Optimization of Waterborne Poly(Urethane-Acrylate) Nanoemulsions Based on Cationic Polymerizable Macrosurfactants with Different Hydrophobic Side Chain Length
Abstract
:1. Introduction
2. Experimental
2.1. Materials
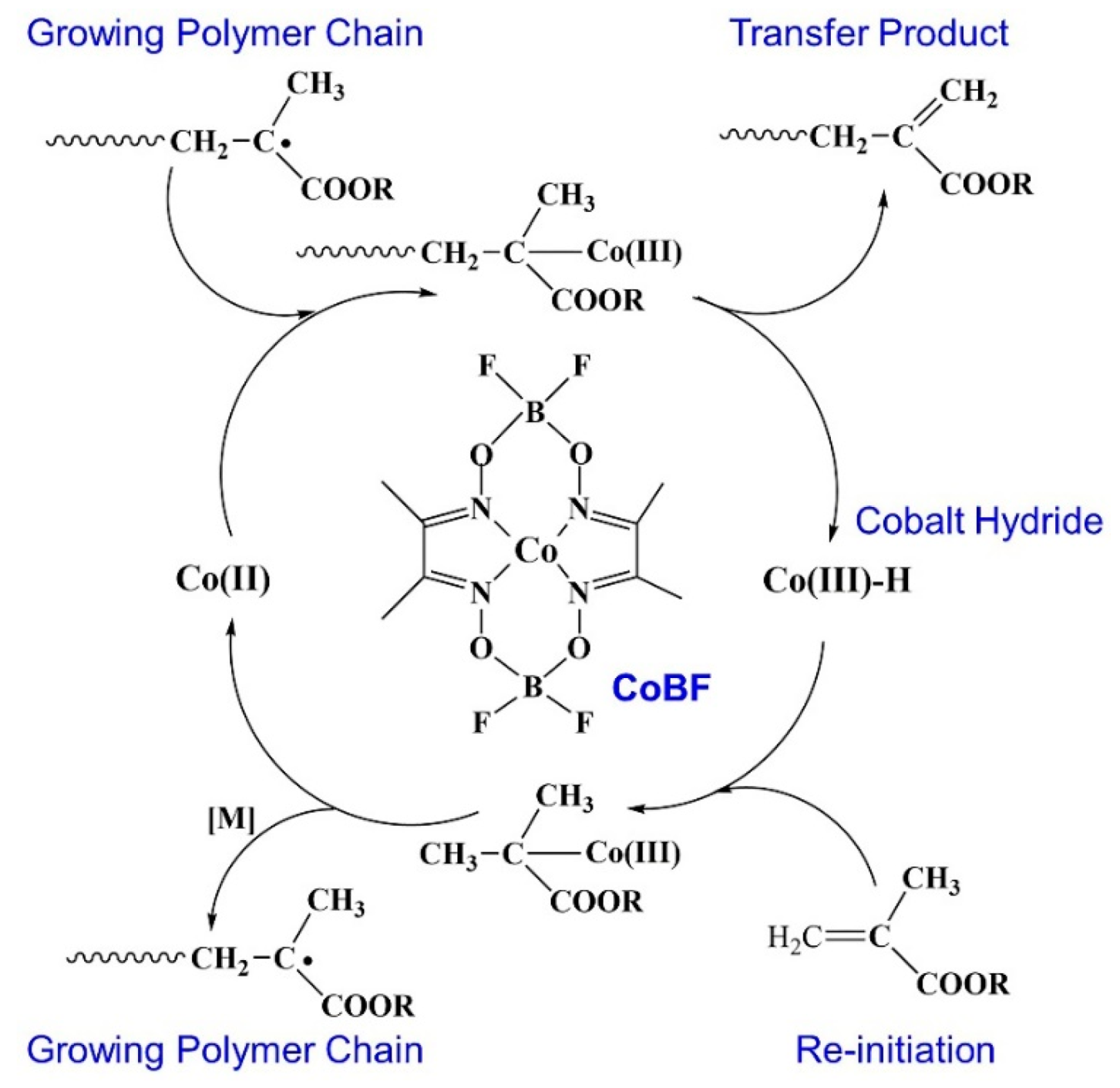
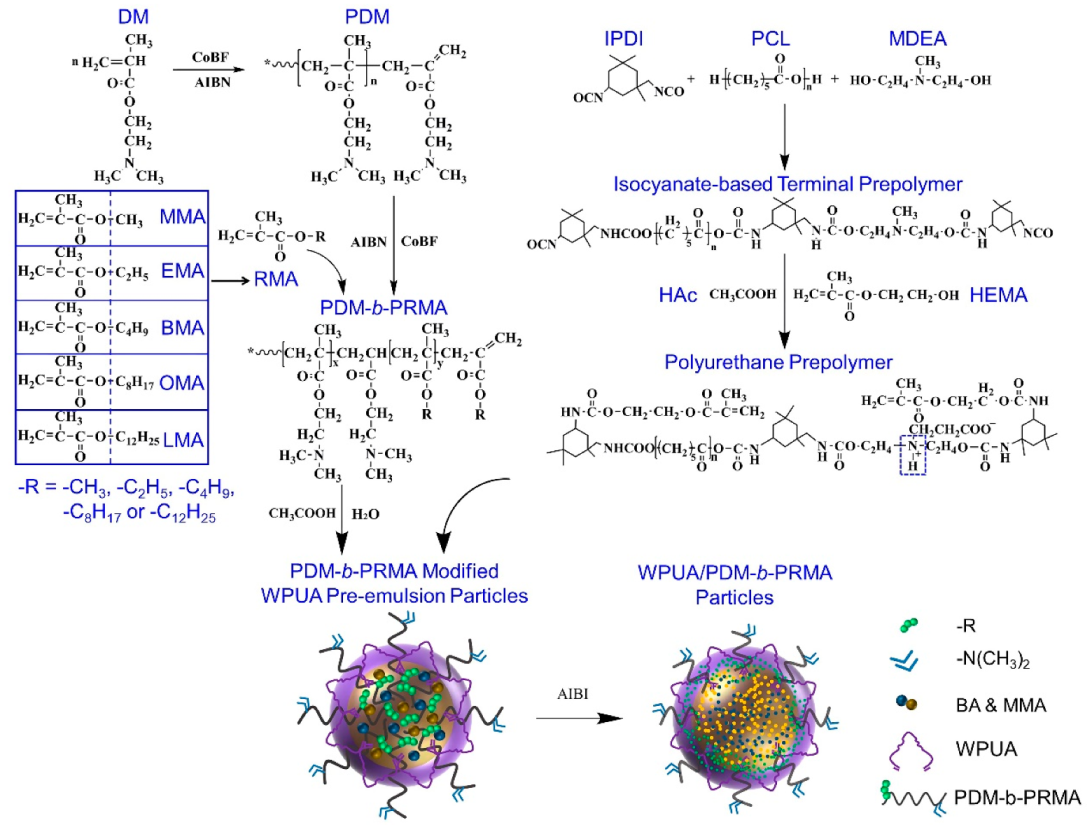
2.2. Preparation of PDM-b-PRMA Macrosurfactants through the CCTP Process
2.3. Preparation of WPUA Emulsion Based on PDM-b-PRMA Macrosurfactants
2.4. Characterizations
3. Results and Discussion
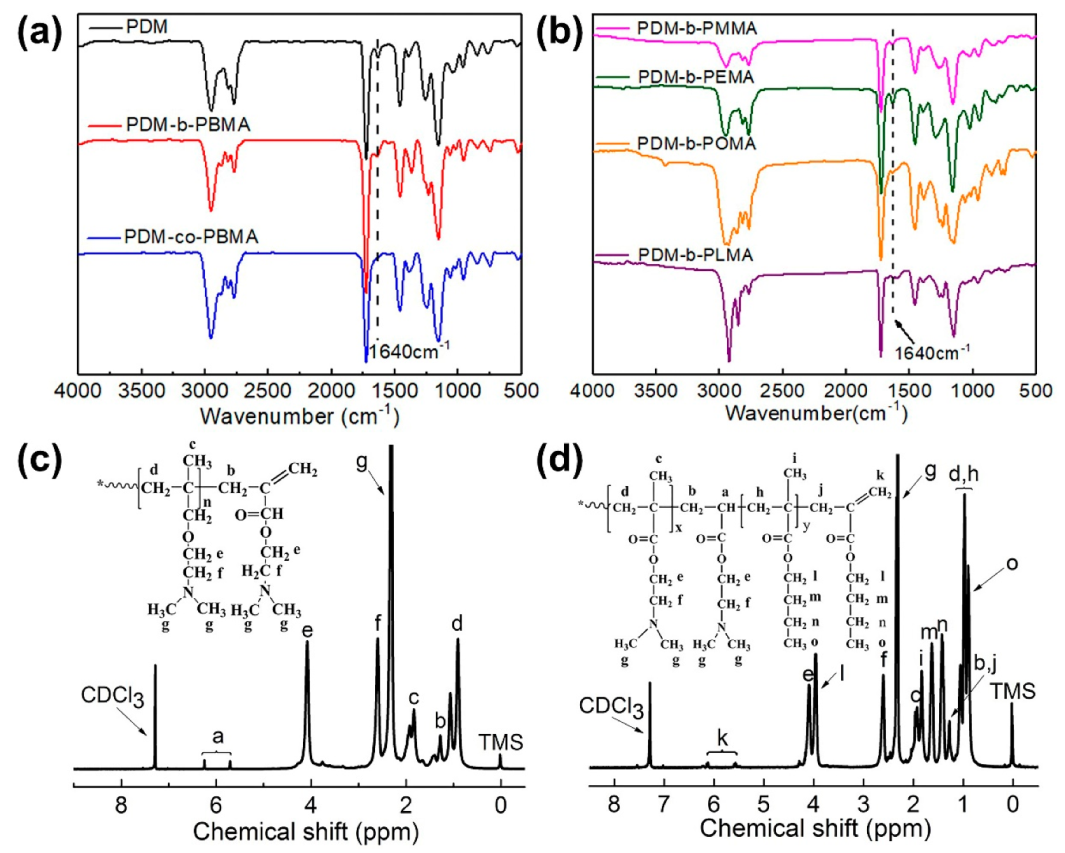
3.1. Structural Analysis
3.2. Thermal Behavior
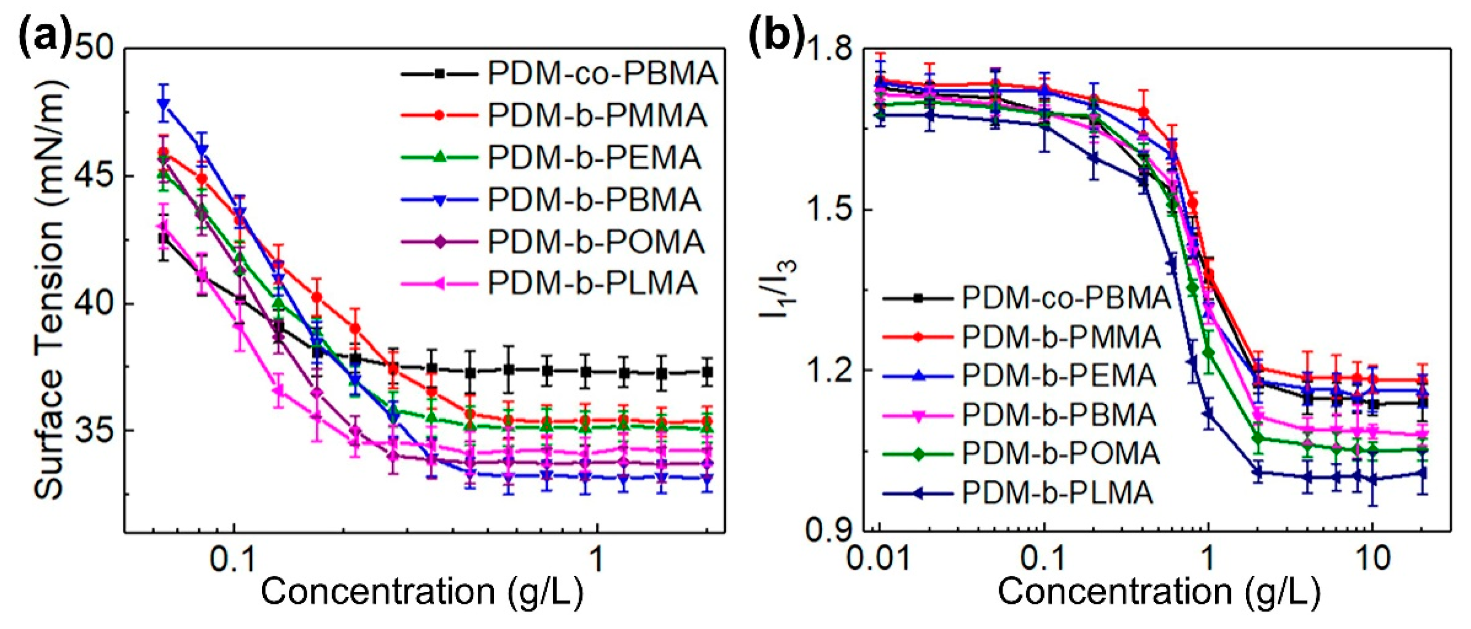
3.3. Solution Property Analysis
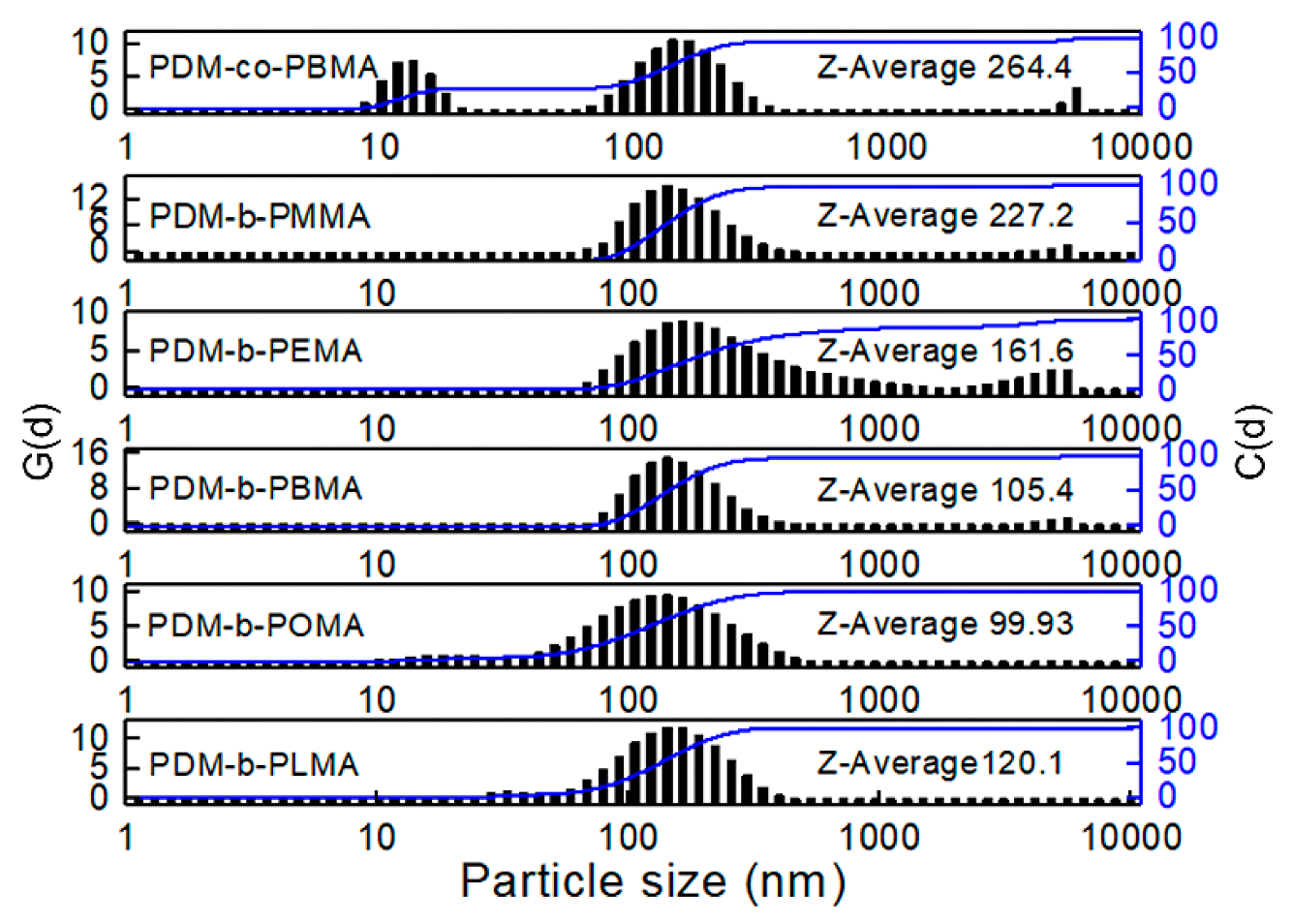
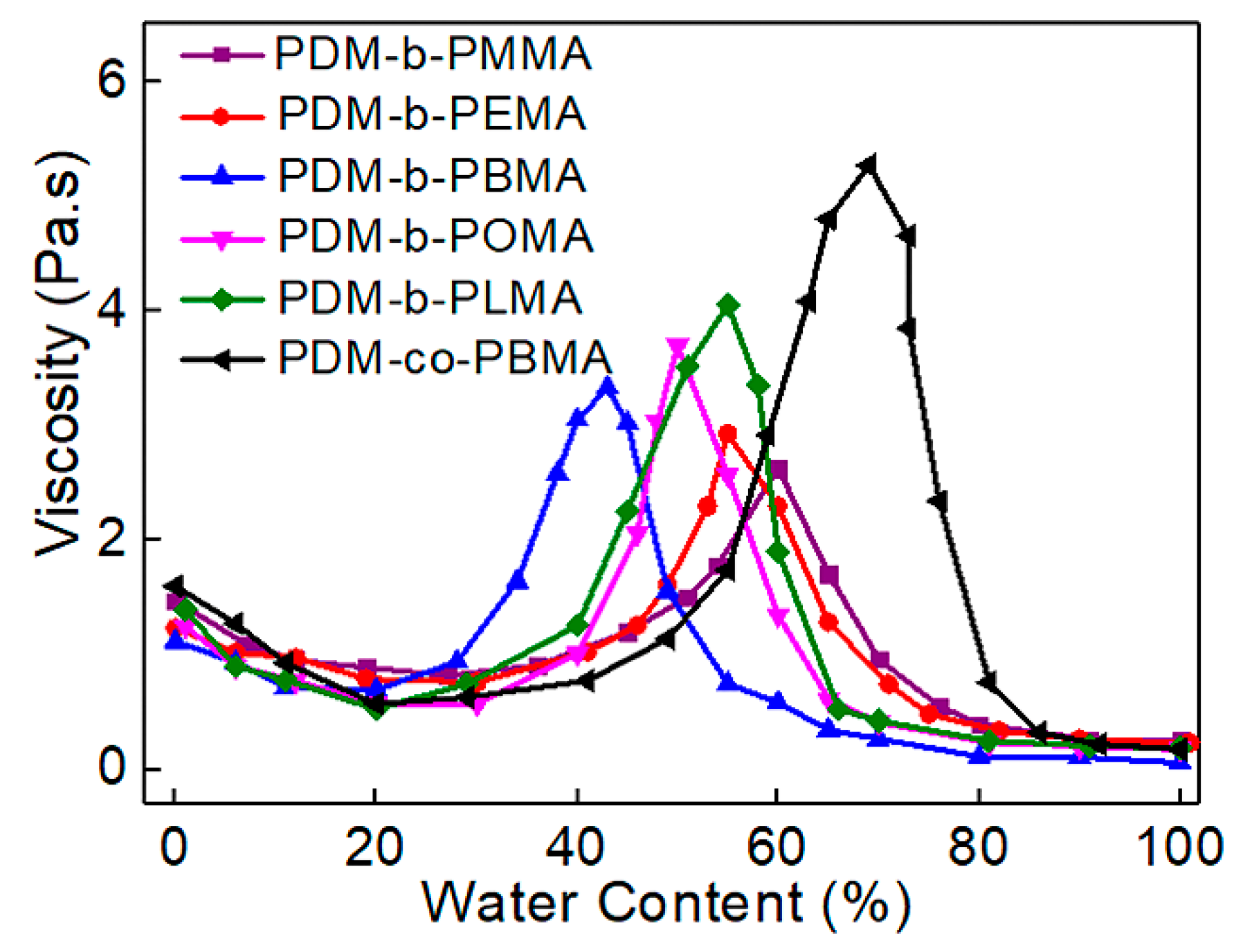
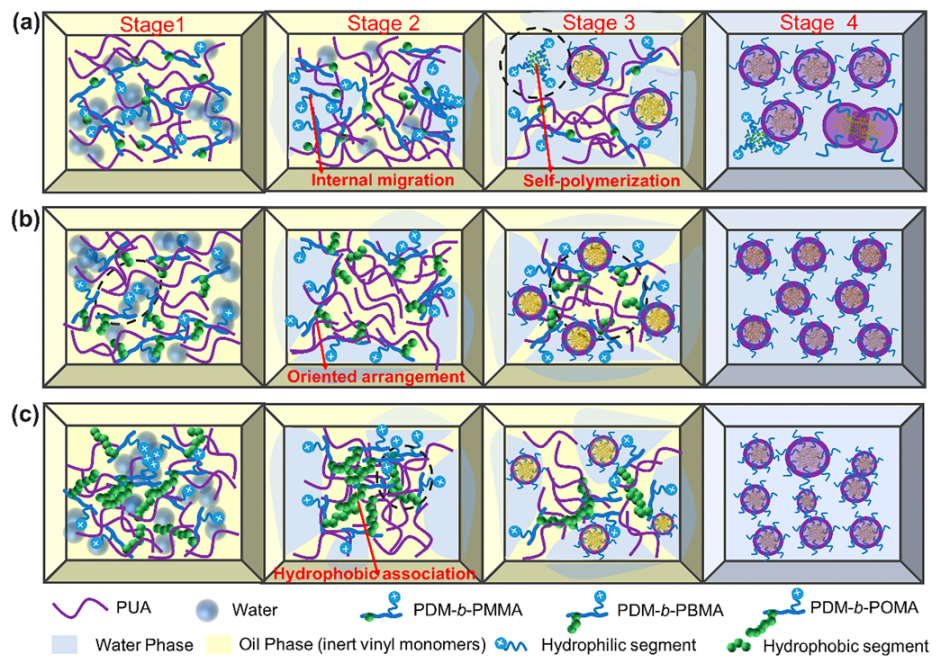
3.4. Phase Inversion Analysis of PUA Prepolymer and Application of Cationic Macrosurfactants to WPUA Nanoemulsions
3.5. Water Resistance, Thermal Stability, and Mechanical Properties Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kang, S.Y.; Ji, Z.X.; Tseng, L.F.; Turner, S.A.; Villanueva, D.A.; Johnson, R.; Albano, A.; Langer, R. Design and synthesis of waterborne polyurethanes. Adv. Mater. 2018, 30, 1706237. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.T.; Hu, J.L.; Xin, Z.Y. In-Situ incorporation of alkyl-grafted silica into waterborne polyurethane with high solid content for enhanced physical properties of coatings. Polymers 2018, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhao, W.J.; Hao, L.F.; Wang, S.; Pei, M.M.; Wang, X.C. Synthesis and characterization of novel fluoroalkyl-terminated hyperbranched polyurethane latex. Appl. Surf. Sci. 2017, 436, 1104–1112. [Google Scholar] [CrossRef]
- Jeong, J.H.; Hanb, Y.C.; Yangb, J.H.; Dong, S.K.; Jeonga, H.M. Waterborne polyurethane modified with poly (ethylene glycol) macromer for waterproof breathable coating. Prog. Org. Coat. 2016, 103, 69–75. [Google Scholar] [CrossRef]
- Xiao, Y.; Bao, L.X.; Fu, X.W.; Wu, B.; Kong, W.B.; Zhou, C.L.; Lei, J.X. Effect of phase separation on water resistance of green waterborne polyurethanes: Unexpected stronger impact compared to hydrophilic segments. Adv. Polym. Technol. 2017, 37, 1618–1624. [Google Scholar] [CrossRef]
- Hu, X.H.; Ding, Y.S.; Liu, J.; Deng, Y.; Cheng, C.L. Synthesis and fluorescence properties of a waterborne polyurethane-acrylic hybrid polymeric dye. Polym. Bull. 2017, 74, 555–569. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, T.; Zhang, J.H.; Chen, H.M.; Yuan, C.Y.; Zhang, W.D.; Zhang, A.M. Synthesis of a waterborne polyurethane-fluorinated emulsion and its hydrophobic properties of coating films. Ind. Eng. Chem. Res. 2014, 53, 19257–19264. [Google Scholar] [CrossRef]
- Yong, Q.W.; Pang, H.; Liao, B.; Mo, W.W.; Huang, F.R.; Huang, H.; Zhao, Y.Y. Preparation and characterization of low gloss aqueous coating via forming self-roughed surface based on waterborne polyurethane acrylate hybrid emulsion. Prog. Org. Coat. 2018, 115, 18–26. [Google Scholar] [CrossRef]
- Chen, K.L.; Gou, W.W.; Wang, X.M.; Zeng, C.J.; Ge, F.Q.; Dong, Z.J.; Wang, C.X. UV-cured fluoride-free polyurethane functionalized textile with pH-induced switchable superhydrophobicity and underwater superoleophobicity for controllable Oil/Water separation. ACS Sustain. Chem. Eng. 2018, 6, 16616–16628. [Google Scholar] [CrossRef]
- Wu, D.M.; Qiu, F.X.; Xu, H.P.; Zhang, J.L.; Yang, D.Y. Preparation, characterization, and properties of environmentally friendly waterborne poly (urethane acrylate)/silica hybrids. J. Appl. Polym. Sci. 2011, 119, 1683–1695. [Google Scholar] [CrossRef]
- Fang, H.G.; Wang, H.L.; Sun, J.; Wei, H.B.; Ding, Y.S. Tailoring elastomeric properties of waterborne polyurethane by incorporation of polymethyl methacrylate with nanostructural heterogeneity. RSC Adv. 2016, 6, 13589–13599. [Google Scholar] [CrossRef]
- Zhang, S.F.; Wang, R.M.; He, Y.F.; Song, P.F.; Wu, Z.M. Waterborne polyurethane-acrylic copolymers crosslinked core-shell nanoparticles for humidity-sensitive coatings. Prog. Org. Coat. 2013, 76, 729–735. [Google Scholar] [CrossRef]
- Fei, G.Q.; Zhu, K.; Wang, H.H.; Shen, Y.D.; Zou, J.; Lan, J. Morphology, dynamic rheology, and cohesive properties of epoxy-modified polyurethane-acrylate microemulsions prepared by in situ surfactant-free polymerization. J. Appl. Polym. Sci. 2014, 131, 39886. [Google Scholar] [CrossRef]
- Yu, F.F.; Cao, L.W.; Meng, Z.H.; Lin, N.B.; Liu, X.Y. Synthesis, characterization and fluorescence performance of a novel SAF-based waterborne polyurethane. Polym. Chem. 2017, 7, 13312. [Google Scholar]
- Qiu, F.X.; Xu, H.P.; Wang, Y.Y.; Xu, J.C.; Yang, D. Preparation, characterization and properties of UV-curable waterborne polyurethane acrylate/SiO2 coating. J. Coat. Technol. Res. 2012, 9, 503–514. [Google Scholar] [CrossRef]
- Yousefi, E.; Ghadimi, M.R.; Amirpoor, S.; Dolati, A. Preparation of new superhydrophobic and highly oleophobic polyurethane coating with enhanced mechanical durability. Appl. Surf. Sci. 2018, 454, 201–209. [Google Scholar] [CrossRef]
- Lee, E.J.; Park, K.H.; Lee, Y.H.; Kim, K.G.; Jeong, Y.U.; Kim, S.Y.; Kim, H.D. Poly(urethane acrylate)-based gel polymer films for mechanically stable, transparent, and highly conductive polymer electrolyte applications. J. Appl. Polym. Sci. 2017, 134, 45009. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Li, R.Q.; Zhang, C.Y.; Gong, S.L. Preparation and properties of high solid content and low viscosity waterborne polyurethane-acrylate emulsion with a reactive emulsifier. Polymers 2018, 10, 154. [Google Scholar] [CrossRef]
- Ma, L.; Song, L.N.; Wang, H.; Fan, L.Q.; Liu, B.H. Synthesis and characterization of poly(propylene carbonate) glycol-based waterborne polyurethane with a high solid content. Prog. Org. Coat. 2018, 122, 38–44. [Google Scholar] [CrossRef]
- Lai, X.J.; Shen, Y.D.; Wang, L. Synthesis and Properties of Waterborne Polyurethane-Acrylate Hybrid Emulsion Modified by Organic Silicane. Adv. Mater. Res. 2011, 287, 1532–1537. [Google Scholar] [CrossRef]
- Cui, M.Y.; Liu, C.; Xu, Q.; Lia, R.H. Effect of hybrid emulsifier (reactive coupling with anionic) on the properties of acrylic emulsion. J. Adhes. Sci. Technol. 2015, 29, 1758–1769. [Google Scholar] [CrossRef]
- Li, K.B.; Shen, Y.D.; Fei, G.Q.; Wang, H.H.; Li, J.Y. Preparation and properties of castor oil/pentaerythritol triacrylate-based UV curable waterborne polyurethane acrylate. Prog. Org. Coat. 2015, 78, 146–154. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, Y.H.; Park, H.; Kim, H.D. Effect of total acrylic/fluorinated acrylic monomer contents on the properties of waterborne polyurethane/acrylic hybrid emulsions. Macromol. Res. 2013, 21, 709–718. [Google Scholar] [CrossRef]
- Hou, L.J.; Ding, Y.T.; Zhang, Z.L.; Sun, Z.S.; Shan, Z.H. Synergistic effect of anionic and nonionic monomers on the synthesis of high solid content waterborne polyurethane. Colloids and Surfaces A: Physicochem. Eng. Aspects 2015, 467, 46–56. [Google Scholar]
- Zheng, Y.; Zhang, X.; Li, J. Synthesis and photochromism properties of anionic waterborne polyurethane containing azobenzene chromophores. J. Macromol. Sci. 2015, 52, 942–949. [Google Scholar] [CrossRef]
- Fei, G.Q.; Yan, T.; Wang, H.H.; Shen, Y.D.; Zou, J. Micromorphology, phase behavior, and properties of environmental, multi-cross-linked polyurethane/polyacrylate microemulsions based on in situ surfactant-free polymerization. Colloid. Polym. Sci. 2017, 295, 1743–1755. [Google Scholar] [CrossRef]
- Hou, Z.S.; Qu, W.Q.; Kan, C.Y. Synthesis and properties of triethoxysilane-terminated anionic polyurethane and its waterborne dispersions. J. Polym. Res. 2015, 22, 111. [Google Scholar] [CrossRef]
- Xi, H.; Shen, Y.D.; Li, X.R. Novel cationic polyurethane-fluorinated acrylic hybrid latexes: Synthesis, characterization and properties. Colloids Surf. A. 2011, 384, 205–211. [Google Scholar]
- Dong, C.H.; Xin, W.; Luo, Y.J. Synthesis and application of a cationic waterborne polyurethane fixative using quaternary ammonium diol as a chain extender. RSC Adv. 2018, 8, 42041–42048. [Google Scholar] [CrossRef]
- Wang, H.H.; Shen, Y.D.; Fei, G.Q.; Li, X.R.; Liang, Y. Micromorphology and phase behavior of cationic polyurethane segmented copolymer modified with hydroxysilane. J. Colloid Interface Sci. 2008, 324, 36–41. [Google Scholar] [CrossRef]
- Li, M.; Liu, F.; Li, Y.; Qiang, X.H. Synthesis of stable cationic waterborne polyurethane with a high solid content: Insight from simulation to experiment. RSC Adv. 2017, 7, 13312–13324. [Google Scholar] [CrossRef]
- Daemia, H.; Rad, R.R.; Barikanic, M.; Adib, M. Catalytic activity of aqueous cationic polyurethane dispersions: A novel feature of polyurethanes. Appl. Catal. A Gen. 2013, 468, 10–17. [Google Scholar] [CrossRef]
- Sheng, L.X.; Zhang, X.T.; Ge, Z.; Liang, Z.; Liu, X.L.; Chai, C.P.; Luo, Y.J. Preparation and properties of waterborne polyurethane modified by stearyl acrylate for water repellents. J. Coat. Technol. Res. 2018, 15, 1283–1292. [Google Scholar] [CrossRef]
- Luo, X.M.; Zhang, P.; Liu, R.; Li, W.H.; Ge, B.H.; Cao, M. Preparation and physical properties of functionalized graphene/waterborne polyurethane UV-curing composites by click chemistry. Polym. Int. 2016, 65, 415–422. [Google Scholar] [CrossRef]
- Clamena, G.; Ferrari, T.; Fu, Z.W.; Hejl, A.; Larson, G.; Procopio, L.; Rosano, W.; Sheppard, A.; Swartz, A. Protection of metal with a novel waterborne acrylic/urethane hybrid technology. Prog. Org. Coat. 2011, 72, 144–151. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, J.S.; Cheong, I.W.; Kim, J.H. Synthesis, characterization, and mechanical property of poly(urethane-glycidyl methacrylate-methyl methacrylate) hybrid polymers. J. Appl. Polym. Sci. 2011, 121, 3111–3121. [Google Scholar] [CrossRef]
- Li, M.R.; Zheng, Z.; Liu, S.J.; Su, Y.Z.; Wei, W.; Wang, X.L. Synthesis and properties of poly(acrylates-co-urethane) adhesives for low surface energy materials. Int. J. Adhes. Adhes. 2011, 31, 565–570. [Google Scholar] [CrossRef]
- Assanvo, E.F.; Baruah, S.D. Synthesis and properties of ricinodendron heudelotii oil based hybrid alkyd–acrylate latexes via miniemulsion polymerization. Prog. Org. Coat. 2015, 86, 25–32. [Google Scholar] [CrossRef]
- Wang, H.H.; Niu, Y.K.; Fei, G.Q.; Shen, Y.D.; Lan, J. In-situ polymerization, rheology, morphology and properties of stable alkoxysilane-functionalized poly (urethane-acrylate) microemulsion. Prog. Org. Coat. 2016, 99, 400–411. [Google Scholar] [CrossRef]
- Wang, H.H.; Li, B.; Fei, G.Q.; Shen, Y.D.; Zhu, K. Phase inversion, formation and stability mechanism of poly (urethane-acrylate) nanoemulsions based on block-copolymer surfmer. Appl. Surf. Sci. 2018, 456, 307–317. [Google Scholar] [CrossRef]
- Sukhawipat, N.; Saetung, N.; Pilard, J.F.; Bistac, S. Synthesis and characterization of novel natural rubber based cationic waterborne polyurethane—Effect of emulsifier and diol class chain extender. J. Appl. Polym. Sci. 2017, 135, 45715. [Google Scholar] [CrossRef]
- Udagama, R.; Degrandi-Contraires, E.; Creton, C.; Graillat, C.; McKenna, T.F.L.; Bourgeat-Lami, E. Synthesis of acrylic-polyurethane hybrid latexes by miniemulsion polymerization and their pressure-sensitive adhesive applications. Macromolecules 2011, 44, 2632–2642. [Google Scholar] [CrossRef]
- Michael, G.H.; Damien, G. Introduction of highly tunable end-groups in polyethylene via chain-transfer polymerization using a cobalt(III) catalyst. Organometallics 2019, 38, 788–796. [Google Scholar]
- Adlington, K.; Jones, G.J.; Harfi, J.E.; Dimitrakis, G.; Smith, A.; Kingman, S.W.; Robinson, J.P.; Irvine, D.J. Mechanistic investigation into the accelerated synthesis of methacrylate oligomers via the application of catalytic chain transfer polymerization and selective microwave heating. Macromolecules 2013, 46, 3922–3930. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, F.; Wang, Y.; Wu, W.; Yang, D.; Guo, Q. UV-curable waterborne polyurethane-acrylate: Preparation, characterization and properties. Prog. Org. Coat. 2012, 73, 47–53. [Google Scholar] [CrossRef]
- Heinze, D.; Mang, T.; Popescu, C.; Weichold, O. Effect of side chain length and degree of polymerization on the decomposition and crystallization behaviour of chlorinated poly(vinyl ester) oligomers. Thermochim. Acta 2016, 637, 143–153. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Inaba, A.; Brown, J.E.; Hatada, K.; Kitayama, T.; Masuda, E. Effects of weak linkages on the thermal and oxidative degradation of poly(methyl methacrylates). Macromolecules 1986, 19, 2160–2168. [Google Scholar] [CrossRef]
- Jing, C.; Suzuki, Y.; Matsumoto, A. Thermal decomposition of methacrylate polymers containing tert-butoxycarbonyl moiety. Polym. Degrad. Stab. 2019, 166, 145–154. [Google Scholar] [CrossRef]




| Polymer | RMA/g | PDM/g | CoBF Dosage | AIBN/g |
|---|---|---|---|---|
| PDM-b-PMMA | 10.0 (MMA) | 15.7 | 100 ppm | 0.08 |
| PDM-b-PEMA | 11.4 (EMA) | 15.7 | 100 ppm | 0.08 |
| PDM-b-PBMA | 14.4 (BMA) | 15.7 | 100 ppm | 0.08 |
| PDM-b-POMA | 19.8 (OMA) | 15.7 | 100 ppm | 0.08 |
| PDM-b-PLMA | 25.4 (LMA) | 15.7 | 100 ppm | 0.08 |
| Sample | Mn | Mw | PDI | Mn′ | X | C | D |
|---|---|---|---|---|---|---|---|
| PDM | 1200 | 1400 | 1.18 | 1400 | 18.52 | 87.5 | / |
| PDM-b-PMMA | 2000 | 2400 | 1.21 | 2400 | 10.54 | 84.4 | 10.00 |
| PDM-b-PEMA | 2300 | 2800 | 1.24 | 2700 | 9.28 | 82.6 | 12.28 |
| PDM-b-PBMA | 2600 | 3500 | 1.33 | 3200 | 7.85 | 81.1 | 14.79 |
| PDM-b-POMA | 3400 | 4900 | 1.42 | 4900 | 5.17 | 69.5 | 17.68 |
| PDM-b-PLMA | 4200 | 7000 | 1.67 | 8900 | 2.85 | 47.3 | 22.05 |
| PDM-co-PBMA | 12,500 | 23,900 | 1.93 | / | / | / | 158.45 |
| Sample | CMC (g/L) | γCMC (mN/m) | Γ (mol/m2) | A (nm2) |
|---|---|---|---|---|
| PDM-co-PBMA | 0.16 | 37.36 | 0.63 × 10−6 | 2.62 |
| PDM-b-PMMA | 0.31 | 35.44 | 1.15 × 10−6 | 1.44 |
| PDM-b-PEMA | 0.26 | 35.14 | 1.36 × 10−6 | 1.21 |
| PDM-b-PBMA | 0.33 | 33.27 | 2.43 × 10−6 | 0.68 |
| PDM-b-POMA | 0.22 | 33.71 | 1.96 × 10−6 | 0.85 |
| PDM-b-PLMA | 0.19 | 34.22 | 1.62 × 10−6 | 1.03 |
| Sample | The Water Content at PIP | Maximum Solid Content |
|---|---|---|
| WPUA/PDM-b-PMMA | 60% | 30% |
| WPUA/PDM-b-PEMA | 55% | 35% |
| WPUA/PDM-b-PBMA | 43% | 40% |
| WPUA/PDM-b-POMA | 50% | 35% |
| WPUA/PDM-b-PLMA | 55% | 35% |
| WPUA/PDM-co-PBMA | 68% | 25% |
| Sample | Ra0/nm | Ra/nm |
|---|---|---|
| WPUA/PDM-b-PBMA | 1.603 | 1.497 |
| WPUA/PDM-co-PBMA | 1.542 | 1.164 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, G.; Geng, H.; Wang, H.; Liu, X.; Liao, Y.; Shao, Y.; Wang, M. Optimization of Waterborne Poly(Urethane-Acrylate) Nanoemulsions Based on Cationic Polymerizable Macrosurfactants with Different Hydrophobic Side Chain Length. Polymers 2019, 11, 1922. https://doi.org/10.3390/polym11121922
Fei G, Geng H, Wang H, Liu X, Liao Y, Shao Y, Wang M. Optimization of Waterborne Poly(Urethane-Acrylate) Nanoemulsions Based on Cationic Polymerizable Macrosurfactants with Different Hydrophobic Side Chain Length. Polymers. 2019; 11(12):1922. https://doi.org/10.3390/polym11121922
Chicago/Turabian StyleFei, Guiqiang, Huanqiong Geng, Haihua Wang, Xuan Liu, Yong Liao, Yanming Shao, and Mengxi Wang. 2019. "Optimization of Waterborne Poly(Urethane-Acrylate) Nanoemulsions Based on Cationic Polymerizable Macrosurfactants with Different Hydrophobic Side Chain Length" Polymers 11, no. 12: 1922. https://doi.org/10.3390/polym11121922






