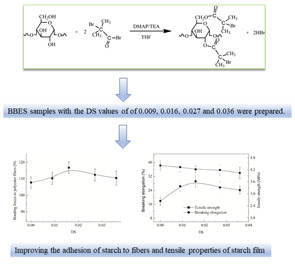
Investigation on the Synthesis Process of Bromoisobutyryl Esterified Starch and Its Sizing Properties: Viscosity Stability, Adhesion and Film Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation Method and Characterization of BBES for Studying Its Synthesis Process
2.2.1. Preparation
2.2.2. Measurement of DS
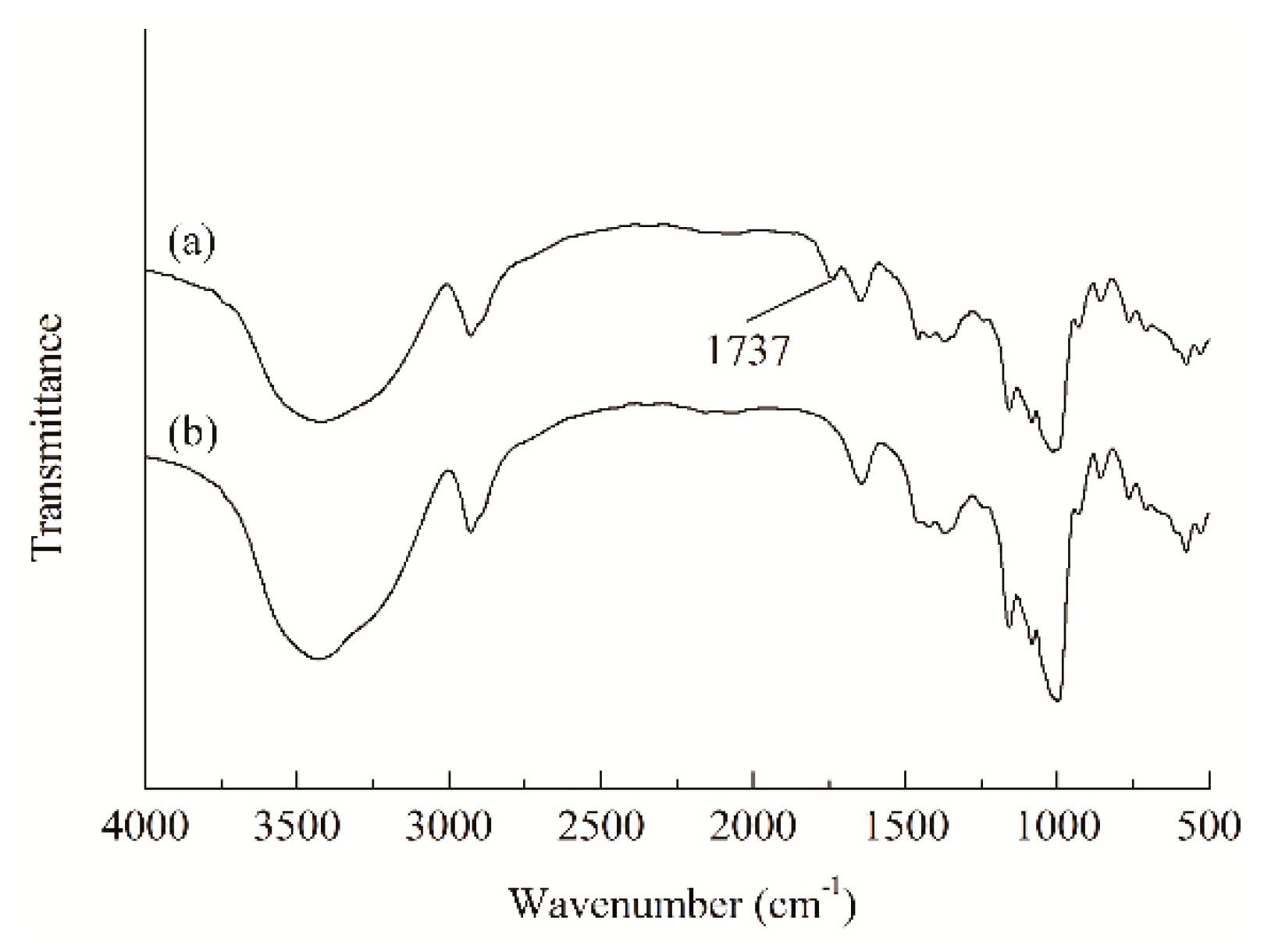
2.2.3. Infrared Spectral Analysis
2.3. Preparation Method of BBES under a Suitable Synthesis Process
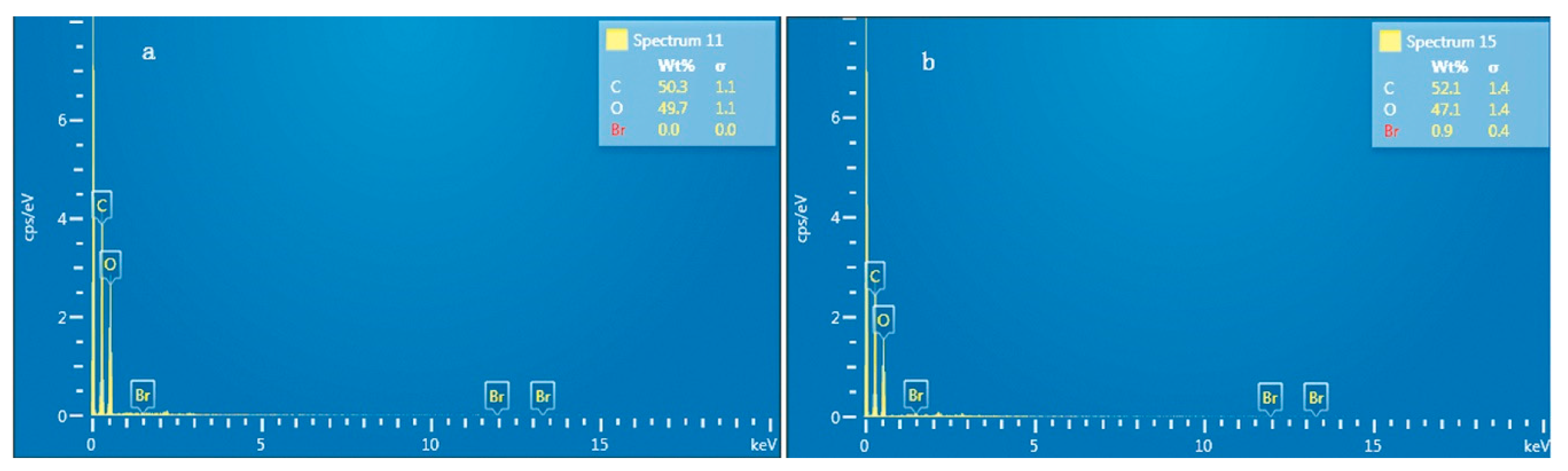
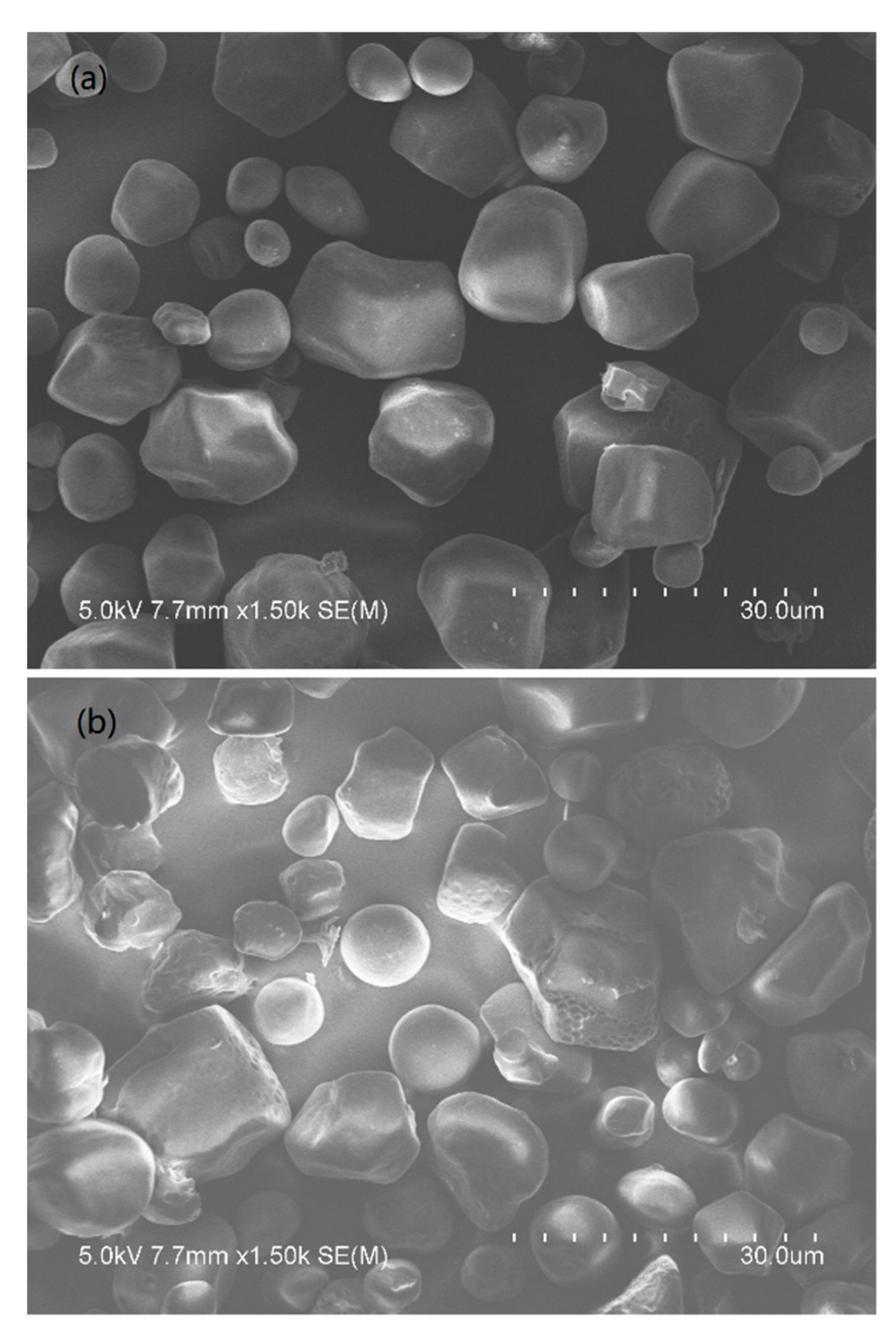
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Apparent Viscosity and Viscosity Stability
2.6. Adhesion Test
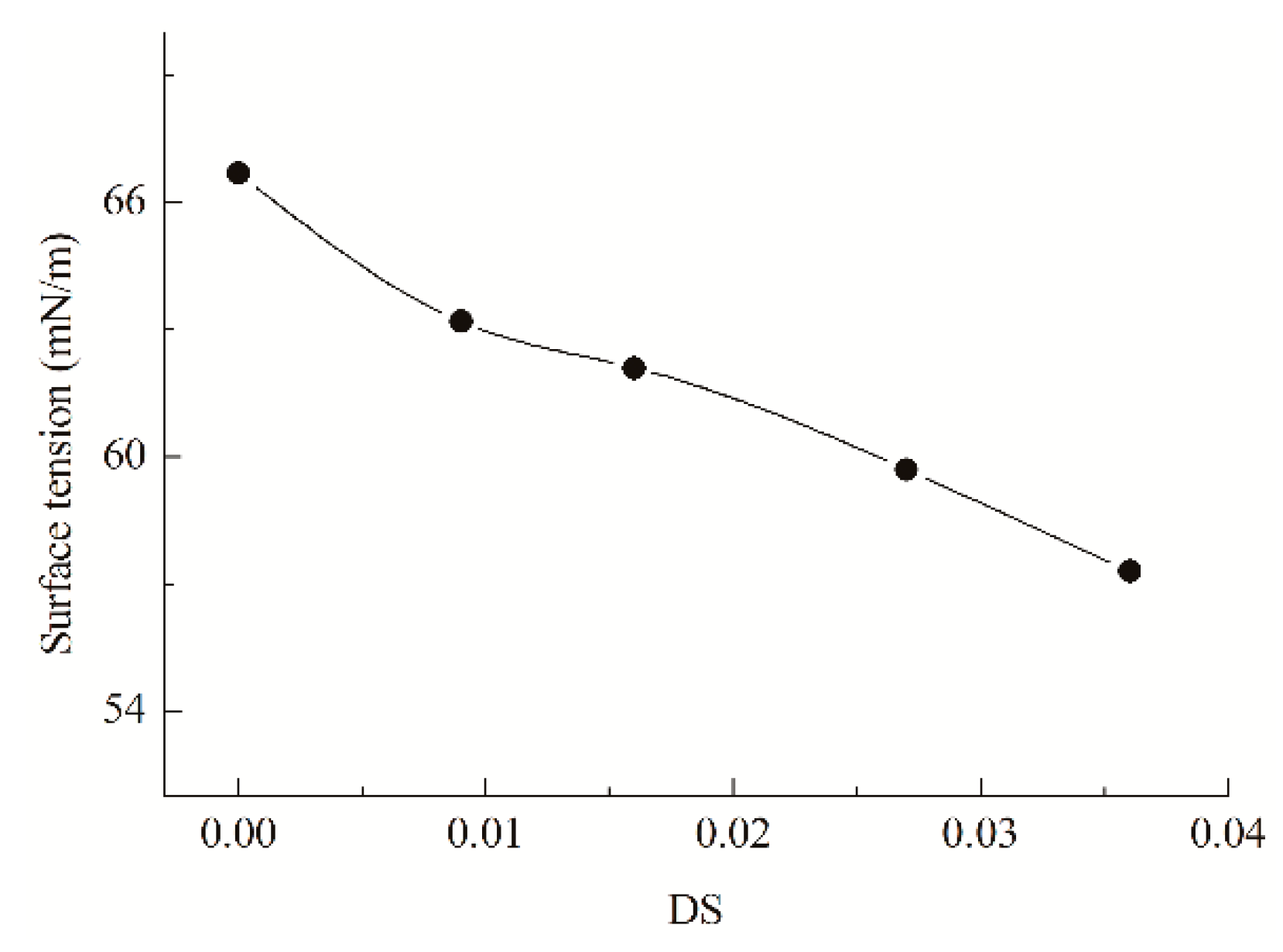
2.7. Surface Tension
2.8. Preparation and Measurement of Starch Films
2.8.1. Preparation
2.8.2. Measurement
2.9. X-Ray Diffraction (XRD) Analysis of Starch Film
3. Results and Discussion
3.1. Process Research of Synthesizing BBES
3.1.1. Influence of the Amount of BIBB on the DS
3.1.2. Influence of the Amount of DMAP on the DS
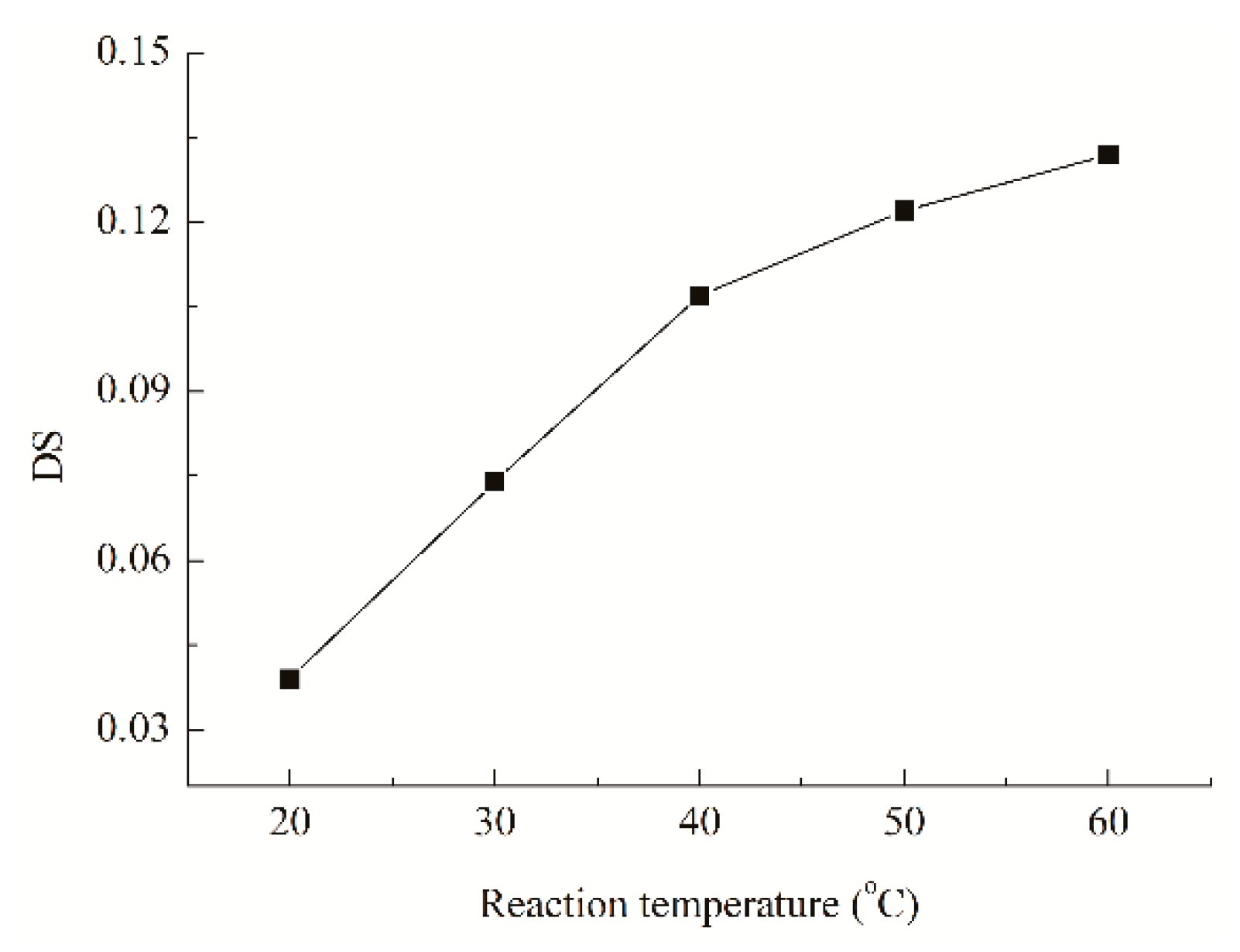
3.1.3. Influence of Reaction Temperature on the DS
3.1.4. Influence of Reaction Time on the DS
3.2. SEM and DS Analyses of the BBES Samples with the Suitable Synthesis Process
3.3. Influence of Bromoisobutyryl Esterification
3.3.1. Influence on Apparent Viscosity and Its Stability
3.3.2. Influence on Adhesion
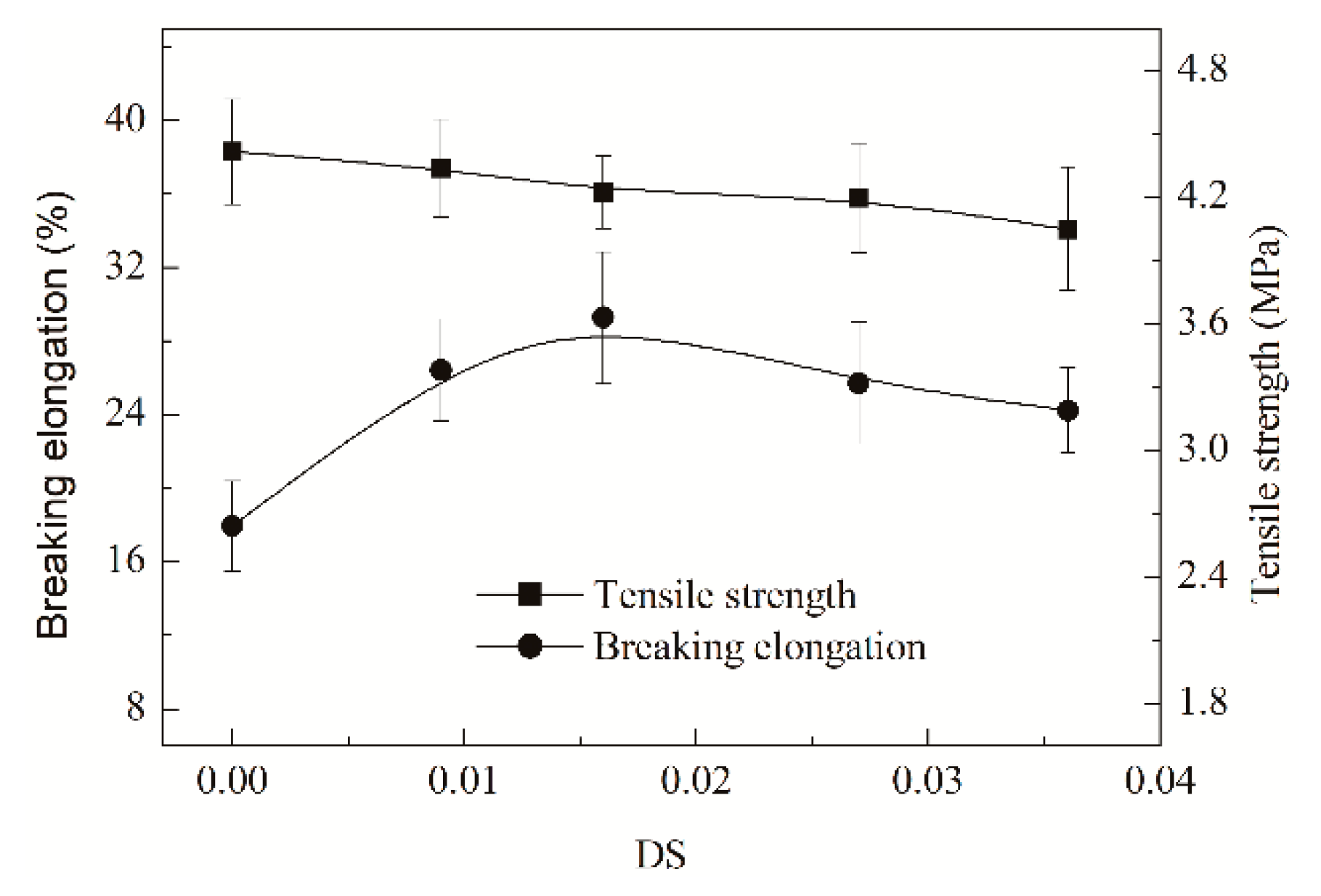
3.3.3. Influence on Film Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| degree of substitution | DS |
| bromoisobutyryl esterified cornstarch | BBES |
| Scanning electron microscopy | SEM |
| polylactic acid | PLA |
| 4-dimethylaminopyridine | DMAP |
| 2-bromoisobutyryl bromide | BIBB |
| acid-converted starch | ACS |
| electron transfer atom transfer radical polymerization | ARGET ATRP |
| Fourier transform infra-red | FTIR |
| X-ray diffraction | XRD |
| tetrahydrofuran | THF |
| triethylamine | TEA |
| relative humidity | RH |
| scanning electron microscopy with an energy-dispersive X-ray spectrometer | SEM-EDS |
References
- Ramaraj, B. Crosslinked poly (vinyl alcohol) and starch composite films. II. Physicomechanical, thermal properties and swelling studies. J. Appl. Polym. Sci. 2007, 103, 909–916. [Google Scholar] [CrossRef]
- Nurmi, L.; Holappa, S.; Mikkonen, H.; Seppälä, J. Controlled grafting of acetylated starch by atom transfer radical polymerization of MMA. Eur. Polym. J. 2007, 43, 1372–1382. [Google Scholar] [CrossRef]
- Cano, A.; Fortunati, E.; Cháfer, M.; Kenny, J.M.; Chiralt, A.; González-Martínez, C. Properties and ageing behaviour of pea starch films as affected by blend with poly(vinyl alcohol). Food Hydrocoll. 2015, 48, 84–93. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Chen, Y.; Chen, Y.; Chang, P.R. Comparative study on the films of poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17. [Google Scholar] [CrossRef]
- Xu, J.Y.; Krietemeyer, E.F.; Finkenstadt, V.L.; Solaiman, D.; Ashby, R.D.; Garcia, R.A. Preparation of starch-poly-glutamic acid graft copolymers by microwave irradiation and the characterization of their properties. Carbohydr. Polym. 2016, 140, 233–237. [Google Scholar] [CrossRef]
- Yao, K.H.; Cai, J.; Liu, M.; Yu, Y.; Xiong, H.G.; Tang, S.W.; Ding, S.Y. Structure and properties of starch/PVA/nano-SiO2, hybrid films. Carbohydr. Polym. 2011, 86, 1784–1789. [Google Scholar] [CrossRef]
- Daniel, J.R.; Whistler, R.L.; Roper, H. Starch. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley–VCH Verlag GmbH and Co.: Weinheim, Germany, 1997; pp. 721–726. [Google Scholar]
- Maurer, H.W.; Kearney, R.L. Opportunities and challenges for starch in the paper industry. Starch-Stärke 1998, 50, 396–402. [Google Scholar] [CrossRef]
- Shamai, K.; Bianco-Peled, H.; Shimoni, E. Polymorphism of resistant starch type III. Carbohydr. Polym. 2003, 54, 363–369. [Google Scholar] [CrossRef]
- Bansal, A.; Kumar, A.; Latha, P.P.; Ray, S.S. Expanded corn starch as a versatile material in atom transfer radical polymerization (ATRP) of styrene and methyl methacrylate. Carbohydr. Polym. 2015, 130, 290–298. [Google Scholar] [CrossRef]
- Wurzburg, O.B. Modified Starches: Properties and Uses; CRC Press: Boca Raton, FL, USA, 1986; pp. 3–16. [Google Scholar]
- Han, T.L.; Kumar, R.N.; Rozman, H.D.; Noor, M.A.M. GMA grafted sago starch as a reactive component in ultra violet radiation curable coatings. Carbohydr. Polym. 2003, 54, 509–516. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Cheng, X.G.; Wu, L.J.; Liu, Z.; Ni, Q.Q.; Lu, Y.H. Hydroxypropylsulfonation/caproylation of corn starch to enhance its adhesion to PLA fibers for PLA sizing. Polymers 2019, 11, 1197. [Google Scholar] [CrossRef]
- Fanta, G.F. Starch Graft Copolymers. In Polymeric Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 7901–7910. [Google Scholar]
- Jenkins, D.W.; Hudson, S.M. Review of vinyl graft copolymerization featuring recent advances toward controlled radical-based reactions and illustrated with chitin/chitosan trunk polymers. Chem. Rev. 2001, 101, 3245–3273. [Google Scholar] [CrossRef]
- Król, P.; Chmielarz, P. Synthesis of PMMA-b-PU-b-PMMA tri-block copolymers through ARGET-ATRP in the presence of air. Express Polym. Lett. 2013, 7, 249–260. [Google Scholar] [CrossRef]
- Yamamoto, S.I.; Matyjaszewski, K. ARGET-ATRP synthesis of thermally responsive polymers with oligo (ethylene oxide) units. Polym. J. 2008, 40, 496–497. [Google Scholar] [CrossRef]
- Hu, L.; Hu, Y.Y.; Chang, J.; Zhang, C.K. Synthesis and self-aggregation of PTRIS-co-MMA polymer films via ARGET-ATRP. Mater. Lett. 2014, 120, 79–81. [Google Scholar] [CrossRef]
- Chalid, M.; Handayani, A.S.; Budianto, E. Functionalization of starch for macro-initiator of atomic transfer radical polymerization (ATRP). Adv. Mater. Res. 2014, 1051, 90–94. [Google Scholar] [CrossRef]
- Wang, L.L.; Shen, J.N.; Men, Y.J.; Wu, Y.; Peng, Q.H.; Wang, X.L.; Yang, R.; Mahmood, K.; Liu, Z.P. Corn starch-based graft copolymers prepared via ATRP at the molecular level. Polym. Chem. 2015, 6, 3480–3488. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Sridhar, R.; Immanuel, V.P. Development of model for mechanical properties of tapioca starch based edible films. Ind. Crop. Prod. 2013, 42, 159–168. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Mechanical and thermal properties of yam starch films. Food Hydrocoll. 2005, 19, 157–164. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Starch blends and their physicochemical properties. Starch-Stärke 2015, 67, 1–13. [Google Scholar] [CrossRef]
- Zhou, Y.Y. Theory of Textile Warp Sizes; China Textile& Apparel Press: Beijing, China, 2004; pp. 115–260. [Google Scholar]
- Davidson, V.J.; Paton, D.; Diosady, L.L.; Larocque, G.J. Degradation of wheat starch in a single-screw extruder: Characteristics of extruded starch polymers. J. Food Sci. 1984, 49, 453–458. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Z.Q.; Wu, J.; Xu, Z.Z.; Liu, Z. Phosphorylation/caproylation of cornstarch to improve its adhesion to PLA and cotton fibers. RSC Adv. 2019, 9, 34880–34887. [Google Scholar] [CrossRef]
- Behera, B.K.; Gupta, R.; Mishra, R. Comparative analysis of mechanical properties of size film. I. Performance of individual size materials. Fibers Polym. 2008, 9, 481–488. [Google Scholar] [CrossRef]
- Beliakova, M.K.; Aly, A.A.; Abdel-Mohdy, F.A. Grafting of poly (methacrylic acid) on starch and poly (vinyl alcohol). Starch-Stärke 2004, 56, 407–412. [Google Scholar] [CrossRef]
- Meshram, M.W.; Patil, V.V.; Mhaske, S.T.; Thorat, B.N. Graft copolymers of starch and its application in textiles. Carbohydr. Polym. 2009, 75, 71–78. [Google Scholar] [CrossRef]
- John, M.J.; Anandjiwala, R.; Oksman, K.; Mathew, A.P. Melt-spun polylactic acid fibers: Effect of cellulose nanowhiskers on processing and properties. J. Appl. Polym. Sci. 2012, 127, 274–281. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Bello-Flores, C.A.; Nunez-Santiago, M.D.C.; Coronel-Aguilera, C.P.; Alvarez-Ramirez, J. Effect of the degree of substitution of octenyl succinic anhydride-banana starch on emulsion stability. Carbohydr. Polym. 2015, 132, 17–24. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Zhuo, R.X. Controlled release of carboxylic-containing herbicides by starch-g-poly (butyl acrylate). J. Appl. Polym. Sci. 2001, 81, 1535–1543. [Google Scholar] [CrossRef]
- Zhu, Z.F.; Cheng, Z.Q. Effect of inorganic phosphates on the adhesion of mono-phosphorylated cornstarch to fibers. Starch-Stärke 2008, 60, 315–320. [Google Scholar] [CrossRef]
- Bismark, S.; Zhu, Z.F. Amphipathic starch with phosphate and octenylsuccinate substituents for strong adhesion to cotton in warp sizing. Fibers Polym. 2018, 19, 1850–1860. [Google Scholar] [CrossRef]
- Varavinit, S.; Chaokasem, N.; Shobsngob, S. Studies of flavor encapsulation by agents produced from modified sago and tapioca starches. Starch-Stärke 2001, 53, 281–287. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.Z.; Wang, Z.Q.; Liu, X.H.; Li, C.L.; Ruan, F.T. Double etherification of corn starch to improve its adhesion to cotton and polyester fibers. Int. J. Adhes. Adhes. 2018, 84, 101–107. [Google Scholar] [CrossRef]
- Chang, Y.J.; Choi, H.W.; Kim, H.S.; Lee, H.; Kim, W.; Kim, D.O.; Kim, B.Y.; Baik, M.Y. Physicochemical properties of granular and non-granular cationic starches prepared under ultra high pressure. Carbohydr. Polym. 2014, 99, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Z.F. Electroneutral maize starch by quaterization and sulfosuccination for strong adhesion-to-viscose fibers and easy removal. J. Adhes. 2016, 92, 257–272. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.Z.; Wang, Z.Q.; Xing, J. One-step quaternization/hydroxypropylsulfonation to improve paste stability, adhesion and film properties of oxidized starch. Polymers 2018, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Ge, W. Chemical analysis of starch-like mineral crystals to eliminate misidentification in ancient residue research. Archaeometry 2013, 55, 1122–1131. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yoshimura, R.; Seki, C.; Fujioka, R. Synthesis and characterization of biodegradable hydrogels based on starch and succinic anhydride. Carbohydr. Polym. 2006, 64, 345–349. [Google Scholar] [CrossRef]
- Chang, P.R.; Qian, D.Y.; Anderson, D.P.; Ma, X.F. Preparation and properties of the succinic ester of porous starch. Carbohydr. Polym. 2012, 88, 604–608. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.; Guo, Y.; Lu, Y.H. Impact of hydroxypropylsulfonation on adhesion-to-fibers and film properties of corn starch. J. Text. Inst. 2019, 110, 1679–1686. [Google Scholar] [CrossRef]
- Xie, W.L.; Wang, Y.B. Synthesis of high fatty acid starch esters with 1-butyl-3-methylimidazolium chloride as a reaction medium. Starch-Stärke 2011, 63, 190–197. [Google Scholar] [CrossRef]
- Li, W.; Xu, W.Z.; Wei, A.F.; Xu, Z.Z.; Zhang, C.H. Quaternization/maleation of cornstarch to improve its adhesion and film properties for warp sizing. Fibers Polym. 2016, 17, 1589–1597. [Google Scholar] [CrossRef]
- Wurzburg, O.; Szymanski, C. Modified starches for the food industry. J. Agric. Food Chem. 1970, 18, 997–1001. [Google Scholar]
- Doublier, J.L.; Llamas, G.; Meur, M.L. A rheological investigation of cereal starch pastes and gels. Effect of pasting procedures. Carbohydr. Polym. 1987, 7, 251–275. [Google Scholar] [CrossRef]
- Wong, R.B.K.; Lelievre, J. Rheological characteristics of wheat starch pastes measured under steady shear conditions. J. Appl. Polym. Sci. 1982, 27, 1433–1440. [Google Scholar] [CrossRef]
- Wu, S.H. Polymer Interface and Adhesion; Marcel Dekker: New York, NY, USA, 1982; pp. 359–448. [Google Scholar]
- Shen, S.Q.; Zhu, Z.F.; Liu, F.D. Introduction of poly [(2-acryloyloxyethyl trimethyl ammonium chloride)-co-(acrylic acid)] branches onto starch for cotton warp sizing. Carbohydr. Polym. 2016, 138, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Z.F. Effect of sulfosuccinylation of corn starch on the adhesion to viscose fibres at lower temperature. Indian J. Fibre Text. Res. 2014, 39, 314–321. [Google Scholar]
- Zhu, Z.F.; Zhang, L.Y.; Feng, X.M. Introduction of 3-(trimethylammonium chloride)-2-hydroxypropyls onto starch chains for improving the grafting efficiency and sizing property of starch-g-poly (acrylic acid). Starch-Stärke 2016, 68, 742–752. [Google Scholar] [CrossRef]
- Zhang, K. Interface Science of Polymers; China Petrochemical Press: Beijing, China, 1996; p. 130. [Google Scholar]
- Zhu, Z.F.; Liu, Z.J.; Li, M.L.; Xu, D.S.; Li, C.L. Mono-phosphorylation of cornstarch to improve the properties of wool yarns sized at reduced temperature. J. Appl. Polym. Sci. 2013, 127, 127–135. [Google Scholar] [CrossRef]
- Zhu, Z.F. Chemistry in Textile Engineering; Donghua University Press Co. Ltd.: Shanghai, China, 2010; pp. 179–217. [Google Scholar]
- Seydel, P.V.; Hunt, J.R. Textile Warp Sizing; Phoenix Printing Inc.: Atlanta, GA, USA, 1981; pp. 5–16, 247–267. [Google Scholar]
- Thiré, R.M.S.; Simão, M.R.A.; Andrade, C.T. High resolution imaging of the microstructure of maize starch films. Carbohydr. Polym. 2003, 54, 149–158. [Google Scholar] [CrossRef]
- Li, W.; Xu, Z.Z.; Wang, Z.Q.; Li, C.L.; Feng, Q.; Zhu, Y.N. Tertiary amination/hydroxypropylsulfonation of cornstarch to improve the adhesion-to-fibers and film properties for warp sizing. Fibers Polym. 2018, 19, 1386–1394. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wu, J.; Zhang, Z.; Wu, L.; Lu, Y. Investigation on the Synthesis Process of Bromoisobutyryl Esterified Starch and Its Sizing Properties: Viscosity Stability, Adhesion and Film Properties. Polymers 2019, 11, 1936. https://doi.org/10.3390/polym11121936
Li W, Wu J, Zhang Z, Wu L, Lu Y. Investigation on the Synthesis Process of Bromoisobutyryl Esterified Starch and Its Sizing Properties: Viscosity Stability, Adhesion and Film Properties. Polymers. 2019; 11(12):1936. https://doi.org/10.3390/polym11121936
Chicago/Turabian StyleLi, Wei, Jie Wu, Zhengqiao Zhang, Lanjuan Wu, and Yuhao Lu. 2019. "Investigation on the Synthesis Process of Bromoisobutyryl Esterified Starch and Its Sizing Properties: Viscosity Stability, Adhesion and Film Properties" Polymers 11, no. 12: 1936. https://doi.org/10.3390/polym11121936
APA StyleLi, W., Wu, J., Zhang, Z., Wu, L., & Lu, Y. (2019). Investigation on the Synthesis Process of Bromoisobutyryl Esterified Starch and Its Sizing Properties: Viscosity Stability, Adhesion and Film Properties. Polymers, 11(12), 1936. https://doi.org/10.3390/polym11121936