An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents Used
2.2. Preparation of Silk Fibroin/Chitosan Hydrogel
2.2.1. Preparation of Silk Fibroin Solution
2.2.2. Preparation of Chitosan Solution
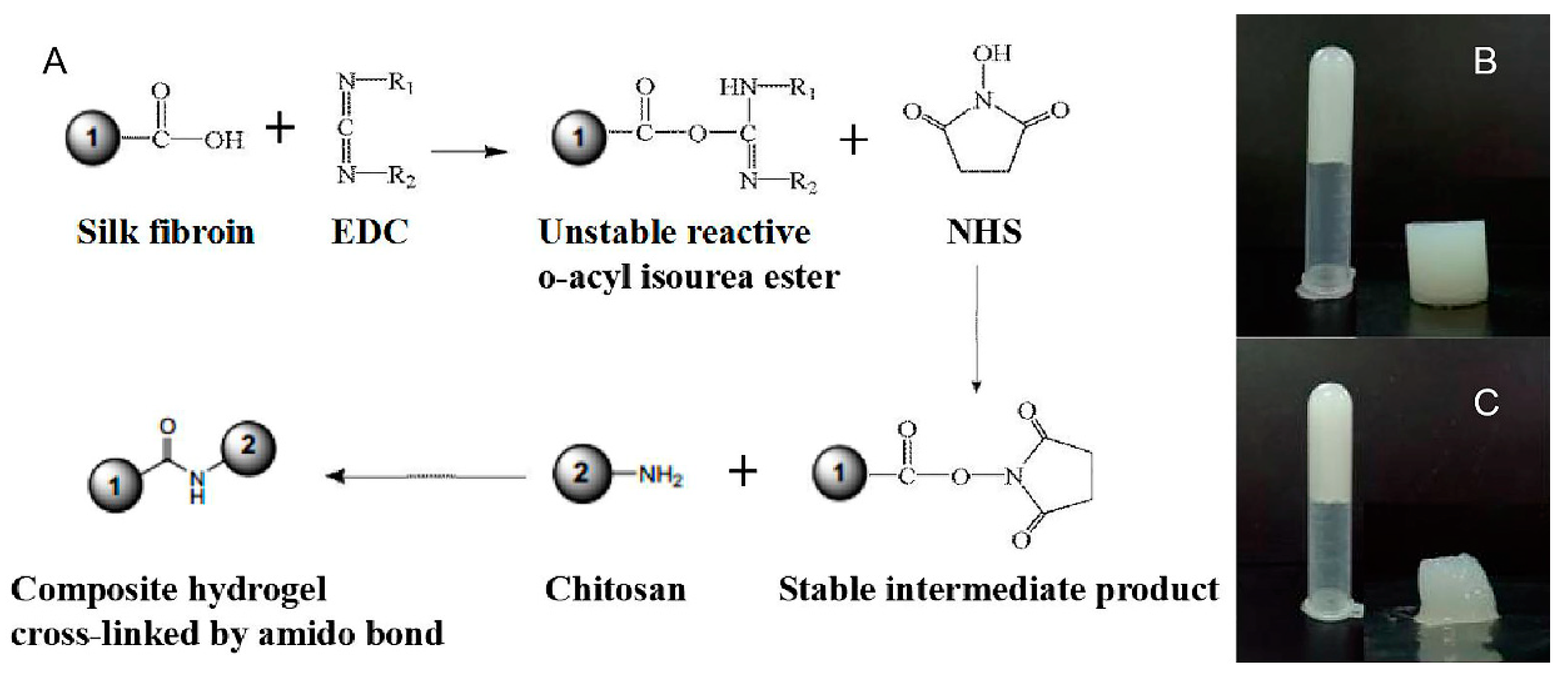
2.2.3. Preparation of the Composite Hydrogel
2.3. Characterization of the Hydrogel
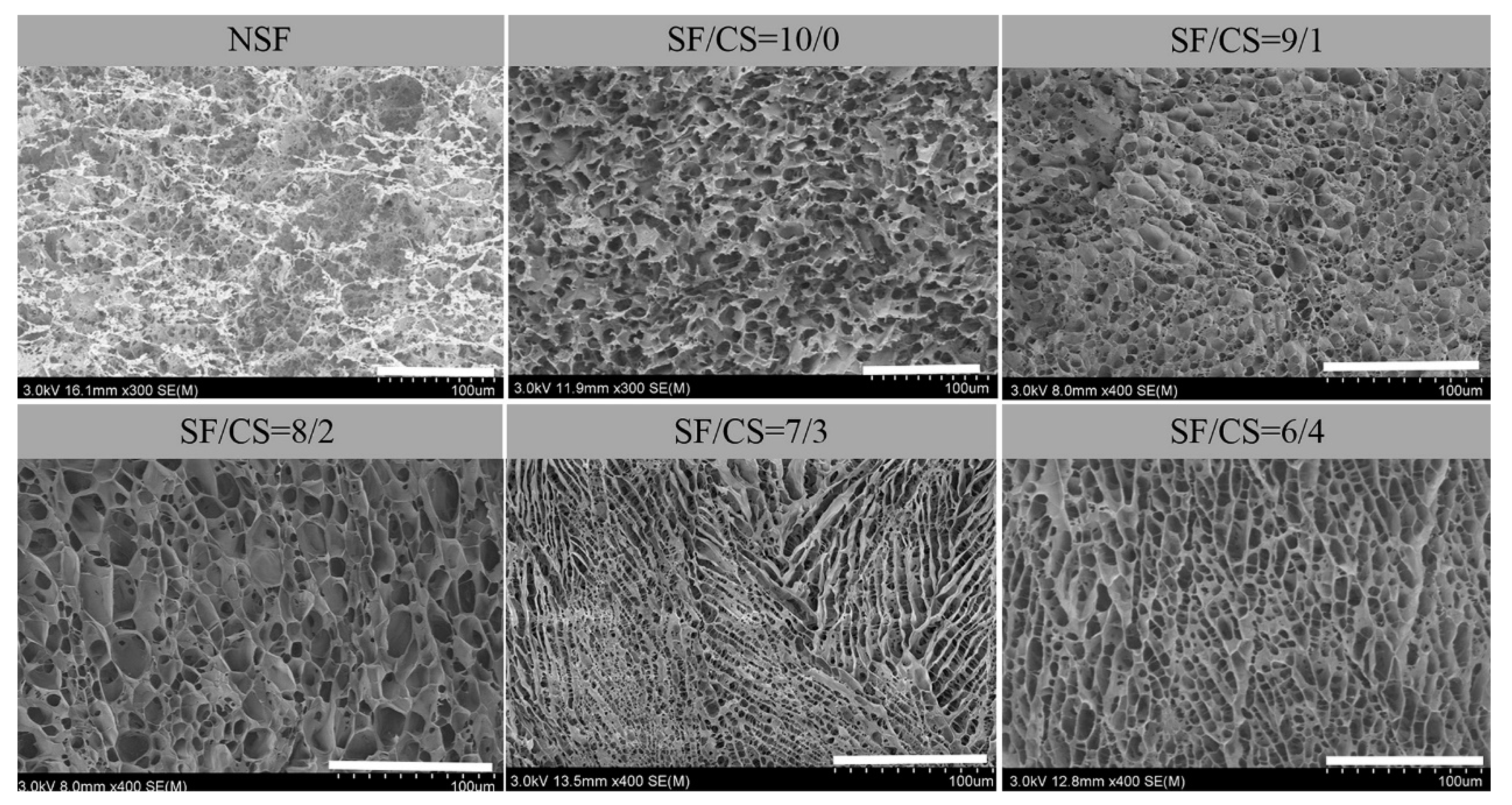
2.3.1. Scanning Electron Microscope (SEM)
2.3.2. N2 Adsorption-Desorption Experiment
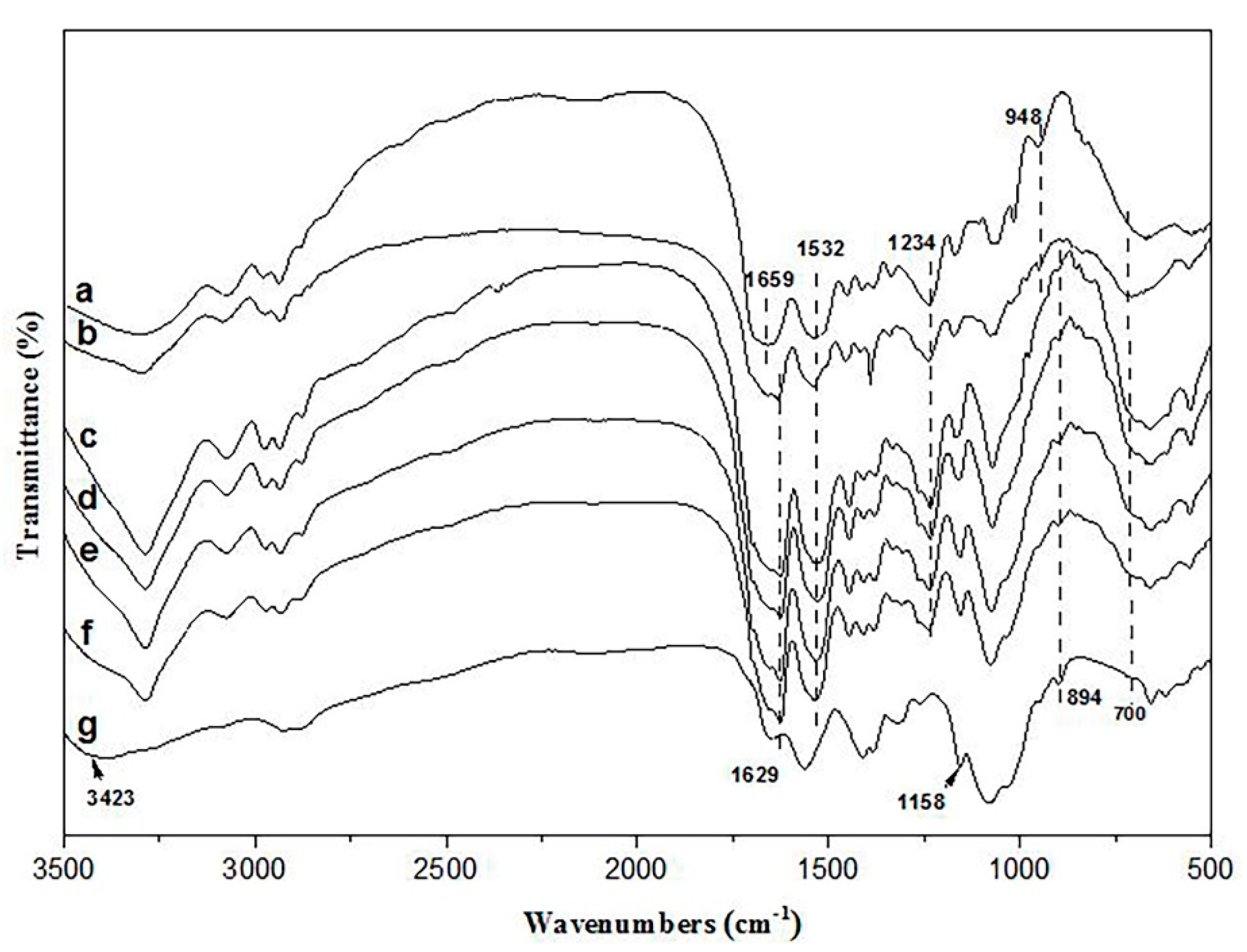
2.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
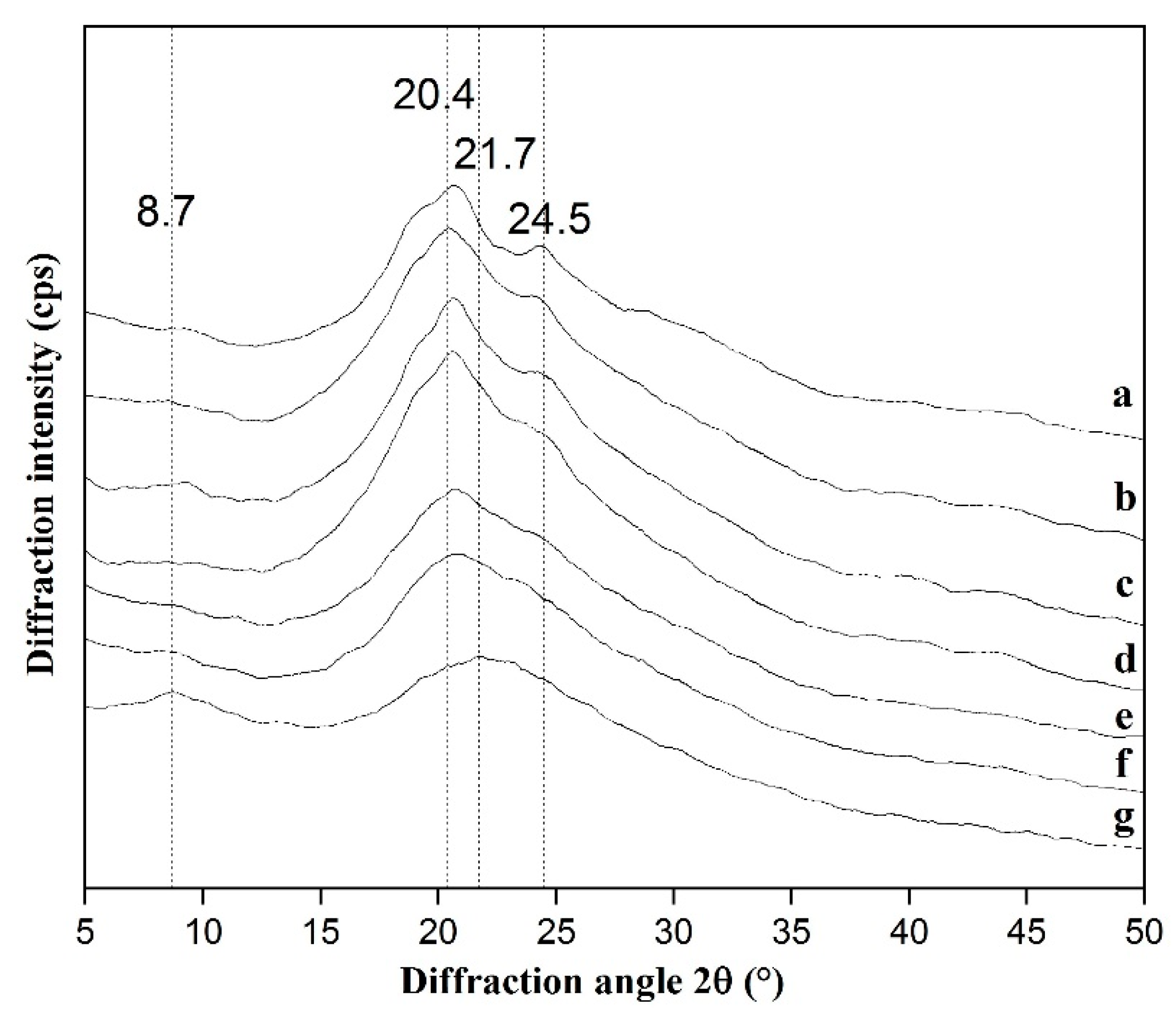
2.3.4. X-ray Diffraction (XRD)
2.3.5. Thermogravimetric Analysis (TGA)
2.3.6. Rheological Studies
2.4. Mechanical Properties
2.5. Swelling Performance and Stimulus Responsive Behaviors
2.6. Drug Loading and In Vitro Release
2.7. Statistical Analysis
3. Result and Discussion
3.1. Preparation of Silk Fibroin/Chitosan Hydrogels
3.2. Characterization of the Hydrogel
3.2.1. SEM
3.2.2. Analysis of the Pore Structure
3.2.3. FTIR
3.2.4. XRD
3.2.5. TGA
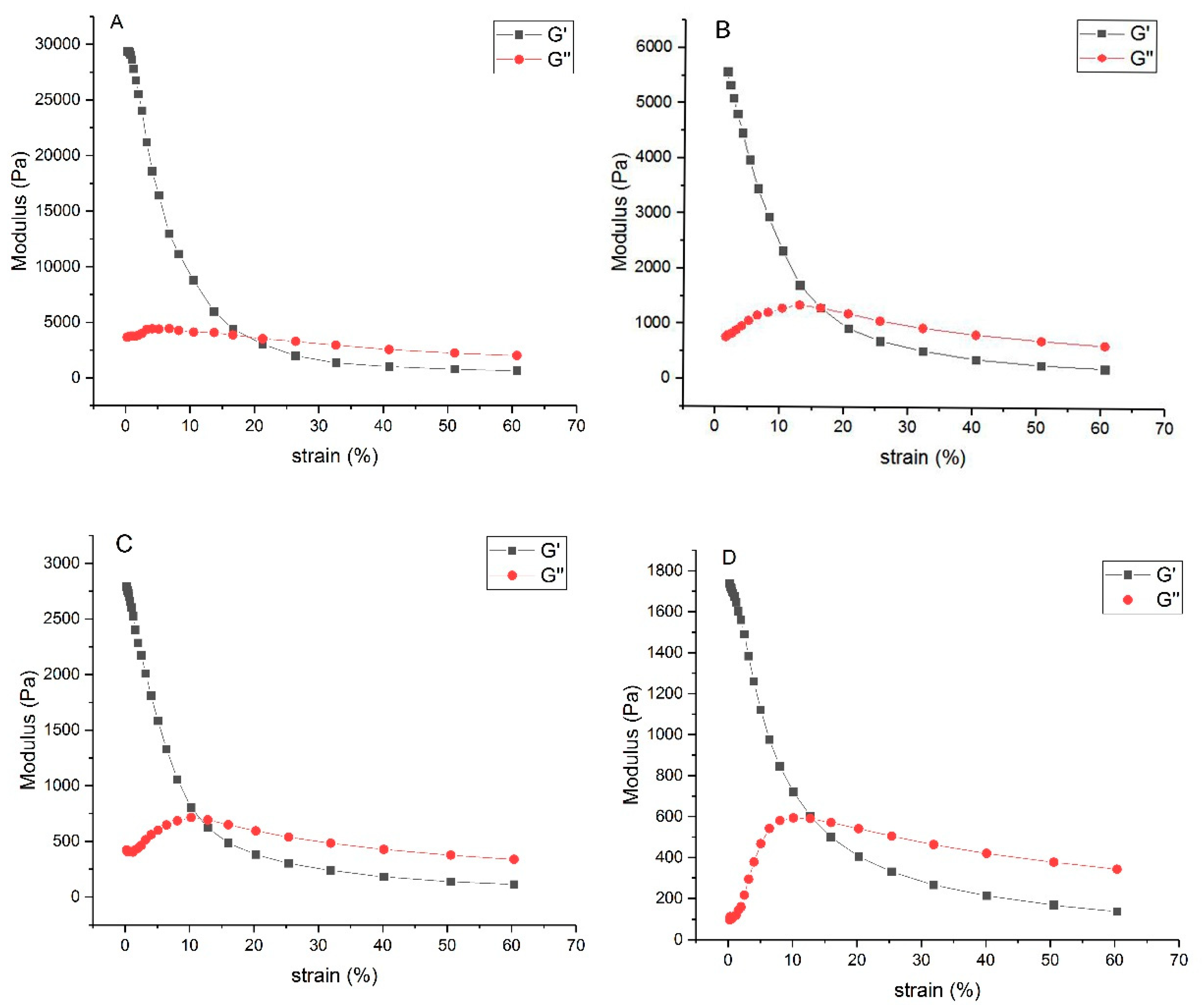
3.2.6. Rheological Properties
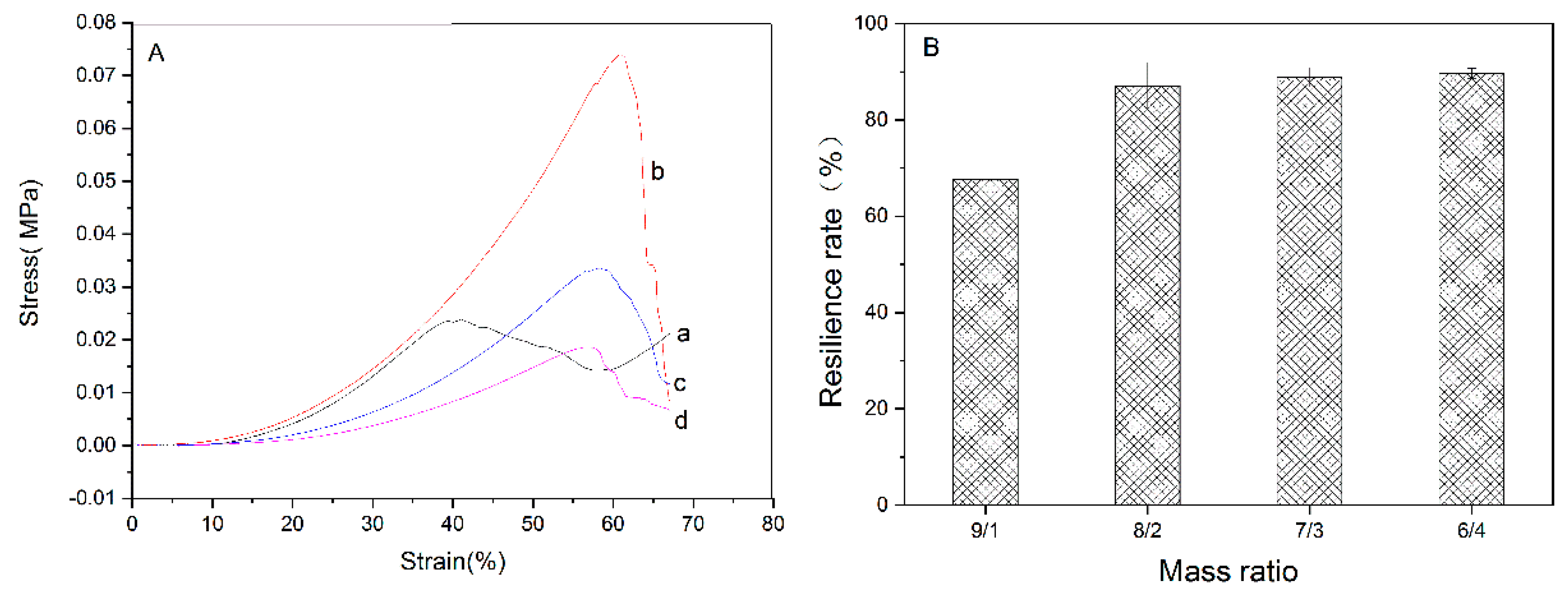
3.3. Mechanical Property
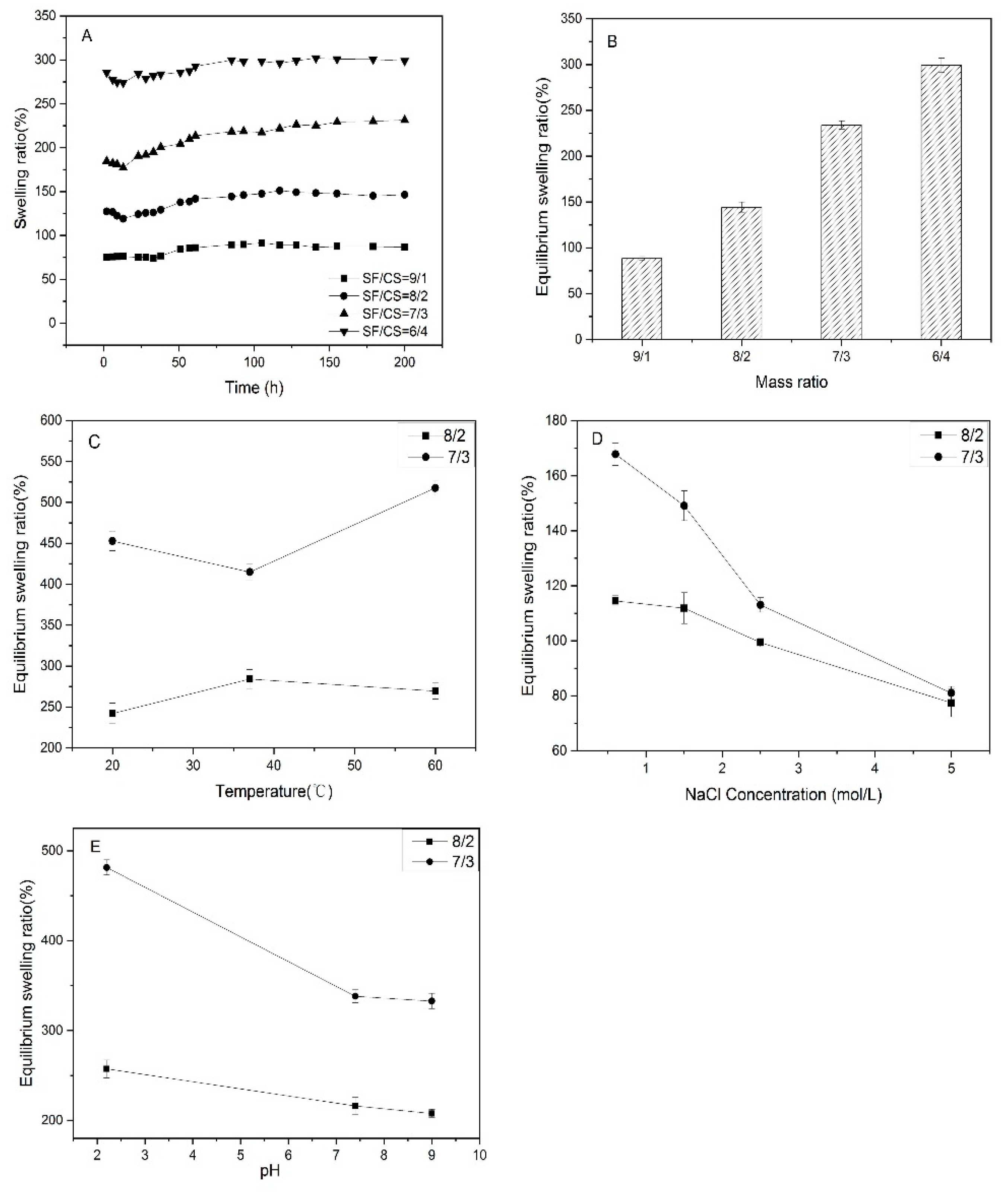
3.4. Swelling Performance and Stimulus Responsive Behaviors
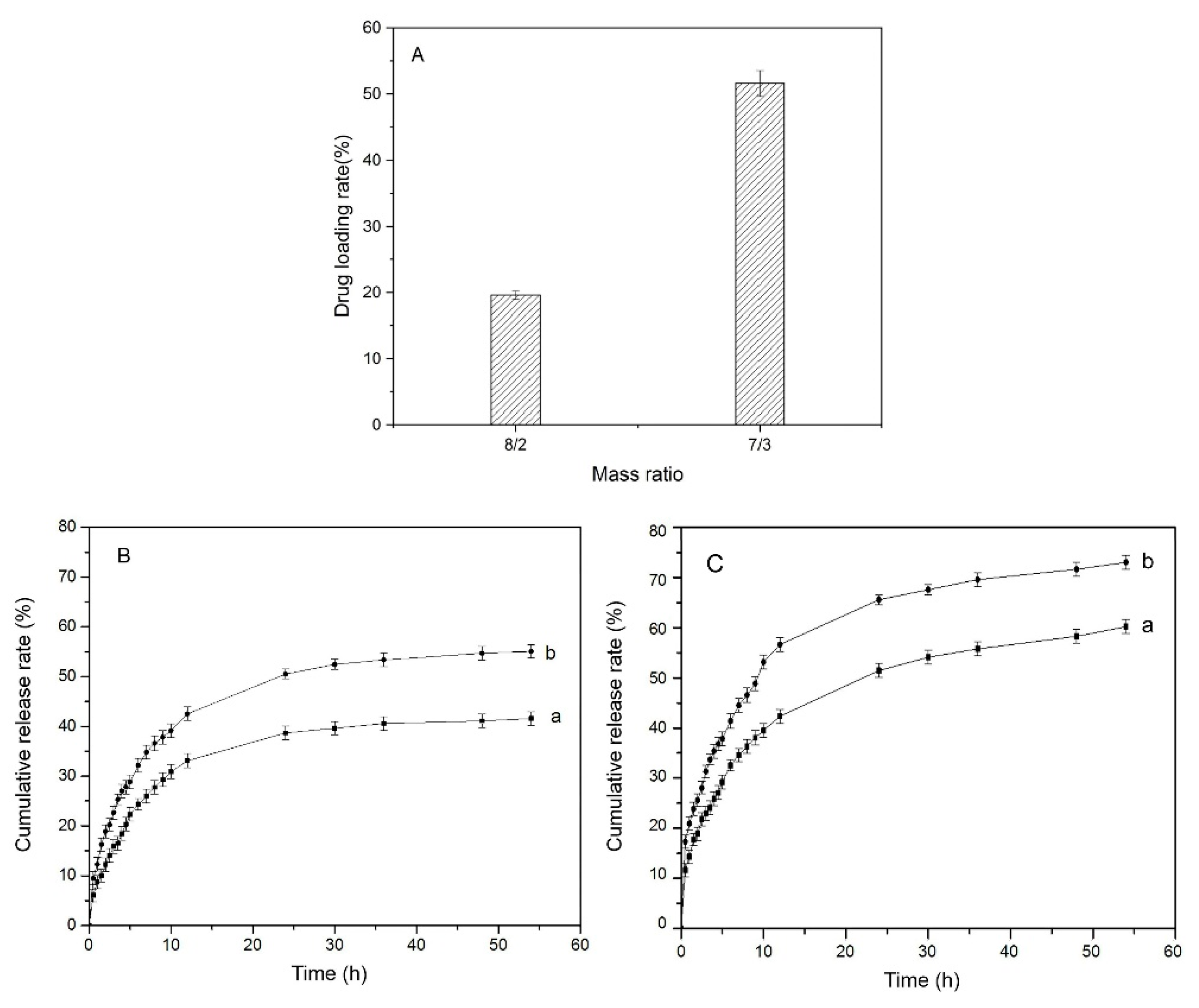
3.5. Drug Loading and In Vitro Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sugano, K.; Terada, K. Rate- and Extent-Limiting Factors of Oral Drug Absorption: Theory and Applications. J. Pharm. Sci. 2015, 104, 2777–2788. [Google Scholar] [CrossRef]
- Loira-Pastoriza, C.; Todoroff, J.; Vanbever, R. Delivery strategies for sustained drug release in the lungs. Adv. Drug Deliv. Rev. 2014, 75, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Yallapu, M.M.; Sadiku, R. A mini review on hydrogels classification and recent developments in miscellaneous applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, X.; Li, C.; Sun, X.; Meng, Q. Chemically Crosslinked Hydrogel Film Leads to Integrated Flexible Supercapacitors with Superior Performance. Adv. Mater. 2015, 27, 7451–7457. [Google Scholar] [CrossRef]
- Billard, A.; Pourchet, L.; Malaise, S.; Alcouffe, P.; Montembault, A.; Ladavière, C. Liposome-loaded chitosan physical hydrogel: Toward a promising delayed-release biosystem. Carbohydr. Polym. 2015, 115, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhou, W.; Wang, J.; Tang, R.; Zhang, D.; Wang, X. Hypromellose succinate-crosslinked chitosan hydrogel films for potential wound dressing. Int. J. Biol. Macromol. 2016, 91, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ulberg, Z.; Korotych, O. Multipurpose smart hydrogel systems. Adv. Colloid Interface Sci. 2011, 168, 247–262. [Google Scholar]
- Kost, J.; Langer, R. Responsive polymeric delivery systems. Adv. Drug Deliv Rev. 2012, 64, 327–341. [Google Scholar] [CrossRef]
- Koetting, M.C.; Peters, J.T.; Steichen, S.D.; Peppas, N.A. Stimulus-responsive hydrogels: Theory, modern advances, and applications. Mater. Sci. Eng. R Rep. A Rev. J. 2015, 93, 1–49. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.H.; Javed, F.; Ahmad, Z.; Akil, H.M. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Bacelar, A.H.; Cengiz, I.F.; Silva-Correia, J.; Sousa, R.A.; Oliveira, J.M.; Reis, R.L. “Smart” Hydrogels in Tissue Engineering and Regenerative Medicine Applications. In Handbook of Intelligent Scaffolds for Regenerative Medicine, 2nd ed.; Panstanfor: Singapore, 2017. [Google Scholar]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.P.; Oliveira, J.M.; Oliveira, A.L.; Caridade, S.G.; Mano, J.F.; Reis, R.L. Macro/microporous silk fibroin scaffolds with potential for articular cartilage and meniscus tissue engineering applications. Acta Biomater. 2012, 8, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Wandrey, A.J.; Merkle, H.P.; Meinel, L. Silk fibroin spheres as a platform for controlled drug delivery. J. Control. Release Off. J. Control. Release Soc. 2008, 132, 26–34. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, X.G.; Kong, M.; Liu, C.S. Preparation of chitosan-based thermosensitive hydrogels for drug delivery. J. Appl. Polym. Sci. 2010, 112, 1509–1515. [Google Scholar] [CrossRef]
- Aycan, D.; Alemdar, N. Development of ph-responsive chitosan-based hydrogel modified with bone ash for controlled release of amoxicillin. Carbohydr. Polym. 2018, 184, 401–407. [Google Scholar] [CrossRef]
- Siti, Z.M.R.; Hazizan, M.A.; Abbas, K.; Zuratul, A.A.H. Controlled release studies through chitosan-based hydrogel synthesized at different polymerization stages. Int. J. Biol. Macromol. 2019, 128, 531–536. [Google Scholar]
- Guaresti, O.; García-Astrain, C.; Aguirresarobe, R.H.; Eceiza, A.; Gabilondo, N. Synthesis of stimuli-responsive chitosan-based hydrogels by diels-alder cross-linking ‘click’ reaction as potential carriers for drug administration. Carbohydr. Polym. 2017, 183, 278–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.S.; Guex, A.G.; Liu, S.S.; Müller, E.; Malini, R.I. A compliant and biomimetic three-layered vascular graft for small blood vessels. Biofabrication 2017, 9, 025010. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M. Chapter Fourteen-Surface Area: Brunauer-Emmett-Teller (BET). Prog. Filtr. Sep. 2015, 585–608. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Tafazzoli, S.M.; Shokrgozar, M.A. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. 2013, 33, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Jang, L.S.; Keng, H.K. Modified Fabr. process of protein chips using a short-chain self-assembled monolayer. Biomed. Microdevices 2008, 10, 203–211. [Google Scholar] [CrossRef]
- Arsiccio, A.; Sparavigna, A.C.; Pisano, R.; Barresi, A.A. Measuring and predicting pore size distribution of freeze-dried solutions. Dry. Technol. 2018, 37, 1–13. [Google Scholar] [CrossRef]
- Huang, B.; Liu, M.; Zhou, C. Chitosan composite hydrogels reinforced with natural clay nanotubes. Carbohydr. Polym. 2017, 175, 689–698. [Google Scholar] [CrossRef]
- Li, X.; You, R.; Luo, Z.; Chen, G.; Li, M. Silk fibroin scaffolds with a micro-/nano-fibrous architecture for dermal regeneration. J. Mater. Chem. B 2016, 4, 2903–2912. [Google Scholar] [CrossRef]
- Wen, D.; Hui, W.; Zhu, X.; Sun, J. Conformation and crystallinity of silk fibroin. J. Text. Res. 2005, 1, 110–112. [Google Scholar]
- Yang, Y.; Cui, J.; Zheng, M.; Hu, C.; Tan, S.; Xiao, Y. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem. Commun. 2011, 48, 380–382. [Google Scholar] [CrossRef]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J. Silk-based biomaterials. Biomater. 2003, 24, 401–416. [Google Scholar] [CrossRef]
- Magoshi, J.; Nakamura, S. Studies on physical properties and structure of silk. glass transition and crystallization of silk fibroin. J. Appl. Polym. Sci. 2010, 19, 1013–1015. [Google Scholar] [CrossRef]
- Tsukada, M.; Freddi, G.; Minoura, N.; Allara, G. Preparation and application of porous silk fibroin materials. J. Appl. Polym. Sci. 1994, 54, 507–514. [Google Scholar] [CrossRef]
- Neto, C.G.T.; Giacometti, J.A.; Job, A.E.; Ferreira, F.C.; Fonseca, J.L.C.; Pereira, M.R. Thermal analysis of chitosan based networks. Carbohydr. Polym. 2005, 62, 97–103. [Google Scholar] [CrossRef]
- Sionkowska, A.; Płanecka, A. Preparation and characterization of silk fibroin/chitosan composite sponges for tissue engineering. J. Mol. Liq. 2013, 178, 5–14. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Tseng, T.C.; Tao, L.; Hsieh, F.Y.; Wei, Y.; Chiu, I.M.; Hsu, S.H. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015, 27, 3518–3524. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Ma, P.X.; Guo, B. Injectable conducting interpenetrating polymer network hydrogels from gelatin-graft-polyaniline and oxidized dextran with enhanced mechanical properties. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Yoshida, R.; Kaneko, Y.; Sakai, K.; Okano, T.; Sakurai, Y.; Bae, Y.H. Positive thermosensitive pulsatile drug release using negative thermosensitive hydrogels. J. Control. Release 1994, 32, 97–102. [Google Scholar] [CrossRef]
- Kozlowska, J.; Pauter, K.; Sionkowska, A. Carrageenan-based hydrogels: Effect of sorbitol and glycerin on the stability, swelling and mechanical properties. Polym. Test. 2018, 67, 7–11. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xu, G.; Hao, W. Swelling behaviours and mechanical properties of silk fibroin-polyurethane composite hydrogels. Compos. Sci. Technol. 2013, 84, 15–22. [Google Scholar] [CrossRef]
- Eman, A.M.A.; Heba, E.H.; Eslam, A.M.; Nabel, A.N. Synthesis, characterization, swelling and antimicrobial efficacies of chemically modified chitosan biopolymer. J. Mol. Liq. 2019, 284, 748–754. [Google Scholar]
- Muller, M.J.; Hollyoak, M.A.; Moaveni, Z.; Brown, T.L.H.; Heggers, J.P. Retardation of wound healing by silver sulfadiazine is reversed by aloe vera and nystatin. Burns 2003, 29, 834–836. [Google Scholar] [CrossRef]
- Li, X.; Fan, D. A novel ccag hydrogel: The mechanical strength and crosslinking densities. Asian J. Chem. 2014, 26, 6567–6570. [Google Scholar] [CrossRef]
- Rasool, N.; Yasin, T.; Heng, J.Y.Y.; Akhter, Z. Synthesis and characterization of novel ph-, ionic strength and temperature- sensitive hydrogel for insulin delivery. Polymer 2010, 51, 1687–1693. [Google Scholar] [CrossRef]
- Zhang, G.X.; Lu, C. Studies on Charges of Silk Fibroin. Sci. Seric. (Acta Sericologica Sin.) 2009, 35, 99–105. [Google Scholar]
- Dharmalingam, K.; Anandalakshmi, R. Fabrication, characterization and drug loading efficiency of citric acid crosslinked NaCMC-HPMC hydrogel films for wound healing drug delivery applications. Int. J. Biol. Macromol. 2019, 134, 815–829. [Google Scholar] [CrossRef]
- Siangsanoh, C.; Ummartyotin, S.; Sathirakul, K.; Rojanapanthu, P.; Treesuppharat, W. Fabrication and characterization of triple-responsive composite hydrogel for targeted and controlled drug delivery system. J. Mol. Liq. 2018, 256, 90–99. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Tang, E.; Zhao, H. An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors. Polymers 2019, 11, 1980. https://doi.org/10.3390/polym11121980
Xu Z, Tang E, Zhao H. An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors. Polymers. 2019; 11(12):1980. https://doi.org/10.3390/polym11121980
Chicago/Turabian StyleXu, Zhangpeng, Erni Tang, and Huijing Zhao. 2019. "An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors" Polymers 11, no. 12: 1980. https://doi.org/10.3390/polym11121980
APA StyleXu, Z., Tang, E., & Zhao, H. (2019). An Environmentally Sensitive Silk Fibroin/Chitosan Hydrogel and Its Drug Release Behaviors. Polymers, 11(12), 1980. https://doi.org/10.3390/polym11121980





