10.3390/polym11121921