Correlation of Montmorillonite Sheet Thickness and Flame Retardant Behavior of a Chitosan–Montmorillonite Nanosheet Membrane Assembled on Flexible Polyurethane Foam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedures and Methods
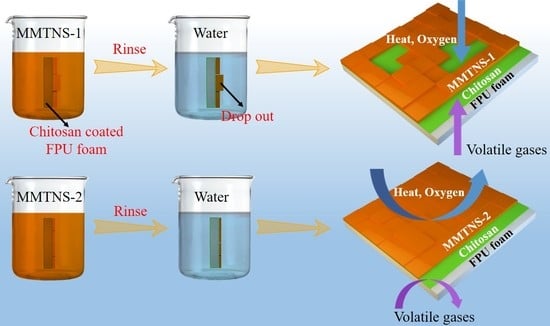
2.2.1. Preparation of MMTNS-1 and MMTNS-2
2.2.2. Preparation of Chitosan and PAA Solutions
2.2.3. LbL Assembly Deposition Process
2.3. Characterization
3. Results and Discussion
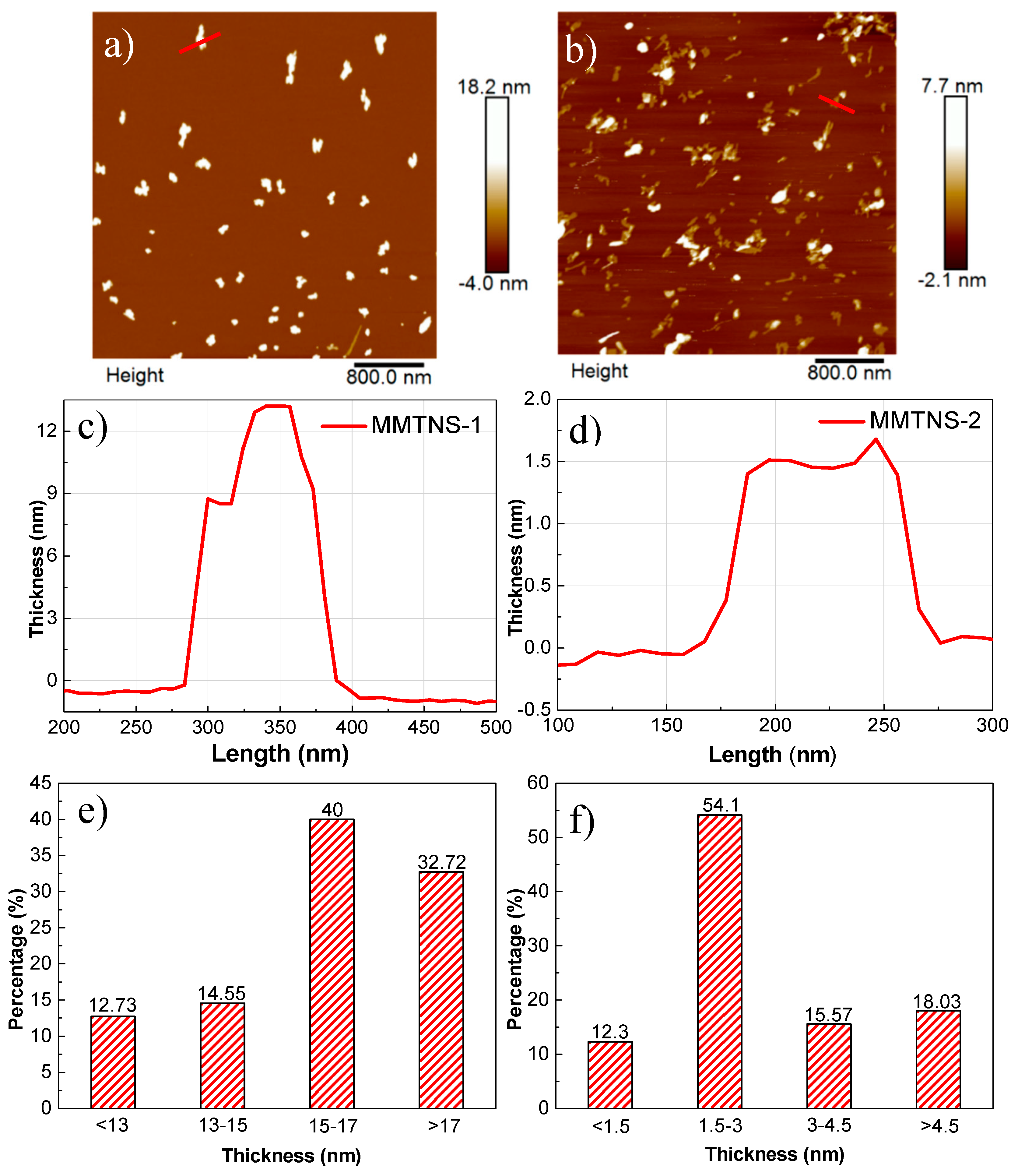
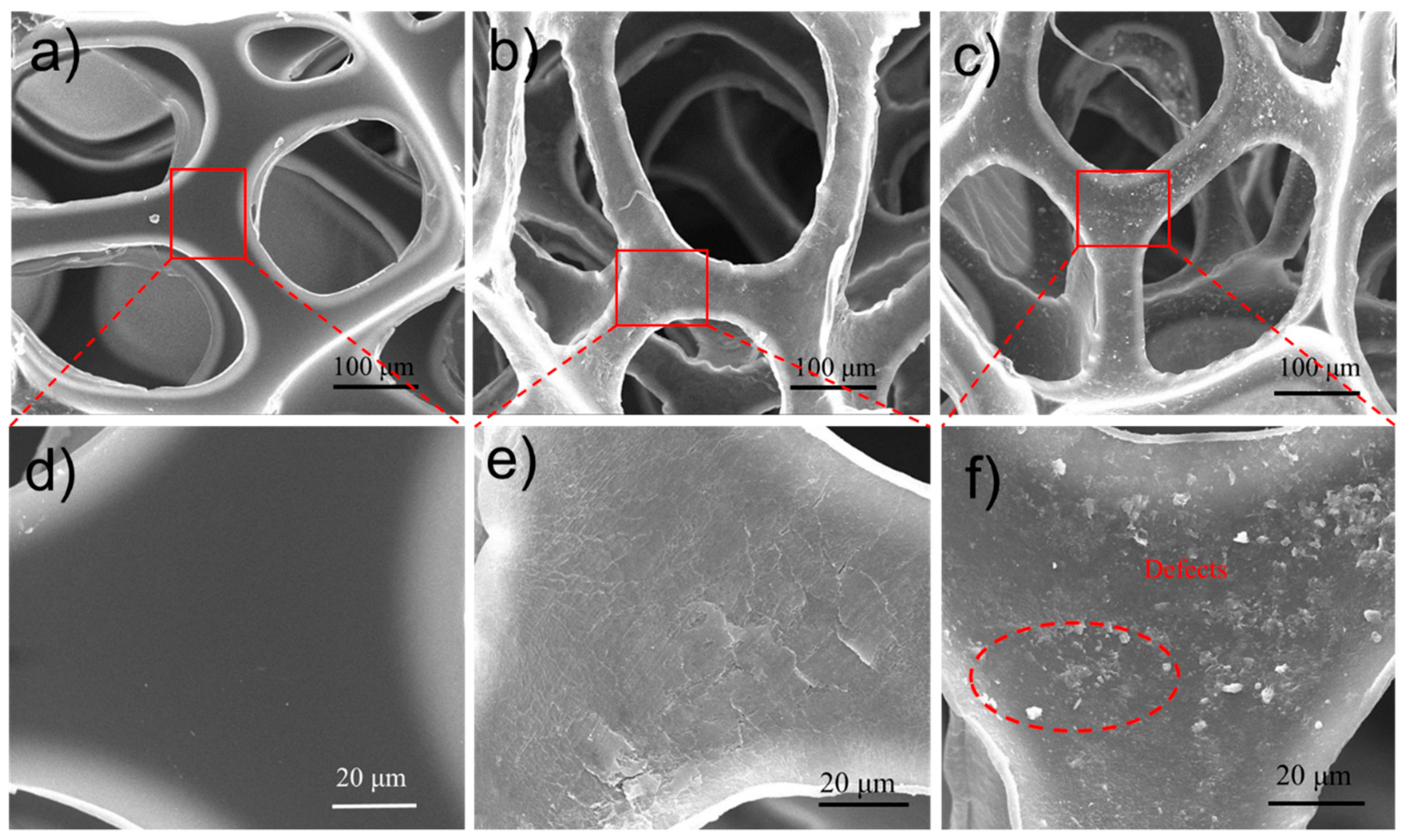
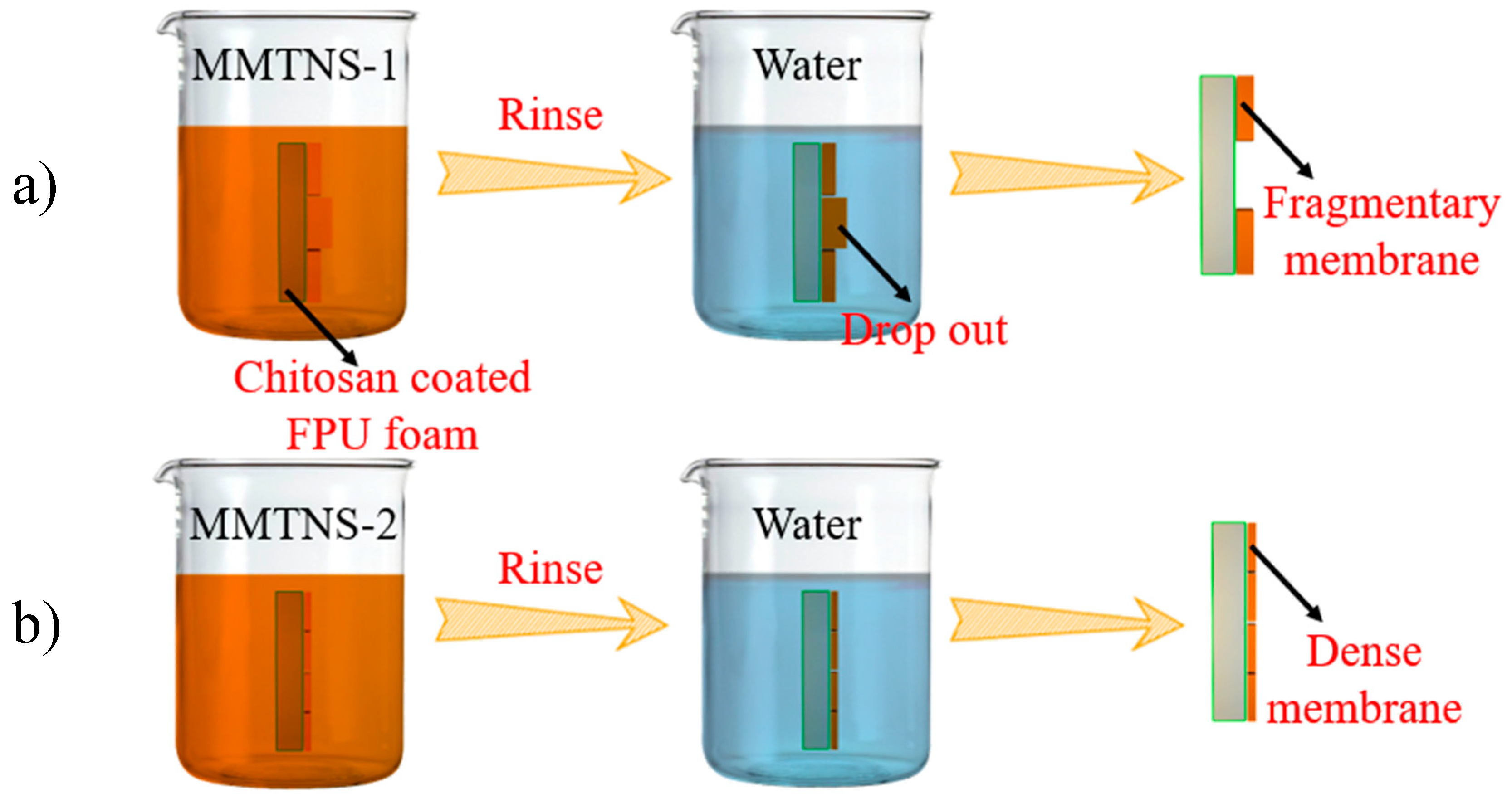
3.1. Characterization of MMTNS
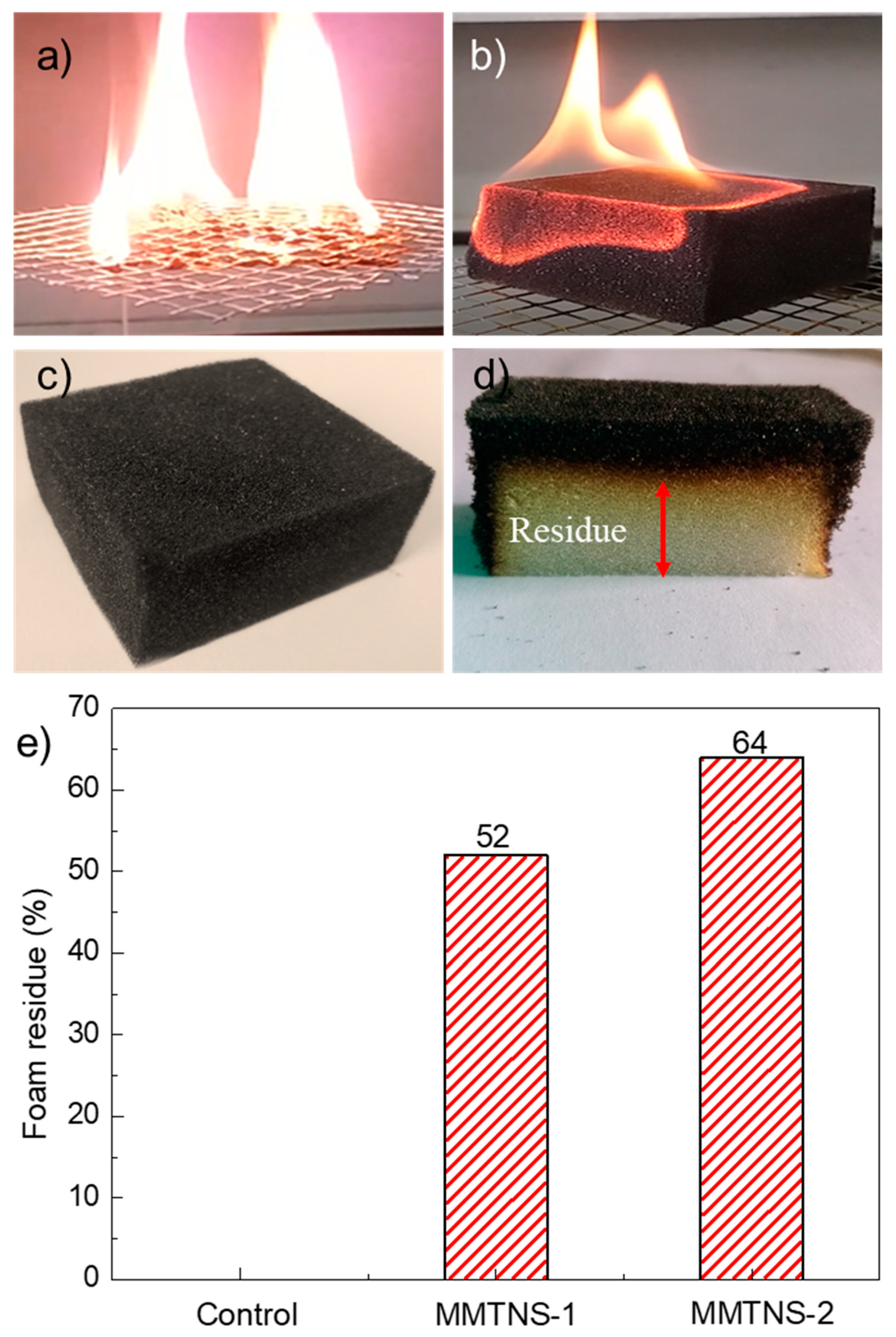
3.2. Characterization of Thermal Stability and Flame Retardancy
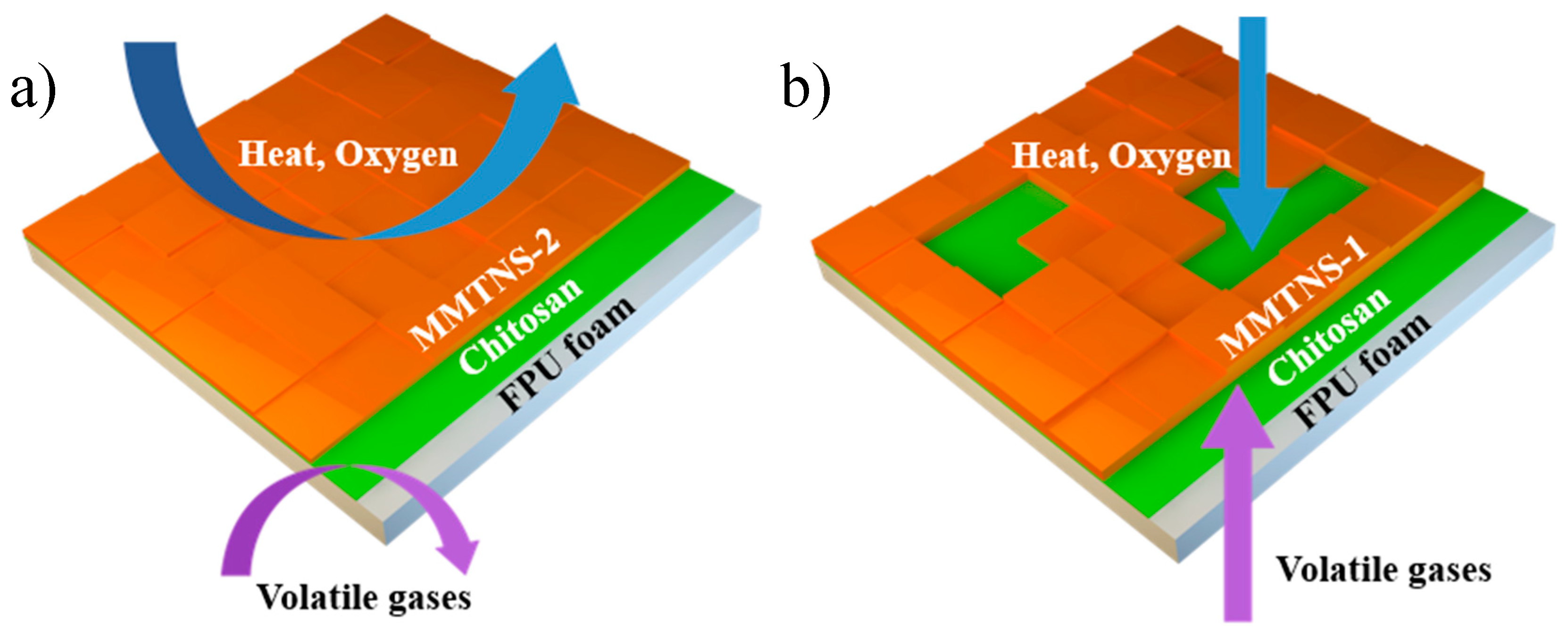
3.3. Mechanism by Which MMTNS Thickness Affects the Flame Retardancy of CH–MMTNS membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lefebvre, J.; Bastin, B.; le Bras, M.; Duquesne, S.; Paleja, R.; Delobel, R. Thermal stability and fire properties of conventional flexible polyurethane foam formulations. Polym. Degrad. Stab. 2005, 88, 28–34. [Google Scholar] [CrossRef]
- Konig, A.; Fehrenbacher, U.; Hirth, T.; Kroke, E. Flexible Polyurethane Foam with the Flame-retardant Melamine. J. Cell. Plast. 2008, 44, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Luo, S.; Zhang, B.; Huang, Z.; Tang, X. Electroless preparation and characterization of magnetic Ni–P plating on polyurethane foam. Appl. Surf. Sci. 2008, 254, 7427–7430. [Google Scholar] [CrossRef]
- Huang, G.; Gao, J.; Wang, X. Preparation and characterization of montmorillonite modified by phosphorus–nitrogen containing quaternary ammonium salts. Appl. Surf. Sci. 2012, 258, 4054–4062. [Google Scholar] [CrossRef]
- Holder, K.M.; Smith, R.J.; Grunlan, J.C. A review of flame retardant nanocoatings prepared using layer-by-layer assembly of polyelectrolytes. J. Mater. Sci. 2017, 52, 12923–12959. [Google Scholar] [CrossRef]
- Patra, D.; Vangal, P.; Cain, A.A.; Cho, C.; Regev, O.; Grunlan, J.C. Inorganic nanoparticle thin film that suppresses flammability of polyurethane with only a single electrostatically-assembled bilayer. ACS Appl. Mater. Interfaces 2014, 6, 16903–16908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, K.; Jiao, T.; Xing, R.; Ma, X.; Hu, J.; Huang, H.; Zhang, L.; Yan, X. Fabrication of Hierarchical Layer-by-Layer Assembled Diamond-based Core-Shell Nanocomposites as Highly Efficient Dye Absorbents for Wastewater Treatment. Sci. Rep. 2017, 7, 44076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haifeng, P.; Wei, W.; Ying, P.; Lei, S.; Yuan, H.; Meow, L.K. Formation of layer-by-layer assembled titanate nanotubes filled coating on flexible polyurethane foam with improved flame retardant and smoke suppression properties. ACS Appl. Mater. Interfaces 2015, 7, 101–111. [Google Scholar]
- Kim, Y.S.; Davis, R. Multi-walled carbon nanotube layer-by-layer coatings with a trilayer structure to reduce foam flammability. Thin Solid Films 2014, 550, 184–189. [Google Scholar] [CrossRef]
- Feng, S.S.; Mei, L.; Anitha, P.; Gan, C.W.; Zhou, W. Poly(lactide)–vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials 2009, 30, 3297–3306. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Li, X.; Ouyang, J.; Yang, H. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Kang, S.; Zhao, Y.; Wang, W.; Zhang, T.; Chen, T.; Yi, H.; Rao, F.; Song, S. Removal of Methylene Blue from Water with Montmorillonite Nanosheets/Chitosan Hydrogels as Adsorbent. Appl. Surf. Sci. 2018, 448, 203–211. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L. Performance and mechanisms of fire retardants in polymers—A review. Polym. Degrad. Stab. 1988, 20, 271–294. [Google Scholar] [CrossRef]
- Wang, W.; Pan, H.; Shi, Y.; Yu, B.; Pan, Y.; Liew, K.M.; Song, L.; Hu, Y. Sandwichlike Coating Consisting of Alternating Montmorillonite and β-FeOOH for Reducing the Fire Hazard of Flexible Polyurethane Foam. ACS Sustain. Chem. Eng. 2015, 3, 3214–3223. [Google Scholar] [CrossRef]
- Laufer, G.; Kirkland, C.; Cain, A.A.; Grunlan, J.C. Clay–Chitosan Nanobrick Walls: Completely Renewable Gas Barrier and Flame-Retardant Nanocoatings. ACS Appl. Mater. Interfaces 2012, 4, 1643–1649. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi, H.; Jia, F.; Li, H.; Peng, C.; Song, S. A novel method for determining the thickness of hydration shells on nanosheets: A case of montmorillonite in water. Powder Technol. 2017, 306, 74–79. [Google Scholar] [CrossRef]
- Yi, H.; Zhan, W.; Zhao, Y.; Qu, S.; Wang, W.; Chen, P. Solar Energy Materials and Solar Cells A novel core-shell structural montmorillonite nanosheets/stearic acid composite PCM for great promotion of thermal energy storage properties. Sol. Energy Mater. Sol. Cells 2019, 192, 57–64. [Google Scholar] [CrossRef]
- Yi, H.; Jia, F.; Zhao, Y.; Wang, W.; Song, S.; Li, H.; Liu, C. Applied Surface Science Surface wettability of montmorillonite (0 0 1) surface as a ff ected by surface charge and exchangeable cations: A molecular dynamic study. Appl. Surf. Sci. 2018, 459, 148–154. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Q.; Zhang, X.; Pan, H.; Lu, Y. Graphene oxide-filled multilayer coating to improve flame-retardant and smoke suppression properties of flexible polyurethane foam. J. Mater. Sci. 2016, 51, 10361–10374. [Google Scholar] [CrossRef]
- Polyphosphate, M. Preparation and Flame Retardance of Polyurethane Composites Containing Microencapsulated Melamine Polyphosphate. Polymers 2017, 9, 407. [Google Scholar] [CrossRef]
- Li, Y.C.; Schulz, J.; Mannen, S.; Delhom, C.; Condon, B.; Chang, S.C.; Zammarano, M.; Grunlan, J.C. Flame Retardant Behavior of Polyelectrolyte−Clay Thin Film Assemblies on Cotton Fabric. ACS Nano 2010, 4, 3325–3337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, X.; Hui, P.; Fang, W.; Xiu, L.; Yang, Y.; Hao, J. Layer-by-Layer Assembly of Multifunctional Flame Retardant Based on Brucite, 3-Aminopropyltriethoxysilane, and Alginate and Its Applications in Ethylene-Vinyl Acetate Resin. ACS Appl. Mater. Interfaces 2016, 8, 9925–9935. [Google Scholar] [CrossRef]
- Cain, A.A.; Nolen, C.R.; Li, Y.C.; Davis, R.; Grunlan, J.C. Phosphorous-filled nanobrick wall multilayer thin film eliminates polyurethane melt dripping and reduces heat release associated with fire. Polym. Degrad. Stab. 2013, 98, 2645–2652. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-Cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Chao, P.; Li, Y.; Gu, X.; Han, D.; Jia, X.; Wang, M.; Zhou, T.; Wang, T. Novel phosphorus–nitrogen–silicon flame retardants and their application in cycloaliphatic epoxy systems. Polym. Chem. 2015, 6, 2977–2985. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Song, S. Electrophoretic mobility study for heterocoagulation of montmorillonite with fluorite in aqueous solutions. Powder Technol. 2016, 309, 61–67. [Google Scholar] [CrossRef]


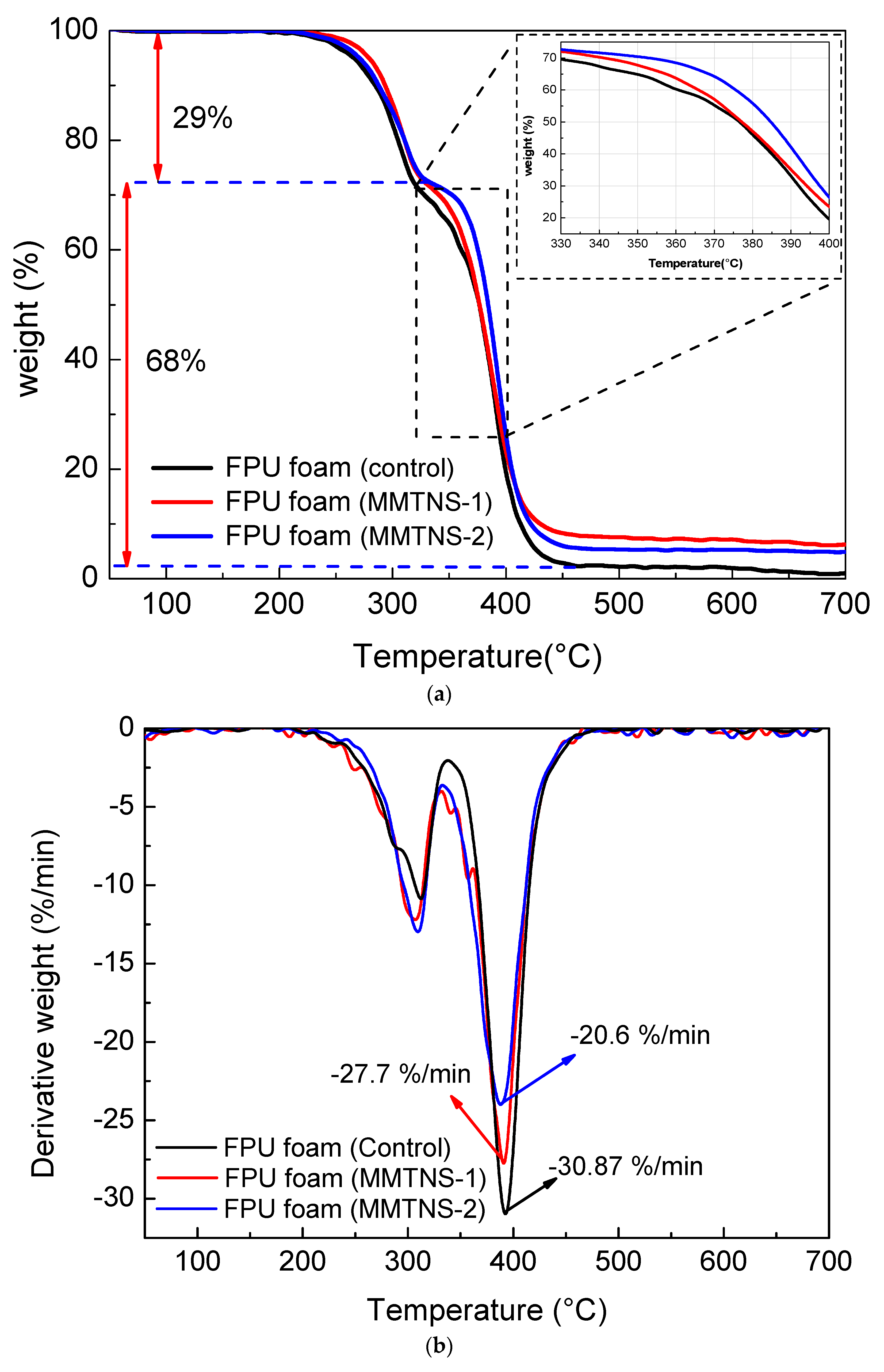


| Sample | Addition (%) | T5% (°C) | T10% (°C) | T50% (°C) | Residue Mass (%) | Residue Char (%) |
|---|---|---|---|---|---|---|
| Control | 0 | 265.7 | 284.6 | 376.4 | 0.988 | 0.988 |
| MMTNS-1 | 1.59 | 279 | 290.3 | 376.7 | 6.24 | 1.59 |
| MMTNS-2 | 2.47 | 277.5 | 287.6 | 384.2 | 4.91 | 2.47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Zhao, Y.; Wang, W.; Zhang, T.; Song, S. Correlation of Montmorillonite Sheet Thickness and Flame Retardant Behavior of a Chitosan–Montmorillonite Nanosheet Membrane Assembled on Flexible Polyurethane Foam. Polymers 2019, 11, 213. https://doi.org/10.3390/polym11020213
Chen P, Zhao Y, Wang W, Zhang T, Song S. Correlation of Montmorillonite Sheet Thickness and Flame Retardant Behavior of a Chitosan–Montmorillonite Nanosheet Membrane Assembled on Flexible Polyurethane Foam. Polymers. 2019; 11(2):213. https://doi.org/10.3390/polym11020213
Chicago/Turabian StyleChen, Peng, Yunliang Zhao, Wei Wang, Tingting Zhang, and Shaoxian Song. 2019. "Correlation of Montmorillonite Sheet Thickness and Flame Retardant Behavior of a Chitosan–Montmorillonite Nanosheet Membrane Assembled on Flexible Polyurethane Foam" Polymers 11, no. 2: 213. https://doi.org/10.3390/polym11020213
APA StyleChen, P., Zhao, Y., Wang, W., Zhang, T., & Song, S. (2019). Correlation of Montmorillonite Sheet Thickness and Flame Retardant Behavior of a Chitosan–Montmorillonite Nanosheet Membrane Assembled on Flexible Polyurethane Foam. Polymers, 11(2), 213. https://doi.org/10.3390/polym11020213